
Clinical and bronchoscopic aspects of bronchial healing after sleeve resection for lung cancer: a multivariate analysis on 541 cases
Introduction
The purpose of sleeve lobectomy is to avoid pneumonectomy and thus to preserve functional lung tissue. In specialized centers, the systematic application of sleeve resection can reduce the rate of pneumonectomies below 10% (1). Patients benefit from lower postoperative morbidity, better quality of life and higher long-term survival (2,3).
Anastomotic insufficiency is a severe complication following sleeve resection. Completion pneumonectomy performed due to anastomotic complications is associated with high morbidity and mortality (4,5). Previously, various factors influencing anastomotic healing have been listed, among these neoadjuvant mediastinal radiation, non-radical resection and postoperative infection (6-10). However, systematic investigations were carried out only for preoperative irradiation, where a negative influence on anastomotic healing could be demonstrated (1,8,10).
In our institution, a five-level bronchoscopic classification was developed to assess anastomotic healing on the seventh postoperative day (Figure 1). The classification is based on the degree of ischemic changes in the mucosa of the distal part of the anastomosis. From circular endobronchial signs of mucosal ischemia onwards (grade 3 or higher), the anastomosis is considered to be at high risk for the development of insufficiency (1). Based on the grading of the anastomosis, patients are discharged (grade 1 and 2) or are kept in hospital for systemic antibiotics and re-bronchoscopy 4 days later (grade 3 or higher). This procedure was based on clinical experience and has not yet been systematically validated.
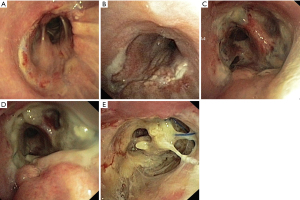
Therefore the aims of this study were first, to investigate the predictive power of our anastomosis classification for the development of insufficiencies and second, to identify negative predictors of anastomotic healing based on this classification. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1627/rc).
Methods
The study was approved by the ethics committee of the University of Witten Herdecke (Nr. 51/2017). All participants gave informed consent before taking part in the study. This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
From January 2006 to December 2019 3883 anatomic resections (including lobectomies, bilobectomies, segmentectomies and pneumonectomies) for the treatment of lung cancer were performed in our hospital. Of these, 621 (16%) were performed as sleeve resections. To focus on a homogenous group of patients, sleeve pneumonectomies (n=16), isolated bronchial sleeve resections (n=21), carinal resections (n=20) and segmentectomies (n=1) were not included in the analysis. For the same reason, patients with carcinoid tumors (n=26) were excluded because they differ from other lung cancer patients in terms of age, pretreatment, smoking habits and prognosis. Finally, 541 consecutive patients who underwent sleeve lobectomy or sleeve bilobectomy for lung cancer between January 2006 and December 2019 were included in this study and retrospectively analyzed.
Patients’ characteristics and perioperative data were collected prospectively using a standardized data entry form. Patients consented preoperatively for anonymous data collection.
Preoperative staging consisted in whole body MRI or PET-CT, brain MRI and mediastinal staging by mediastinoscopy or EBUS bronchoscopy.
Neoadjuvant chemotherapy was based on a platinum-containing treatment combined with vinorelbine or pemetrexed. In case of neoadjuvant chemoradiation two cycles of chemotherapy were applied simultaneously to radiotherapy. Depending on histology, tumor size and location two cycles of induction chemotherapy were optional.
Radiotherapy was applied using a fractionated radiation therapy with a single daily dose of 1.8 to 2 Gy with 5 fractions per week up to a total dose of 60–66 Gy.
Preoperative pulmonary function testing (FEV1) and C-reactive protein (CRP) levels were obtained prior to resection.
The surgical procedures included lobectomies and bilobectomies with complete bronchus sleeve resection and radical lymphadenectomy. Angioplastic or intrapericardial resections were performed when necessary. The bronchial anastomosis was performed by a continuous suture with 4-0 polydioxanone (PDS). In the case of neoadjuvant radiotherapy, the anastomosis was routinely covered with vital pedicled tissue. In addition to pedicled thymic flaps, which are standard practice at our hospital, flaps from intercostal muscle, pectoralis major, latissimus dorsi, and pericardium were used. Covering of the anastomosis in non-pretreated patients was performed at the surgeon’s preference.
