
Influence of body composition measures on chyle leak after oesophagectomy
Introduction
With a reported incidence between 1.1–3.8%, chyle leak (CL) is an infrequent but potentially serious complication of oesophagectomy (1-5). Whilst mild CL may be managed conservatively, surgical management is often required (5,6), for example in high output leaks (7). It is therefore desirable to identify any preoperative factors that increase the risk of CL after oesophagectomy, to improve individualised preoperative counselling and risk stratification.
Preoperative nutritional status has been shown to be significantly related to risk of complications after oesophagectomy (8-10). Given that nutritional status can be accurately assessed using preoperative cross-sectional imaging (11), body composition measures such as sarcopenia are increasingly being used as preoperative risk assessment tools for major surgery such as oesophagectomy (12-15).
However, the interaction between CL and body composition is not known. Therefore, the aim of this study was to identify the influence of body composition and related factors on CL following oesophagectomy. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1580/rc).
Methods
A prospectively collected and maintained departmental database of oesophageal cancer resections between January 2006–December 2020 was reviewed retrospectively to identify patients who developed CL after oesophageal cancer resection. A control group of patients who underwent oesophagectomy, who did not experience CL, for cancer during the same time period was also collected from the same departmental database to allow for investigation of risk factors. Oesophagectomy was performed in accordance with the operating surgeon’s expertise and case. If the thoracic duct is visualized intraoperatively it was ligated with ties or clips.
Relationships between CL and demographics, operative factors and body composition measures were investigated as primary outcomes. Risk factors for severe CL, defined as need for reintervention or greater than 1 litre of output per day, were evaluated as a secondary outcome.
Sarcopenia and myosteatosis evaluation
Computed tomography (CT) scan has proven to be accurate for measuring human body composition (11). Regional muscle tissue was measured on CT scans performed on diagnosis as part of the staging process preoperatively.
Preoperative CT scans, that were previously used in the staging process, were reviewed to evaluate body composition measures such as sarcopenia and myosteatosis. A transverse CT image from L3 was assessed from each scan. Images were analysed with SliceOmatic V4.3 software (Tomovision, Montreal, QB, Canada), which enables specific tissue demarcation using previously reported Hounsfield unit (HU) thresholds (11). Skeletal muscle is identified and quantified by HU thresholds of −29 to +150. Muscles in the L3 region encompass psoas, erector spinae, quadratus lumborum, transversus abdominus, external and internal obliques, and rectus abdominus. The following HU thresholds were used for adipose tissues: −190 to −30 for subcutaneous and intermuscular adipose tissues and −150 to −50 for visceral adipose tissues (16). Cross-sectional areas (cm2) were automatically computed by summing tissue pixels and multiplying by pixel surface area. Cross-sectional area of muscle and adipose tissue was normalized for stature (cm2/m2) as reported elsewhere (17), and this value is referred to as the L3 (Skeletal Muscle Index) SMI. Cut-offs for sarcopenia were based on a CT-based study in patients with solid tumours using optimal stratification, a statistical method similar to receiver operator curve analysis, to solve specific threshold values for L3 SMI in relation to an outcome (death) (L3 SMI: ≤41 cm2/m2 for women and ≤53 cm2/m2 for men with BMI ≥25 and ≤43 cm2/m2 in patients with BMI <25) (16). Muscle attenuation indirectly measures fat infiltration in muscles. Mean muscle attenuation in HU was reported for the entire muscle area at the third lumbar vertebra. We also, used previous cut-off values for muscle attenuation previously associated with mortality, specifically <41HU in patients with a BMI up to 24.9, and <33 in those with a BMI ≥25 (16). Sarcopenic obesity was defined as those patients with concurrent sarcopenia and overweight or obesity (BMI >25 kg/m2).
Statistical analyses
Comparisons were initially made between the CL and no CL groups. Univariate analyses were performed using Chi-Squared test for categorical variables and Mann-Whitney U test for continuous variables. Multivariate analyses were then performed, to identify independent predictors of CL, using multivariable cox regression modelling with a forward stepwise approach. Receiver operating characteristic (ROC) curves were then used to quantify the relationship of body composition measures to the development of CL. All analyses were performed using IBM SPSS Statistics 26 (IBM Corp. Armonk, NY, USA), with P<0.05 deemed to be indicative of statistical significance.
Institutional approval
This was a retrospective human study so individual patient consent was not required for this study. Institutional approval for this study was acquired from the local Clinical Audit Registration and Management System (No. CARMS-16870). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
There were 26 patients who developed a CL following an oesophagectomy during the study period and 60 patients were included in the control group (Table 1). Age and gender were similar between the CL and no CL cohorts whilst preoperative body mass index (BMI) (P=0.001), subcutaneous fat index (P=0.001) and total fat index (P=0.004) were significantly associated with CL (Table 2).
