Machine learning-based screening of the diagnostic genes and their relationship with immune-cell infiltration in patients with lung adenocarcinoma
Introduction
It is widely acknowledged that early detection and treatment can improve patient outcomes for any disease, and cancer is no exception. Lung adenocarcinoma (LUAD) is the most common type of lung cancer, and has a dismal mortality rate of 80% (1). Significant progress in the screening and diagnostic methods, such as computed tomography (CT) imaging, has been made in recent years (2). However, most patients still miss the optimal therapeutic window, as they are only diagnosed at an advanced stage (3). Previously reported non-invasive approaches for early diagnosis of LUAD included microRNAs (4), DNA methylation markers (5), and autoantibody combined with CT (6). Nevertheless, biomarkers with high specificity, simplicity, and convenience for test in clinical practice are sparse. Thus, novel biomarkers need to be explored and identified.
Immune cells and the immune response have been shown to play very important roles in the occurrence and development of LUAD (7,8). As reported in the literature, tumor cells and immune cells interact with the tumor microenvironment (TME) and affect tumorigenesis (8,9). Notably, different levels of immune-cell infiltration have various effects on prognosis (10). In recent years, machine learning has been used to screen diagnostic genes, which has the ability to decipher complicated connections between multiple sets of test data and diseases (11). However, in some studies, the screened genes were not associated with the absolute value and proportion of the infiltrating immune cells (12,13) and only 1 type of machine-learning method was used (14).
The aim of the present study is to screen the diagnostic genes and analyze their relationship with immune-cell infiltration based on machine-learning in patients with lung adenocarcinoma. We hypotheses that novel diagnostic genes for LUAD could be identified. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-206/rc).
Methods
Data download and preliminary process
The Gene Expression Omnibus (GEO) data sets GSE32863 and GSE75037 were downloaded, normalized, and merged using R packages “limma” and “Sva.” The differential expression analysis was conducted on the merged data using the screening criteria |logFC| >2, and an adjusted P value <0.05. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Functional analysis
The differentially expressed genes (DEGs) were analyzed by GO, KEGG and GSEA R packages with “clusterProfiler”, “org.Hs.eg.db” and “c5.go.v7.4.symbols.gmt”. GO (http://geneontology.org) is a standard recognized classification system for defining the biological processes (BPs), molecular functions (MFs), and cellular components (CCs) of DEGs (15). The KEGG (https://www.kegg.jp/) is a database that provides a manual curation of the pathways associated with genes (16). The screening conditions for the GO annotation and KEGG analysis included P values <0.05 and adjusted P values <0.05. The enrichment of the upregulated or downregulated sets of genes from the REACTOME pathway database was computed by running GSEA against the fold-change ranked list of genes in the experiment (17) The filter conditions were a P value <1 and, and an adjusted P value filter <0.05.
Immune-cell infiltration analysis and the correlations between the immune cells and DEGs
The CIBERSORT deconvolution algorithm is a method used to characterize the cell composition of complex tissues from their gene expression profiles (18). The immune-cell infiltration of the GSE32863 data set was analyzed. The correlations between the immune cells and DEGs are displayed in a lollipop chart. The abscissa represents the correlation coefficient, the left ordinate represents the names of immune cells, and the right ordinate represents the P values. The size of the lollipop head indicates the correlation coefficient, and the color of the lollipop head indicates if the differences were significant (green indicates a P value <0.05, and yellow indicates a P value >0.05).
Statistical analysis
The diagnostic performance of the genes was assessed using area under the receiver operating characteristic curve (AUC). The distribution of the differentially expressed genes was shown by heatmap and volcano map. The differences of gene expression between the two groups were compared by t-test and expressed by boxplot. All the statistical analyses were performed by using R software (Version 4.1.1). A P value <0.05 was considered as statistical significance.
Results
Results of the DEG analysis
In total, 384 DEGs were identified from the GSE32863 data set, including 91 upregulated and 293 downregulated genes. The flowchart of the study is shown in Figure 1. The expression of the screened DEGs and the differences in details in each sample showed in https://cdn.amegroups.cn/static/public/jtd-22-206-1.xls, https://cdn.amegroups.cn/static/public/jtd-22-206-2.xls.