In all patients the healing of the anastomosis was assessed by bronchoscopy at the 7th postoperative day, using our classification from grade 1 to 5 (Figure 1). Anastomotic insufficiency was defined as grade 5 anastomosis (bronchial wall necrosis with perforation, insufficiency).
Bronchoscopy was performed by the surgeon who performed the operation or by a member of the surgical team. Intraoperative bronchoscopy to examine the anastomosis was not performed as a standard procedure. Bronchoscopies before postoperative day seven were performed when clinically necessary.
Grade 1 and 2 healing were considered satisfactory and the patients were discharged. An anastomosis healing of grade 3 or higher was considered critical. These patients received systemic antibiotic treatment and re-bronchoscopy was performed 4 days later to monitor bronchial healing.
Anastomotic insufficiencies were treated conservatively with antibiotics or surgically. The decision regarding the approach was made on an individual basis.
Statistical analysis
Statistical evaluation was conducted with MedCalc 20.013 (MedCalc Software Ltd., Belgium). Categorical data between groups was compared made using the χ²-test. Comparison of means was performed by the unpaired t-test.
In order to evaluate the independence of factors on anastomotic healing (grade <3 vs. grade ≥3), all variables showing statistical significance (P<0.05) were included in a logistic regression analysis. The classifying cut-off value was set to 0.5. P values <0.05 were considered significant.
Results
Anastomosis grading on the seventh postoperative day is shown in Table 1. Clinicopathological characteristics are summarized in Table 2. Anastomotic healing was satisfactory (grade 1 or 2) in 441 patients (81.5%). In 100 patients (18.5%) the anastomosis was classified as critical (grade ≥3). Patients with critical anastomotic healing spent more time in hospital (16.7 vs. 12.0 days, P<0.0001) and had a longer drainage treatment time (8.2 vs. 6.8 days, P<0.05) compared to patients with a satisfactory anastomotic healing. Furthermore, patients with critical anastomoses suffered more complications (61.0% vs. 40.1%, P<0.001) and had a higher postoperative mortality (6.0% vs. 0.7%, P<0.001) than patients with satisfactory anastomosis. The most frequent complications were prolonged air leak (18.0% vs. 16.1%, P=0.64 and pneumonia (22.0% vs. 7.9%, P=0.001).
Table 1
| Anastomosis grading | n (%) |
|---|---|
| Grade 1 | 234 (43.3) |
| Grade 2 | 207 (38.3) |
| Grade 3 | 75 (13.9) |
| Grade 4 | 21 (3.9) |
| Grade 5 | 4 (0.7) |
Table 2
| Variables | Anastomosis grade <3 (n=441) | Anastomosis grade ≥3 (n=100) | P value |
|---|---|---|---|
| Male sex | 290 (65.8%) | 75 (75.0%) | 0.08 |
| Age ≥70 years | 124 (28.1%) | 32 (32.0%) | 0.44 |
| Preop. CRP | |||
| >3 mg/L | 402 (91.2%) | 95 (95.0%) | 0.21 |
| >20 mg/L | 153 (34.7%) | 48 (48.0%) | 0.013 |
| >40 mg/L | 103 (23.4%) | 40 (40.0%) | <0.001 |
| FEV1 <80% | 276 (62.6%) | 83 (83.0%) | <0.001 |
| Neoadjuvant therapy | |||
| No neoadjuvant therapy | 330 (74.8%) | 64 (64.0%) | 0.03 |
| Chemotherapy | 38 (8.6%) | 6 (6%) | 0.39 |
| Radiation/chemoradiation | 73 (16.6%) | 30 (30%) | 0.002 |
| Current or former smoker | 410 (93%) | 97 (97.0%) | 0.14 |
| Tumour size, cm | 4.52±2.56 | 4.71±2.82 | 0.53 |