Table 1
| Factor | Chyle leak (n=26) | No chyle leak (n=60) | P |
|---|---|---|---|
| Age | 71 [62–76] | 66 [59–70] | 0.085 |
| Gender, male | 22 [85] | 48 [80] | 0.767 |
| BMI | 24 [22–27] | 27 [25–32] | 0.001 |
| Neoadjuvant chemotherapy | 20 [77] | 49 [82] | 0.769 |
Given as n (%) or median (Q1–Q3). Univariate analyses performed using Chi-Squared test (categorical variables) and Mann-Whitney U test (continuous variables). BMI, body mass index.
Table 2
| Factor | Chyle leak (n=26) | No chyle leak (n=60) | P |
|---|---|---|---|
| Preoperative sarcopenia | 10 [46] | 36 [60] | 0.316 |
| Preoperative myosteatosis | 13 [59] | 33 [56] | 1 |
| Preoperative BMI | 24 [22–27] | 27 [25–32] | 0.001 |
| Preoperative subcutaneous fat index | 34 [25–47] | 71 [42–103] | 0.001 |
| Preoperative visceral fat index | 54 [39–92] | 68 [48–96] | 0.135 |
| Preoperative total fat index | 95 [71–136] | 148 [103–184] | 0.004 |
| Preoperative muscle HU | 36 [32–41] | 34 [29–38] | 0.072 |
Given as n (%) or median (Q1–Q3). Fat indices calculated by diving fat area by height in metres squared. (cross-sectional area of skeletal muscle was normalized for stature (cm2/m2) as reported elsewhere (17). Univariate analyses performed using Chi-Squared test (categorical variables) and Mann-Whitney U test (continuous variables). BMI, body mass index; HU, Hounsfield units.
On multivariate analysis, a lower preoperative subcutaneous fat index was a significant independent predictor of CL (P=0.003). Sarcopenia was not found to be a significant predictor of developing CL (Table 3).
Table 3
| Factor | Odds ratio (95% CI) | P |
|---|---|---|
| Preoperative BMI | – | 0.214 |
| Preoperative subcutaneous fat index | 1.026 (1.009–1.043) | 0.003 |
| Preoperative total fat index | – | 0.925 |
Multivariate analyses performed using multivariable cox regression modelling with forwards stepwise approach. CI, confidence interval; BMI, body mass index.
The relationship between body composition measures and CL were further investigated with ROC curves. Decreasing preoperative subcutaneous fat index [area under the receiver operating characteristic curve (AUROC) 0.744, P=0.001] and BMI (AUROC 0.728, P=0.001) were significantly associated with CL (Figure 1A,1B).
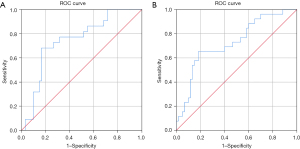
There were 10 (38%) patients who developed a severe CL but no significant predictors of severe CL were identified (Table 4).
Table 4
| Factor | Mild chyle leak (n=16) | Severe chyle leak (n=10) | P |
|---|---|---|---|
| Demographics | |||
| Age | 69 [63–77] | 73 [62–73] | 0.816 |
| Male | 14 [88] | 8 [80] | 0.625 |
| BMI | 23 [22–25] | 25 [23–28] | 0.310 |
| Oesophagectomy type | 0.723 | ||
| Open | 3 [19] | 3 [30] | |
| Hybrid | 6 [38] | 4 [40] | |
| MIO | 7 [44] | 3 [30] | |
| Neoadjuvant Therapy | |||
| Neoadjuvant Chemotherapy | 14 [88] | 6 [60] | 0.163 |
| Neoadjuvant Chemoradiotherapy | 0 (0) | 2 [20] | 0.138 |
| Preoperative Bloods | |||
| Albumin | 41 [35–46] | 44 [37–46] | 0.484 |
| CRP | 5 [3–7] | 8 [3–14] | 0.464 |
| NLR | 3.0 (2.6–3.7) | 2.7 (2.1–4.2) | 0.938 |
| Preoperative sarcopenia | 5 [31] | 5 [50] | 0.378 |
| Preoperative myosteatosis | 8 [50] | 5 [50] | 1.000 |
| Preoperative subcutaneous fat index | 34 [25–38] | 39 [26–58] | 0.616 |
| Preoperative visceral fat index | 54 [39–73] | 68 [36–100] | 0.714 |
| Preoperative total fat index | 95 [66–136] | 103 [75–139] | 0.570 |
| Preoperative Mus HU | 35 [33–41] | 38 [32–41] | 0.920 |
| Preoperative SMI | 49 [44–53] | 43 [40–46] | 0.145 |
Given as n (%) or median (Q1-Q3). Fat indices calculated by diving fat area by height in metres squared. HU, Hounsfield units. SMI, skeletal muscle index [cross-sectional area of skeletal muscle was normalized for stature (cm2/m2) as reported elsewhere (17)]. Univariate analyses performed using Chi-Squared test (categorical variables) and Mann-Whitney U test (continuous variables). MIO, minimally invasive oesophagectomy; BMI, body mass index; NLR, neutrophil:lymphocyte ratio; CRP, C-reactive protein.