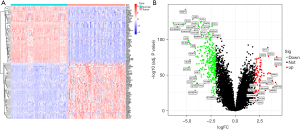
The top 50 DEGs are shown in Figure 2A (heat map) and Figure 2B (volcano map). We found that the genes recombinant glutathione peroxidase 2 (GPX2), Purkinje cell protein 4 (PCP4), locus649841 (LOC649841), fucosyltransferase 3 (FUT3), and transmembrane protein 45B (TMEM45B) were upregulated, while the genes intelectin 1 (ITLN1), metallothionein 1M (MT1M), nterleukin-6 (IL-6), surfactant protein A (SFTPA), and ficolin 3 (FCN3) were downregulated in the tumor group.
GO, KEGG and GSEA enrichment
During the GO annotation, the DEGs were found to be significantly enriched in terms of the CCs, including the endocytic vesicles, extracellular matrix, and collagen-containing extracellular matrix, BPs, including the extracellular matrix organization, humoral-immune response and neutrophil activation, and the MFs, including glycosaminoglycan binding, oxygen carrier activity, and haptoglobin binding (see Figure 3A). The KEGG analysis showed that these genes were significantly enriched in the signaling pathways related to malaria, complement and coagulation cascades, and leukocyte transendothelial migration (see Figure 3B). GO and KEGG pathway enrichment analyses were conducted by GSEA, and the GO terms “adaptive-immune-response”, “humoral-immune-response”, and “extracellular-signal-regulated kinases (ERK) 1 and 2 cascade” were significantly expressed in the normal group (see Figure S1A), while the GO terms “nuclear-chromosome”, “DNA-conformation-change”, and “chromosomal region” were significantly expressed in the tumor group (see Figure S1B). In the KEGG pathway analysis, the terms “chemokine-signaling-pathway”, “cytokine-cytokine-receptor-interaction”, and “graft-versus-host-disease” were significantly expressed in the normal group (see Figure S1C), while the terms “base-excision-repair”, “cell-cycle”, and “DNA-replication” were significantly expressed in the tumor group (see Figure S1D).
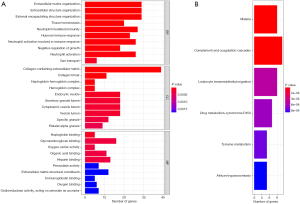
Screening of the diagnostic genes by LASSO and SVM regression analyses and the validation
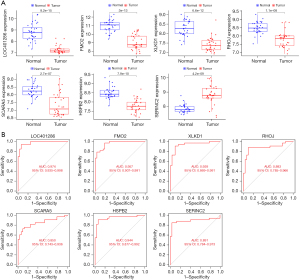
The intersection results of the LASSO (see Figure 4A) and SVM (Figure 4B) regression analyses revealed 7 DEGs that were considered diagnostic genes (see Figure 4C); that is, LOC401286, flavin-containing monooxygenase 2 (FMO2), XLKD1, RHOJ, SCARA5, HSPB2, and serine incorporator 2 (SERINC2) (see Table 1). There were significant differences in the expression of these 7 genes between the normal and tumor groups (see Figure 4D). SERINC2 was significantly upregulated in the tumor group, but the remaining 6 genes were significantly downregulated.
Table 1
| Different genes of the LASSO regression model | Different genes of the SVM regression model | Intersection genes |
|---|---|---|
| FABP4 | HBA2 | LOC401286 |
| LOC401286 | MFAP4 | FMO2 |
| FMO2 | FAM107A | XLKD1 |
| XLKD1 | AGER | RHOJ |
| RHOJ | XLKD1 | SCARA5 |
| MMRN1 | TEK | HSPB2 |
| SCARA5 | HSPB2 | SERINC2 |
| LGI3 | JAM2 | |
| ETV4 | RASL12 | |
| HSPB2 | HBB | |
| C10orf67 | LDB2 | |
| SOX17 | TCF21 | |
| CCL23 | RHOJ | |
| MT1M | STX11 | |
| IGSF9 | SH3GL3 | |
| C5AR1 | STX1A | |
| MGC34774 | FMO2 | |
| SERINC2 | LOC401286 | |
| PROM2 | C2orf32 | |
| GCNT3 | MS4A7 | |
| RHBDL1 | ANKRD47 | |
| DES | EDG1 | |
| IL6 | PECAM1 | |
| C18orf34 | LOC653463 | |
| SPDEF | LIMS2 | |
| SERINC2 | ||
| FHL1 | ||
| SCN4B | ||
| NAP5 | ||
| MMP11 | ||
| CAV2 | ||
| DNASE1L3 | ||
| COX7A1 | ||
| CFD | ||
| GIMAP5 | ||
| ADH1A | ||
| SCARA5 | ||
| DPEP2 | ||
| PLAC9 | ||
| GSTM5 |
LASSO, least absolute shrinkage and selection operator; SVM, support vector machine.