| Nodal stage (postoperative) | |||
| N0 | 156 (35.4%) | 41 (41.0%) | 0.29 |
| N1 | 163 (37.0%) | 27 (27.0%) | 0.06 |
| N2/N3 | 122 (27.7%) | 32 (32.0%) | 0.39 |
| Stage (postoperative) | |||
| Ia/Ib | 30 (6.8%) | 3 (3.0%) | 0.15 |
| IIa/IIb | 178 (40.3%) | 35 (35.0%) | 0.33 |
| IIIa/IIIb | 215 (48.8%) | 58 (58.0%) | 0.1 |
| IVa/IVb | 18 (4.1%) | 4 (4.0%) | 0.96 |
| Histology | |||
| Adenocarcinoma | 150 (34.0%) | 29 (29.0%) | 0.34 |
| Squamous cell carcinoma | 237 (53.7%) | 65 (65.0%) | 0.04 |
| Other | 47 (10.7%) | 6 (6.0%) | 0.16 |
| Type of resection | |||
| Lobectomy | 412 (93.4%) | 78 (78.0%) | <0.0001 |
| Right upper lobe | 161 (36.5%) | 28 (28.0%) | 0.11 |
| Left upper lobe | 125 (28.3%) | 17 (17.0%) | 0.02 |
| Right lower lobe | 47 (10.7%) | 14 (14.0%) | 0.35 |
| Left lower lobe | 75 (17%) | 19 (19.0%) | 0.63 |
| Middle lobe | 4 (0.9%) | 0 | 0.34 |
| Bilobectomy | 29 (6.6%) | 22 (22.0%) | <0.0001 |
| Upper | 23 (5.2%) | 13 (13.0%) | 0.005 |
| Lower | 6 (1.4%) | 9 (9.0%) | <0.0001 |
| Thoracoscopic resection | 9 (2.0%) | 2 (2.0%) | 0.98 |
| Angioplasty | 166 (37.6%) | 47 (47.0%) | 0.08 |
| R1/R2 resection | 59 (13.4%) | 18 (18.0%) | 0.24 |
| Discharge on postoperative day | 12.0±7.77 | 16.7±10.54 | <0.0001 |
| Postoperative complications | 179 (40.1%) | 61 (61.0%) | <0.001 |
| Prolonged air leak (>7 d) | 71 (16.1%) | 18 (18.0%) | 0.64 |
| Pneumonia | 35 (7.9%) | 22 (22.0%) | 0.001 |
| Atrial fibrillation | 22 (5.0%) | 5 (5.0%) | 1 |
| Respiratory failure | 14 (3.2%) | 6 (6.0%) | 0.18 |
| Re-operation | 12 (2.7%) | 9 (9.0%) | 0.003 |
| Anastomosis insufficiency | 1 (0.2%) | 19 (19.0%) | <0.0001 |
| Bleeding | 11 (2.5%) | 4 (4.0%) | 0.41 |
| Recurrent laryngeal nerve injury | 4 (0.9%) | 1 (1.0%) | 0.93 |
| Pleural empyema | 4 (0.9%) | 0 | 0.34 |
| Other | 37 (8.4%) | 12 (12.0%) | 0.26 |
| Postoperative mortality | 3 (0.7%) | 6 (6.0%) | <0.001 |
CRP, C-reactive protein; FEV1, forced expiratory volume in 1 second.
A total of 20 patients (3.7%) developed anastomotic insufficiency. In four patients, insufficiency (grade 5) was discovered during routine bronchoscopy on the seventh postoperative day, 16 patients developed an insufficiency in the further clinical course. 19 patients with anastomotic insufficiency were in the group with critical anastomotic healing and one in the group with satisfactory anastomotic healing (P<0.0001).
In patients who received neoadjuvant radiation insufficiency rate was 6.8% (7 from 103 patients) and in patients without neoadjuvant radiation 3.0% (13 from 438 patients, P=0.06).
Table 3 shows characteristics, initial surgery and clinical course of the patients with anastomotic insufficiency. Nine patients (45%) with anastomotic insufficiency underwent revision surgery, including four with revision of the anastomosis, four with completion pneumonectomy and one with middle lobe resection and reimplantation of the upper lobe to the main bronchus. One patient initially underwent revision of the anastomosis and later underwent pneumonectomy. In 12 patients (60%), anastomotic insufficiency was treated conservatively with antibiotics. Five patients (25%) with anastomotic insufficiency died during hospitalization. Mortality was 22% (2 of 9) in reoperated patients and 25% (3 of 12) in patients initially treated conservatively.