Discussion
This retrospective descriptive study sought to investigate the aetiology of CL after oesophagectomy using body composition measures. The main finding is that a reduced preoperative body fat composition is a risk factor for CL after oesophagectomy. This is similar to previous studies which have reported a reduced incidence of CL after oesophagectomy amongst those with a higher BMI (18-21). CL seems to be anomalous in this regard, as other complications after oesophagectomy such as anastomotic leakage have been shown to be increased amongst those with a higher BMI (20,22-24).
Sarcopenia did not influence either the occurrence or severity of CL. Given that sarcopenia has frequently been shown to increase prevalence of complications after oesophagectomy (21,25), it was surprising that was not related to the prevalence of CL in this study. Rather, it seems that body fat is the most important factor influencing CL, opposed to muscle mass. Given this important finding and the fact that preoperative CT scans are readily available, clinicians could certainly consider routine preoperative measurement of body fat indices as this may allow some individualised risk stratification.
The mechanism for increased CL rate amongst those with a lower BMI and in this study, specifically a reduced body fat composition, is unclear and previous cohort studies and meta-analyses have also struggled to explain this finding (22). Reviewing the physiology, it is possible to suggest some potential causes. For instance, it is known that fasting can dramatically reduce flow through the thoracic duct, down to 1 mL/minute, which is in contrast to the physiological norm of approximately 200 mL/minute with normal diet (26-28). A reduction in flow could make the thoracic duct and its tributaries more difficult to identify intraoperatively, leading to an increase in iatrogenic injury. Furthermore, it is known that lymph flow is increased with hypertension (26,29). This then may explain why those with a higher BMI, who are more likely to be hypertensive, have a lower incidence of CL.
Previously published evidence indicates that nutritional status may not only be important preoperatively, but also play a vital role in patients’ recovery from CL (30-32). It is likely that this is related to both the loss of plasma proteins, such as albumin, and immune cells, such as lymphocytes. Combined, these will lead to impairment of the patient’s tissue healing and immune function. This emphasises the importance of physiological and nutritional optimisation before oesophagectomy to both reduce the likelihood of CL and to promote its recovery in the event it does occur.
Limitations of this study include its retrospective nature and the fact that CL is a relatively uncommon complication, so the number of patients are small and cases that are included in this study are spread over a large time period of time. Furthermore, in this study sarcopenia was assessed purely on a radiological basis, whereas the European Working Group on Sarcopenia in Older People (EWGSOP) does recommend the use of both low muscle mass and low muscle function to diagnose sarcopenia (33). In this study muscle function was not used but this is certainly an area of development for future studies.