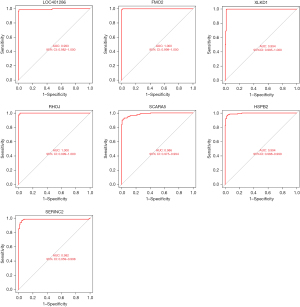
The diagnostic performance of LOC401286, FMO2, XLKD1, RHOJ, SCARA5, HSPB2, and SERINC2 for LUAD was assessed by a ROC curve analysis, which yielded area under the curve (AUC) values of 0.99, 1.00, 0.99, 1.00, 0.99, 0.99, and 0.98, respectively (see Figure 5). Similar results were obtained during the validation using the GSE63459 data set (see Figure 6). Consistently, SERINC2 was significantly upregulated in the tumor group, while the remaining 6 genes were significantly downregulated. The ROC curve analyses for LOC401286, FMO2, XLKD1, RHOJ, SCARA5, HSPB2, and SERINC2 yielded AUC values of 0.97, 0.96, 0.94, 0.88, 0.85, 0.94 and 0.89, respectively.
Immune-cell infiltration analysis and the correlations between immune cells and DEGs
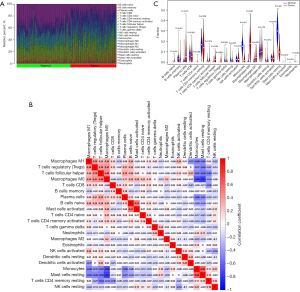
The proportion of immune cells infiltrated in normal and tumor tissues (see Figure 7A), and the correlation between the immune cells (see Figure 7B) was analyzed. Significant differences in the content of the immune cells, naive B cells, memory B cells, plasma cells, naive cluster of differentiation (CD) 4 T cells, T follicular helper cells, regulatory T cells (Tregs), gamma delta T cells, monocytes, M0 macrophages, M1 macrophages, resting mast cells, activated mast cells, and neutrophils were found in normal and tumor tissues (see Figure 7C). The correlations between the infiltrating immune cells and the expression of the 7 diagnostic genes are shown in Figure 8. A negative correlation was found between M0 macrophages, and monocytes and resting mast cells (r=−0.66 and −0.73), while a positive correlation was found between monocytes and resting mast cells (r=0.64). LOC401286, FMO2, XLKD1, RHOJ, SCARA5, and HSPB2 were positively correlated with monocytes and resting mast cells, and negatively correlated with Tregs and macrophages. The opposite results were found for SERINC2.

Discussion
In the present study, the functional enrichment analysis showed that the identified DEGs were enriched in the GO terms of extracellular matrix, glycosaminoglycan binding, complement and coagulation cascades, and leukocyte transendothelial migration. The intersection of the LASSO and SVM regression results identified 7 diagnostic genes (i.e., LOC401286, FMO2, XLKD1, RHOJ, SCARA5, HSPB2, and SERINC2), which shown significant performance for the early diagnosis of LUAD in clinical practice. We also estimated the infiltration of immune cells, and analyzed their correlations with the 7 diagnostic DEGs.
The SVM and LASSO regression methods are machine-learning methods that have been extensively used to screen diagnostic and prognosis-related indicators in recent years. In the present study, we integrated and intersected the regression results. Ultimately, 7 DEGs (i.e., LOC401286, FMO2, XLKD1, RHOJ, SCARA5, HSPB2 and SERINC2) were identified. Consistent with the literature (19), only SERINC2 was upregulated in the tumor group. SERINC2 is a member of the SERINC family of transmembrane proteins that incorporates serine into membrane lipids, including phosphatidylserine, and sphingolipids, during synthesis. These membrane lipids thus act as important indicators of tumorigenesis and cancer progression (20,21). Further, SERINC2 reportedly promotes LUAD proliferation, migration, and invasion, and may involve the phosphatidylinositol 3 kinase (PI3K)/serine threonine kinase (Akt) signaling pathway (22) Additionally, SERINC2 has been correlated with alcohol dependence in Europeans (23). Interestingly, the knockdown of SERINC2 in hepatocellular carcinoma reportedly inhibits cell-cycle progression via the transcriptional activation of Kirsten rat sarcoma (k-Ras) (22). However, the role of SERINC2 in cancer has been largely understudied; thus, further studies and animal experiments need to be conducted to assess the value of SERINC2 as an early diagnostic or therapeutic marker for LUAD.