Table 3
| No. | Sex | Age | Preop. CRP | Preop. FEV1 | Preop. RT | Initial surgery | AG POD 7 | Postoperative course |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 54 | 9 | 66 | No | Lower bilobectomy | 4 | Detection of insufficiency on POD 14. Conservative treatment. Discharged on POD 34 |
| 2 | M | 64 | 17 | 64 | Yes | Upper bilobectomy, thymic flap | 4 | Massive hemoptysis on POD 17. Intraoperative detection of anastomosis insufficiency with broncho-pulmonary artery fistula. Completion pneumonectomy. Patient died shortly after surgery |
| 3 | M | 81 | 29 | 55 | No | Right upper lobe | 4 | Detection of insufficiency on POD 10. No surgery due to critical condition. Patient died of pneumogenic sepsis on POD 11 |
| 4 | M | 55 | 290 | 52 | No | Left lower lobe | 4 | Detection of insufficiency on POD 28. Infarction of the remnant lung in CT-scan. Completion pneumonectomy. Discharged on POD 39 |
| 5 | M | 72 | 180 | 56 | No | Left upper lobe, PA-angioplasty | 4 | Detection of insufficiency on POD 13. Conservative treatment. Discharged on POD 25 |
| 6 | M | 75 | 65 | 80 | Yes | Right upper lobe, thymic flap | 5 | Detection of insufficiency on POD 7. Initially conservative treatment. Reoperation with Revision of the anastomosis on POD 30 due to persistent healing disorders. Reoperation due to empyema on POD 70. Died of pneumogenic sepsis on POD 110 |
| 7 | F | 72 | 14 | 59 | No | Upper bilobectomy | 4 | Detection of insufficiency on POD 10. Conservative treatment. Discharged on POD 30 |
| 8 | M | 66 | 10 | 56 | No | Right lower lobe | 4 | Detection of insufficiency on POD 16. Conservative treatment. Discharged on POD 23 |
| 9 | M | 51 | 116 | 62 | Yes | Right upper lobe, thymic flap | 5 | Detection of insufficiency on POD 7. Conservative treatment. Died due to massive hemoptysis on POD 8 |
| 10 | M | 66 | 90 | 55 | No | Right lower lobe | 4 | Detection of insufficiency on POD 10. Conservative treatment. Discharged on POD 19 |
| 11 | M | 64 | 81 | 70 | No | Right lower lobe | 5 | Detection of insufficiency on POD 7. Conservative treatment. Discharged on POD 12 |
| 12 | M | 70 | 79 | 70 | No | Right upper lobe, PA-angioplasty | 5 | Detection of insufficiency on POD 7. Reoperation with revision of the anastomosis and covering with thymic flap. Completion pneumonectomy on POD 10 due to necrosis of the remnant lung. Died of pneumogenic sepsis on POD 12 |
| 13 | M | 69 | 69 | 79 | Yes | Right upper lobe and thymectomy, pectoralis mayor flap | 3 | Detection of insufficiency on POD 12. Reoperation with revision of the anastomosis. Discharged on POD 22 |
| 14 | F | 65 | 45 | 53 | Yes | Right upper lobe, PA-angioplasty, thymic flap | 3 | Detection of insufficiency on POD 20. Completion pneumonectomy. Discharged POD 62 |
| 15 | M | 62 | 18 | 53 | Yes | Left lower lobe | 4 | Detection of insufficiency on POD 10. Conservative treatment. Discharged on POD 20 |
| 16 | M | 64 | 7 | 78 | Yes | Right lower lobe, thymic flap | 3 | Detection of insufficiency on POD 21. Conservative treatment. Discharged on POD 23 |
| 17 | M | 67 | 49 | 61 | No | Right lower lobe | 4 | Detection of insufficiency on POD 12. Reoperation with middle lobe resection and reimplantation of the upper lobe to the main bronchus. Discharged on POD 30 |
| 18 | M | 59 | 75 | 59 | No | Left lower lobe, PA-angioplasty, aorta en bloc resection and reconstruction. Latissimus dorsi flap | 3 | Detection of insufficiency on POD 10. Reoperation with revision of the anastomosis. Discharge on POD 16 |
| 19 | M | 68 | 0 | 61 | No | Right upper lobe | 1 | Detection of insufficiency on POD 12. Reoperation with revision of the anastomosis. Discharged on POD 19 |
| 20 | M | 61 | 40 | 89 | No | Lower bilobectomy, thymic flap | 3 | Detection of insufficiency on POD 10. Conservative treatment. Discharged on POD 24 |
M, male; F, female; CRP, C-reactive protein; FEV1, forced expiratory volume in 1 second; RT, radiotherapy; PA, pulmonary artery; AG, anastomosis grading; POD, postoperative day.