The knowledge that reduced preoperative body fat increases the risk of CL presents a potentially modifiable risk factor for CL after oesophagectomy. Preoperative nutritional and physical optimisation could help to reduce CL rates after oesophagectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1580/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1580/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1580/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1580/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This was a retrospective human study so individual patient consent was not required for this study. Institutional approval for this study was acquired from the local Clinical Audit Registration and Management System (No. CARMS-16870). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim D, Cho J, Kim K, et al. Chyle leakage patterns and management after oncologic esophagectomy: A retrospective cohort study. Thorac Cancer 2014;5:391-7. [Crossref] [PubMed]
- Lagarde SM, Omloo JM, de Jong K, et al. Incidence and management of chyle leakage after esophagectomy. Ann Thorac Surg 2005;80:449-54. [Crossref] [PubMed]
- Merigliano S, Molena D, Ruol A, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg 2000;119:453-7. [Crossref] [PubMed]
- Rao DV, Chava SP, Sahni P, et al. Thoracic duct injury during esophagectomy: 20 years experience at a tertiary care center in a developing country. Dis Esophagus 2004;17:141-5. [Crossref] [PubMed]
- Bolger C, Walsh TN, Tanner WA, et al. Chylothorax after oesophagectomy. Br J Surg 1991;78:587-8. [Crossref] [PubMed]
- Dougenis D, Walker WS, Cameron EW, et al. Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet 1992;174:501-6. [PubMed]
- Dugue L, Sauvanet A, Farges O, et al. Output of chyle as an indicator of treatment for chylothorax complicating oesophagectomy. Br J Surg 1998;85:1147-9. [Crossref] [PubMed]
- Soma D, Kawamura YI, Yamashita S, et al. Sarcopenia, the depletion of muscle mass, an independent predictor of respiratory complications after oncological esophagectomy. Dis Esophagus 2019;32:doy092. [Crossref] [PubMed]
- Nishigori T, Okabe H, Tanaka E, et al. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol 2016;113:678-84. [Crossref] [PubMed]
- Yassaie SS, Keane C, French SJH, et al. Decreased total psoas muscle area after neoadjuvant therapy is a predictor of increased mortality in patients undergoing oesophageal cancer resection. ANZ J Surg 2019;89:515-9. [Crossref] [PubMed]
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115-22. [Crossref] [PubMed]
- Papaconstantinou D, Vretakakou K, Paspala A, et al. The impact of preoperative sarcopenia on postoperative complications following esophagectomy for esophageal neoplasia: a systematic review and meta-analysis. Dis Esophagus 2020; Epub ahead of print. [Crossref] [PubMed]
- den Boer RB, Jones KI, Ash S, et al. Impact on postoperative complications of changes in skeletal muscle mass during neoadjuvant chemotherapy for gastro-oesophageal cancer. BJS Open 2020;4:847-54. [Crossref] [PubMed]
- Fehrenbach U, Wuensch T, Gabriel P, et al. CT Body Composition of Sarcopenia and Sarcopenic Obesity: Predictors of Postoperative Complications and Survival in Patients with Locally Advanced Esophageal Adenocarcinoma. Cancers (Basel) 2021;13:2921. [Crossref] [PubMed]
- Hagens ERC, Feenstra ML, van Egmond MA, et al. Influence of body composition and muscle strength on outcomes after multimodal oesophageal cancer treatment. J Cachexia Sarcopenia Muscle 2020;11:756-67. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997-1006. [Crossref] [PubMed]
- Miao L, Chen H, Xiang J, et al. A high body mass index in esophageal cancer patients is not associated with adverse outcomes following esophagectomy. J Cancer Res Clin Oncol 2015;141:941-50. [Crossref] [PubMed]
- Morgan MA, Lewis WG, Hopper AN, et al. Prognostic significance of body mass indices for patients undergoing esophagectomy for cancer. Dis Esophagus 2007;20:29-35. [Crossref] [PubMed]
- Blom RL, Lagarde SM, Klinkenbijl JH, et al. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol 2012;19:766-71. [Crossref] [PubMed]
- Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann Surg 2017;266:822-30. [Crossref] [PubMed]
- Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 2013;109:2894-903. [Crossref] [PubMed]
- Grotenhuis BA, Wijnhoven BP, Hötte GJ, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 2010;34:2621-7. [Crossref] [PubMed]
- Healy LA, Ryan AM, Gopinath B, et al. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg 2007;134:1284-91. [Crossref] [PubMed]
- Järvinen T, Ilonen I, Kauppi J, et al. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol 2018;16:27. [Crossref] [PubMed]
- Lv S, Wang Q, Zhao W, et al. A review of the postoperative lymphatic leakage. Oncotarget 2017;8:69062-75. [Crossref] [PubMed]
- Baniel J, Foster RS, Rowland RG, et al. Management of chylous ascites after retroperitoneal lymph node dissection for testicular cancer. J Urol 1993;150:1422-4. [Crossref] [PubMed]
- Su IC, Chen CM. Spontaneous healing of retroperitoneal chylous leakage following anterior lumbar spinal surgery: a case report and literature review. Eur Spine J 2007;16:332-7. [Crossref] [PubMed]
- Valenzuela-Rendon J, Manning RD Jr. Chronic transvascular fluid flux and lymph flow during volume-loading hypertension. Am J Physiol 1990;258:H1524-33. [PubMed]
- Steven BR, Carey S. Nutritional management in patients with chyle leakage: A systematic review. Eur J Clin Nutr 2015;69:776-80. [Crossref] [PubMed]
- Townshend AP, Speake W, Brooks A. Chylothorax. Emerg Med J 2007;24:e11. [Crossref] [PubMed]
- Havas TE, Gullane PJ, Kassel RN. The incidence and management of chylous fistulae. Aust N Z J Surg 1987;57:851-4. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]