Human FMO2 is expressed in the lungs in 2 isoforms (i.e., FMO2*2A and FMO2*1) (24), and acts as a tumor suppressor in LUAD. XLKD1, RHOJ and SCARA5 are reportedly downregulated in patients with LUAD, and their high expression can inhibit the occurrence and development of cancer (25,26). Additionally, HSPB2 has been shown to be correlated with pancreatic cancer and hepatocellular carcinoma (27) via the activation of protein 53. To the best of our knowledge, no study has uncovered a relationship between HSPB2 and LUAD; thus, further investigations need to be conducted.
Over the years, due to unprecedented technological progress, the focus of research has shifted from tumor cells to the TME, and our understanding of tumorigenesis has been refined (28). It is now well established that immune-cell infiltration is an important part of the TME (29,30), and the immune system plays a dual role in tumor cells. At present, immunosuppressive therapy, such as programmed cell death protein 1 (PD-1) inhibitor therapy, has become the mainstay of LUAD treatment along with chemotherapy and surgery (29,31)
Consistent with the literature (28), our immune-cell infiltration analysis showed that naive B cells, memory B cells, plasma cells, naive CD4 T cells, T follicular helper cells, Tregs, gamma delta T cells, monocytes, M0 macrophages, M1 macrophages, resting mast cells, activated mast cells, and neutrophils were significantly different between LUAD and healthy subjects. CD4+ and CD8+ T cells and their secreted cytokines participate in adaptive immunity; PD-1 inhibition has been reported to result in the increased proliferation of all T cell subsets and effector cytokine production by CD4+ T helper 1 cells (32). Our study found that M0 macrophages were negatively correlated with monocytes and resting mast cells (r=−0.66 and −0.73), but a positive correlation was found between monocytes and resting mast cells (r=0.64). LOC401286, FMO2, XLKD1, RHOJ, SCARA5, and HSPB2 were positively correlated with monocytes and resting mast cells, and negatively correlated with Tregs and macrophages. Interestingly, the opposite results were observed for SERINC2. These results are consistent with other reports (33), but further experimental research at the in-vivo and in-vitro levels needs to be conducted to increase the robustness of our finding. The limitation of this study was that the stage of patients from validation dataset was not exactly the same as the stage in training dataset, because the paper needs to identify the diagnostic gene, which was more meaningful in the early stages, and therefore made up for this deficiency.
In conclusion, we identified 7 DEGs in LUAD tissue that can be considered diagnostic genes based on 2 machine-learning regression methods (i.e., the SVM and LASSO regression models). Our findings were successfully validated using another independent data set that contained data from patients with stage I LUAD. Importantly, the infiltrating immune cells were analyzed, and a significant correlation was found to the 7 DEGs, which suggests that these genes affect the occurrence and development of tumors via their interaction with immune cells. Accordingly, our findings could be very helpful for the early diagnosis of LUAD in clinical practice.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81970167 and 81800108).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-206/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-206/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. [Crossref] [PubMed]
- Shao X, Niu R, Jiang Z, et al. Role of PET/CT in Management of Early Lung Adenocarcinoma. AJR Am J Roentgenol 2020;214:437-45. [Crossref] [PubMed]
- Zhang M, Yue J, Cui R, et al. Bright quantum dots emitting at ~1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc Natl Acad Sci U S A 2018;115:6590-5. [Crossref] [PubMed]
- Wang Y, Zhao H, Gao X, et al. Identification of a three-miRNA signature as a blood-borne diagnostic marker for early diagnosis of lung adenocarcinoma. Oncotarget 2016;7:26070-86. [Crossref] [PubMed]
- Li M, Zhang C, Zhou L, et al. Identification and validation of novel DNA methylation markers for early diagnosis of lung adenocarcinoma. Mol Oncol 2020;14:2744-58. [Crossref] [PubMed]
- Meng QC, Gao PR, Ren PF, et al. Early diagnosis of subtype in early clinical stage lung adenocarcinoma by using an autoantibody panel and computed tomography. Zhonghua Yi Xue Za Zhi 2019;99:204-8. [PubMed]