In the univariate analysis (Table 2) male sex, squamous tumor differentiation, sleeve bilobectomy, preoperative elevated CRP, longer operation time, reduced FEV1 and neoadjuvant radiotherapy showed positive correlation with critical anastomotic healing. These variables were included in a logistic regression analysis. The results are summarized in Table 4. Bilobectomy, preoperative serum CRP level >40 mg/L, FEV1 <80% and neoadjuvant radiation were independent predictors of critical anastomotic healing.
Table 4
| Variables | P value | OR | 95% CI |
|---|---|---|---|
| Neoadjuvant radiation/chemoradiation | 0.0043 | 2.181 | 1.277–3.724 |
| Bilobectomy | 0.0001 | 3.508 | 1.851–6.648 |
| CRP >40 mg/L | 0.0024 | 2.139 | 1.309–3.495 |
| FEV1 <80% | 0.0007 | 2.847 | 1.557–5.206 |
OR, odds ratio; CI, confidence interval; CRP, C-reactive protein; FEV1, forced expiratory volume in 1 second.
Long-term survival
Median overall survival was 49 months in the satisfactory anastomosis group and 31 months in the critical anastomosis group (P=0.06). The 5- and 10-year overall survival was 35% and 12% in the critical and 44% and 15% in the satisfactory anastomosis group (Figure 2). Median cancer specific survival was 58 months in the satisfactory and 34 months in the critical anastomosis group (P=0.09). The 5- and 10-year cancer specific survival was 43% and 24% in the critical and 49% and 33% in satisfactory anastomosis group (Figure 3).
Discussion
Anastomotic insufficiency is a feared complication after bronchial sleeve resection and correlates with high mortality and morbidity. Standardization of surgical techniques and improvement of postoperative care led to a reduction in insufficiency rates. Nevertheless, anastomotic complication rates of up to 8% are described in the literature (9,11-14). In our study the insufficiency rate was 3.7%. Insufficiency was thus a rather rare event compared to other complications, but in case of occurrence it had serious consequences. Forty-five percent of the patients with anastomotic insufficiency had to undergo reoperation, resulting in completion pneumonectomy in near half of the cases. One quarter of the patients with anastomosis insufficiency died during the postoperative course.
These figures underline the importance of early detecting impaired bronchial healing. In this regard, an important finding of our study is that the classification of anastomotic healing developed in our institution is a reliable tool for detecting anastomoses at risk (1). Based on bronchoscopic evaluation on the seventh postoperative day, the anastomosis is considered critical when signs of ischemia and/or necrosis in the distal mucosa are present (grade 3 or higher). In our study 100 of 541 patients (18.4%) showed critical healing of the anastomosis. All but one insufficiency (19 of 20) occurred in the critical group which underscores the accuracy of the classification for identifying at-risk patients. In addition, we know that we can safely discharge patients with grade 1 and 2 anastomoses. A higher mortality and morbidity rate in patients with critical anastomosis compared to those with a satisfactory anastomosis emphasize the importance of intensified medical care when impaired bronchial healing is eminent.
The choice of the seventh postoperative day for bronchoscopy was defined based on pathophysiological considerations, according to which revascularization of the distal bronchus occurs after 7 days (1,15). However, systematic validation by means of a study has not been performed. We do not know whether an earlier bronchoscopy, for example on day 5, would have yielded the same results. In this regard, further studies could determine the correct timing for bronchoscopy.
In our analysis, we found that neoadjuvant radiation, bilobectomies, reduced preoperative FEV1 and elevated preoperative CRP levels were negative predictors for anastomotic healing. Circumferential transection, as well as denudation of the bronchus during lymphadenectomy, inevitably leads to ischemia of the distal bronchial mucosa. Healing of the anastomosis ultimately occurs via revascularization, which is mainly induced by surrounding vital tissue (10). A lack of coverage of the anastomosis or deterioration of the natural covering tissue due to external influences may lead to impaired revascularization and thus healing of the anastomosis. An adverse effect of preoperative radiotherapy on bronchial healing could be demonstrated previously. Yamamoto et al. analyzed the effects of radiotherapy, chemotherapy and lymphadenectomy on bronchial mucosal blood flow after lung resection. Patients who received preoperative radiotherapy showed a 70% reduction in mucosal blood flow compared with untreated patients or those who received chemotherapy alone. Patients who received preoperative radiotherapy showed ischemic changes to the bronchial stump more frequently than patients without radiotherapy. In contrast, after lymphadenectomy no significant changes in bronchial mucosal blood flow and satisfactory bronchial healing could be shown. The authors concluded that the reduction in bronchial blood flow due to bronchial artery dissection can be compensated by arteriolar communication from the pulmonary circulation. However, irradiation leads to hyalinization and fibrosis of the arterioles, resulting in deterioration of the bronchial microcirculation (10). We were recently able to demonstrate the effect of neoadjuvant radiation on anastomotic healing after bronchial sleeve resection. In a retrospective analysis on 501 patients, preoperative radiation was associated with a worse anastomotic healing and a higher rate of insufficiencies compared with patients without pretreatment or chemotherapy alone (8).