- Zhang C, Zhang J, Xu FP, et al. Genomic Landscape and Immune Microenvironment Features of Preinvasive and Early Invasive Lung Adenocarcinoma. J Thorac Oncol 2019;14:1912-23. [Crossref] [PubMed]
- Petitprez F, Meylan M, de Reyniès A, et al. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front Immunol 2020;11:784. [Crossref] [PubMed]
- Katsuta E, Rashid OM, Takabe K. Clinical relevance of tumor microenvironment: immune cells, vessels, and mouse models. Hum Cell 2020;33:930-7. [Crossref] [PubMed]
- Xie M, Wei J, Xu J. Inducers, Attractors and Modulators of CD4+ Treg Cells in Non-Small-Cell Lung Cancer. Front Immunol 2020;11:676. [Crossref] [PubMed]
- Wu J, Zan X, Gao L, et al. A Machine Learning Method for Identifying Lung Cancer Based on Routine Blood Indices: Qualitative Feasibility Study. JMIR Med Inform 2019;7:e13476. [Crossref] [PubMed]
- Sun S, Guo W, Wang Z, et al. Development and validation of an immune-related prognostic signature in lung adenocarcinoma. Cancer Med 2020;9:5960-75. [Crossref] [PubMed]
- Chen H, Carrot-Zhang J, Zhao Y, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun 2019;10:5472. [Crossref] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res 2019;47:D330-8. [Crossref] [PubMed]
- Kanehisa M, Furumichi M, Sato Y, et al. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res 2021;49:D545-51. [Crossref] [PubMed]
- Canzler S, Hackermüller J. multiGSEA: a GSEA-based pathway enrichment analysis for multi-omics data. BMC Bioinformatics 2020;21:561. [Crossref] [PubMed]
- Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453-7. [Crossref] [PubMed]
- Wu K, Zhang X, Li F, et al. Frequent alterations in cytoskeleton remodelling genes in primary and metastatic lung adenocarcinomas. Nat Commun 2015;6:10131. [Crossref] [PubMed]
- Digumarti R, Bapsy PP, Suresh AV, et al. Bavituximab plus paclitaxel and carboplatin for the treatment of advanced non-small-cell lung cancer. Lung Cancer 2014;86:231-6. [Crossref] [PubMed]
- Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest 2015;125:1379-87. [Crossref] [PubMed]
- Zeng Y, Xiao D, He H, et al. SERINC2-knockdown inhibits proliferation, migration and invasion in lung adenocarcinoma. Oncol Lett 2018;16:5916-22. [Crossref] [PubMed]
- Zuo L, Wang KS, Zhang XY, et al. Rare SERINC2 variants are specific for alcohol dependence in individuals of European descent. Pharmacogenet Genomics 2013;23:395-402. [Crossref] [PubMed]
- Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol 2006;46:65-100. [Crossref] [PubMed]
- Hsu YL, Hung JY, Lee YL, et al. Identification of novel gene expression signature in lung adenocarcinoma by using next-generation sequencing data and bioinformatics analysis. Oncotarget 2017;8:104831-54. [Crossref] [PubMed]
- Zeng T, Chen C, Yang P, et al. A Protective Role for RHOJ in NonSmall Cell Lung Cancer Based on Integrated Bioinformatics Analysis. J Comput Biol 2020;27:1092-103. [Crossref] [PubMed]
- Liu J, Hu G, Chen D, et al. Suppression of SCARA5 by Snail1 is essential for EMT-associated cell migration of A549 cells. Oncogenesis 2013;2:e73. [Crossref] [PubMed]
- Yu Z, Wang H, Fang Y, et al. Molecular chaperone HspB2 inhibited pancreatic cancer cell proliferation via activating p53 downstream gene RPRM, BAI1, and TSAP6. J Cell Biochem 2020;121:2318-29. [Crossref] [PubMed]
- Yang Z, Zhuang L, Szatmary P, et al. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int J Med Sci 2015;12:256-63. [Crossref] [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22. [Crossref] [PubMed]
- Zhang C, Wang H, Wang X, et al. CD44, a marker of cancer stem cells, is positively correlated with PD-L1 expression and immune cells infiltration in lung adenocarcinoma. Cancer Cell Int 2020;20:583. [Crossref] [PubMed]
- Zuo S, Wei M, Wang S, et al. Pan-Cancer Analysis of Immune Cell Infiltration Identifies a Prognostic Immune-Cell Characteristic Score (ICCS) in Lung Adenocarcinoma. Front Immunol 2020;11:1218. [Crossref] [PubMed]
- Saab S, Zalzale H, Rahal Z, et al. Insights Into Lung Cancer Immune-Based Biology, Prevention, and Treatment. Front Immunol 2020;11:159. [Crossref] [PubMed]
- Fan T, Lu Z, Liu Y, et al. A Novel Immune-Related Seventeen-Gene Signature for Predicting Early Stage Lung Squamous Cell Carcinoma Prognosis. Front Immunol 2021;12:665407. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)