The influence of sleeve bilobectomy on anastomotic complications has not been analyzed yet. However, previous studies demonstrated that lower bilobectomies were associated with an increased rate of bronchial fistula and pleural space complications, whereas upper bilobectomies had the same risks as lobectomies (16,17). The discrepancy between the remnant lung and the volume of the pleural cavity was discussed as a possible cause (16). In our study the rate of sleeve bilobectomies was 9.4%. We could demonstrate that this procedure is associated with a worse anastomotic healing and a higher risk of developing insufficiency. However, because of the small number of lower bilobectomies in our study, it was not possible to make a serious statement about the difference with upper bilobectomies. Technical challenges of sleeve bilobectomies include discrepancies in bronchial diameter, proximity to the pulmonary artery, poor exposure of the mediastinal side of the anastomosis and the short length of the upper lobe bronchus, resulting in higher tension on the anastomosis (9,18,19).
In our study, the preoperative CRP level was an independent predictor of postoperative anastomotic healing. Patients with elevated preoperative CRP exhibited healing disorders more frequently. Significant impairment was found with an increase above 40 mg/L, whereas patients with CRP between 3 mg/L (detection limit) and 40 mg/Lshowed no differences regarding anastomotic healing compared with patients with values below 3 mg/L. CRP is a highly sensitive but non-specific marker of acute phase response. An increase is often observed in lung cancer patients as a sign of inflammation and increased tumor burden (20). A relationship between preoperatively elevated inflammatory markers and postoperative complications has been demonstrated in previous studies. Amar et al. showed an association between postoperative complications after thoracic surgery and preoperatively elevated CRP and IL-6 levels (21). Preoperatively elevated CRP above 40mg/L was an independent risk factor for increased postoperative morbidity and mortality after thoracic surgery for lung cancer (22).
Infectious complications, especially pneumonia, are among the most common complications after pulmonary resection. The prevalence of pneumonia varies from 2% to 40%, depending on type of resection, patient population and diagnostic criteria. After lung surgery pneumonia is associated with a significant increase of morbidity and mortality (23,24). Postoperative pneumonia was the second most frequent complication in our study, affecting 10.9% of all patients. Pneumonia was more frequent (22%) in the group of patients with critical anastomoses compared to those with satisfactory bronchial healing (7.9%). We assume that infectious complications may have been a predisposing factor for the development of anastomotic healing disorders in our study.
We demonstrated an association between decreased FEV1 and impaired anastomotic healing. Several studies have previously demonstrated an association between decreased FEV1 and/or the presence of COPD and elevated CRP levels. This is generally attributed to the fact that chronic obstructive pulmonary disease is associated with systemic inflammation (25-27). Thus, many lung cancer patients have a systemic inflammatory state due to their underlying disease and comorbidities. These patients seem to be predisposed to postoperative complications, as surgery must be considered as an additional trigger for the inflammatory response (22).
Analysis of the clinical courses of anastomotic insufficiencies (Table 3) in our study highlights the severity of this complication and the complexity of its management. Many of the patients have a significantly prolonged hospital stay with infectious complications, reoperations, and high mortality. Regarding conservative treatment of insufficiency, it was successful in 9 cases, secondary surgery was performed in 1 case, and the course was ultimately fatal in 3 cases. Revision of the anastomosis was performed in 5 cases (one case after initially convervative therapy), of which 3 could be discharged and 2 died in the further postoperative course. A general recommendation when an insufficiency should be treated conservatively and when surgically cannot be derived from our data. Neither for the decision whether a revision of the anastomosis or a secondary pneumonectomy should be performed. The decision on how to treat anastomotic insufficiency is always individual and depends on various factors such as the extent of necrosis, the condition of the remaining lung and the clinical condition of the patient.
In patients at increased risk for developing bronchial healing disorders, wrapping the anastomosis with a pedicled flap has been suggested (9,12,13,28). This recommendation is based on pathophysiological studies that demonstrated the ingrowth of capillaries from the flap into the submucosal bronchial plexus (29,30). However, clinical studies showing an effect on anastomotic healing do not exist. Therefore, some centers do not protect the anastomosis even after neoadjuvant radiation, arguing that with careful airway management and preservation of peribronchial tissue, additional anastomotic protection is not required (14).
At our institution, covering the anastomosis is performed as a standard procedure after neoadjuvant radiotherapy. Nevertheless, the insufficiency rate in this group was 6.8%. Therefore, it is reasonable to question whether the benefit of wrapping is not as great as previously thought. Storelli et al. reported a series of 103 sleeve resection over 11 years. No anastomotic complications were reported even after neoadjuvant radiation (14). However, comparability may be limited because only 5 patients underwent preoperative irradiation in this study. Campisi et al. compared patients that underwent bronchial wrapping (n=60) and patients that did not (n=30) after sleeve resection for lung cancer. None of the patients underwent neoadjuvant radiotherapy. Although there was no significant difference regarding overall anastomotic complication rate, anastomotic dehiscence was 3% in the wrapped group and 10% in the non-wrapped group (31). Overall, the question of coverage or non-coverage cannot be answered at present. Only prospective studies could help to clarify this question. Although the benefit of this procedure is controversial, preparing a flap of pericardial fat or intercostal muscle is a simple and fast to perform technique that may prevent serious anastomotic complications or allow conservative treatment in case of initially impaired anastomotic healing (7,14,32).
In our study, patients with critical anastomoses tended to have lower overall and cancer-specific survival than patients with satisfactory anastomoses. There were no significant differences between the two groups with respect to postoperative tumor stage, radicality and histology. However, the higher rate of pretreated patients and bilobectomies in the group with critical anastomoses suggests that larger and more advanced tumors were present prior to therapy. Moreover, risk factors leading to impaired anastomotic healing may also have an impact on survival.
We acknowledge that this study was limited by its single-institutional retrospective design. In addition, our compilation of risk factors for impaired anastomotic healing is incomplete. Our data lack information on preexisting conditions such as diabetes, use of steroids, or the patient‘s performance status. Since it can be assumed that the surgeon‘s experience has an influence on the occurrence of anastomotic complications, it can be considered a limitation that this variable was not included in this study.
Conclusions
In conclusion, sleeve resection can be performed with acceptable morbidity and mortality. Anastomotic insufficiency is a rare but serious complication with a mortality rate of 25%. Therefore, patients must be closely monitored throughout the postoperative course. In this context, bronchoscopic assessment of anastomotic healing has proven to be an effective tool to detect anastomoses at risk. Neoadjuvant radiation, bilobectomies, and acute or chronic inflammation were independent risk factors for impaired bronchial healing and should be considered at the planning stage of surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1627/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1627/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1627/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1627/coif). ES is a past president of the German Society of Thoracic surgery (2017-2019). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee of the University of Witten Herdecke (Nr. 51/2017). All participants gave informed consent before taking part in the study. This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ludwig C, Stoelben E. A new classification of bronchial anastomosis after sleeve lobectomy. J Thorac Cardiovasc Surg 2012;144:808-12. [Crossref] [PubMed]
- Chen J, Soultanis KM, Sun F, et al. Outcomes of sleeve lobectomy versus pneumonectomy: A propensity score-matched study. J Thorac Cardiovasc Surg 2021;162:1619-1628.e4. [Crossref] [PubMed]
- Stallard J, Loberg A, Dunning J, et al. Is a sleeve lobectomy significantly better than a pneumonectomy? Interact Cardiovasc Thorac Surg 2010;11:660-6. [Crossref] [PubMed]
- Van Schil PE, Brutel de la Rivière A, Knaepen PJ, et al. Completion pneumonectomy after bronchial sleeve resection: incidence, indications, and results. Ann Thorac Surg 1992;53:1042-5. [Crossref] [PubMed]
- Jungraithmayr W, Hasse J, Olschewski M, et al. Indications and results of completion pneumonectomy. Eur J Cardiothorac Surg 2004;26:189-96. [Crossref] [PubMed]
- Bylicki O, Vandemoortele T, Orsini B, et al. Incidence and management of anastomotic complications after bronchial resection: a retrospective study. Ann Thorac Surg 2014;98:1961-7. [Crossref] [PubMed]
- Ludwig C. Prophylaxis and management of postoperative complications after tracheobronchial surgery. J Thorac Dis 2020;12:6179-84. [Crossref] [PubMed]
- Koryllos A, Lopez-Pastorini A, Zalepugas D, et al. Bronchus Anastomosis Healing Depending on Type of Neoadjuvant Therapy. Ann Thorac Surg 2020;109:879-86. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Yamamoto R, Tada H, Kishi A, et al. Effects of preoperative chemotherapy and radiation therapy on human bronchial blood flow. J Thorac Cardiovasc Surg 2000;119:939-45. [Crossref] [PubMed]
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112-7. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Rendina EA, Venuta F, de Giacomo T, et al. Parenchymal sparing operations for bronchogenic carcinoma. Surg Clin North Am 2002;82:589-609. vii. [Crossref] [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Stoelben E, Harpering H, Haberstroh J, et al. Heterotopic transplantation of cryopreserved tracheae in a rat model. Eur J Cardiothorac Surg 2003;23:15-20. [Crossref] [PubMed]
- Thomas PA, Falcoz PE, Bernard A, et al. Bilobectomy for lung cancer: contemporary national early morbidity and mortality outcomes. Eur J Cardiothorac Surg 2016;49:e38-43; discussion e43. [Crossref] [PubMed]
- Nagahiro I, Aoe M, Sano Y, et al. Bronchopleural fistula after lobectomy for lung cancer. Asian Cardiovasc Thorac Ann 2007;15:45-8. [Crossref] [PubMed]
- Ludwig C. Editorial comment on "Reimplantation of the upper lobe bronchus after lower sleeve lobectomy or bilobectomy: long-term results" by Maurizi et al. J Thorac Dis 2018;10:6427-9. [Crossref] [PubMed]
- Maurizi G, Ciccone AM, Vanni C, et al. Reimplantation of the upper lobe bronchus after lower sleeve lobectomy or bilobectomy: long-term results. Eur J Cardiothorac Surg 2018;53:1180-5. [Crossref] [PubMed]
- Lee JG, Cho BC, Bae MK, et al. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer 2009;63:106-10. [Crossref] [PubMed]
- Amar D, Zhang H, Park B, et al. Inflammation and outcome after general thoracic surgery. Eur J Cardiothorac Surg 2007;32:431-4. [Crossref] [PubMed]
- Lopez-Pastorini A, Riedel R, Koryllos A, et al. The impact of preoperative elevated serum C-reactive protein on postoperative morbidity and mortality after anatomic resection for lung cancer. Lung Cancer 2017;109:68-73. [Crossref] [PubMed]
- Radu DM, Jauréguy F, Seguin A, et al. Postoperative pneumonia after major pulmonary resections: an unsolved problem in thoracic surgery. Ann Thorac Surg 2007;84:1669-73. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Fogarty AW, Jones S, Britton JR, et al. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax 2007;62:515-20. [Crossref] [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. [Crossref] [PubMed]
- Kony S, Zureik M, Driss F, et al. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax 2004;59:892-6. [Crossref] [PubMed]
- Burfeind WR Jr, D'Amico TA, Toloza EM, et al. Low morbidity and mortality for bronchoplastic procedures with and without induction therapy. Ann Thorac Surg 2005;80:418-21; discussion 422. [Crossref] [PubMed]
- Ishihara T, Nemoto E, Kikuchi K, et al. Does pleural bronchial wrapping improve wound healing in right sleeve lobectomy? J Thorac Cardiovasc Surg 1985;89:665-72. [Crossref] [PubMed]
- Turrentine MW, Kesler KA, Wright CD, et al. Effect of omental, intercostal, and internal mammary artery pedicle wraps on bronchial healing. Ann Thorac Surg 1990;49:574-8; discussion 579. [Crossref] [PubMed]
- Campisi A, Ciarrocchi AP, Congiu S, et al. Sleeve Lobectomy: To Wrap or Not to Wrap the Bronchial Anastomosis? Ann Thorac Surg 2022;113:250-5. [Crossref] [PubMed]
- Van Schil PE. Wrapping of bronchial anastomoses: something of the past? Eur J Cardiothorac Surg 2012;42:81-2. [Crossref] [PubMed]




