Clinical significance of tumor necrosis and viability in non-small cell lung cancer
Introduction
Cancer prognosis generally depends on accurate TNM staging system, which reflects the extent of cancer (1). However, great differences in outcomes after surgery and various prognostic courses are observed in patients with the same TNM stage (2,3). Therefore, auxiliary parameters along with the TNM staging system are necessary to predict prognosis of patients (1,3). A greater understanding of the tumor biology of non-small cell lung cancer (NSCLC) would aid in the identification of additional parameters that better predict prognosis (2,3). Moreover, these insights could help individualize treatment (2). Of tumor biology characteristics, tumor necrosis (TN) has been shown to be related to a poor prognosis in a variety of tumor types (4,5). Consequently, TN has been included in pathological classifications and prognostic parameters for several solid organ cancers (4,5). The presence of TN has further been shown to affect management strategy and prognosis in some inflammatory and hematopoietic disorders (2,4,5). However, our understanding of TN in NSCLC is poor and TN is not used widely in the clinical context of NSCLC (6,7). In addition, tumor viability (TV), which refers to the amount of viable cancer cells in the tumor, and its effects on cancer prognosis remain unclear (8). In the present study, we investigated the prognostic significance of TN and TV in NSCLC by investigating the associations between these two biological tumor parameters and clinicopathological parameters. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1597/rc).
Methods
Subjects and study methods
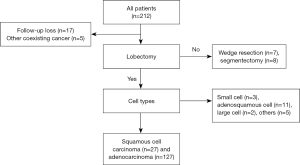
We assessed TN and TV as aspects of tumor biology and analyzed the associations between these parameters and various clinicopathological parameters associated with prognosis in NSCLC. These clinicopathological parameters included neoadjuvant therapy, cancer cell types, cell differentiation, involvement of mediastinal lymph nodes, and pathologic stage, vascular, lymphatic, and perineural invasion, tumor size, epidermal growth factor receptor (EGFR) and ALK mutations, and Ki-67 antigen level. Medical records of all subjects who underwent surgical resection for lung cancer between 2015 to 2016 were reviewed retrospectively, and all consecutive cases who met our inclusion criteria were included in the present study (Figure 1). All patients underwent a lobectomy with standard mediastinal lymph node dissection for NSCLC. Inclusion/exclusion criteria for the present study were as follows: (I) one of two cancer cell types (squamous cell carcinoma or invasive adenocarcinoma); (II) no other coexisting primary cancer; (III) complete resection with curative intent, and (IV) no immediate postoperative death within one month due to postoperative complications. Preoperative assessments included chest computed tomography (CT), PET-CT, and brain magnetic resonance imaging (MRI). Ultrasound-guided transbronchial aspiration or mediastinoscopic biopsy was performed in cases with clinically suspicious lymph nodes. Three thoracic surgeons primarily conducted video-assisted thoracoscopic surgery with standard mediastinal lymph node dissection. Postoperative cancer stages were determined by the eighth American Joint Committee on Cancer staging system. Neoadjuvant therapy or adjuvant treatment was conducted in accordance with the National Comprehensive Cancer Network guidelines and the proposals of a multidisciplinary team who reviewed clinical data. Neoadjuvant therapy primarily consists of a combination of chemotherapy (two cycles of cisplatin and paclitaxel) and radiation therapy (over 5 weeks for a total of 44–45 Gray).
Disease status was monitored regularly by medical oncologists, and monitoring examinations included physical examination, chest radiography, chest CT, and routine blood tests. PET-CT, bone scan, or brain MRI was performed when any symptoms developed. Pathologic confirmation was obtained when clinically required.
Histopathological considerations
NSCLC cell types were determined by the World Health Organization Classification of Lung Tumors (9,10). After formalin fixation and paraffin embedding of tumor samples, multiple histologic sections were made. Immunohistochemistry studies were performed to evaluate histopathologic findings. Further tests of EGFR and K-ras mutation status as well as level of Ki-67 antigen were performed as necessary (11). Two expert pathologists reviewed the data independently and blindly. Tumors are composed of viable cancer tissue, necrotic tissue, calcifications, and fibrosis, and the relative amounts of these components in each tumor sample were described as ratio for all cases (1,12,13). TV was defined as the amount of viable cancer cells relative to the area of the slice (8,13). TN was defined as the presence of coagulative necrosis as indicated by the formation of homogeneous clusters of necrotic cells, and the necrotic ratio was defined as the amounts of necrotic area relative to the area of the slice (1,6,7). Values are reported semi-quantitatively in 5% increments. The presence of histological TN in specimens was defined as greater than 5% necrosis. In addition, adenocarcinoma subtypes were classified using the lung adenocarcinoma multidisciplinary classification system proposed by the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society, and each subtype component ratio in the tumor was described semi-quantitatively in 5% increments (9,10,12,14,15). Adenocarcinoma subtypes were lepidic, acinar, papillary, solid, micropapillary, and variant (9,12,16). The predominant subtype of adenocarcinoma cases was defined based on the subtype with the largest component ratio (14). Vascular invasion was considered present when cancer cells are observed in vascular lumens (1,3,7). The presence of perineural invasion was defined as tumoral invasion of the epineurium (1,7). EGFR mutation was examined by PNA clamping-assisted fluorescence melting curve analysis using PANAMutyperTM R EGFR (Panagene, Daejeon, Korea). Fluorescent in situ hybridization (FISH) with Vysis ALK Break Apart FISH probe kit (Abbott, IL, USA) and immunohistochemistry with ALK D5F3 CDx assay (Ventana Medical Systems. Inc., Tucson, AZ, USA) were used to examine ALK mutation. Monoclonal mouse Anti-Human Ki-67 Antigen/FITC is used for a flow cytometry for identification of cells expressing the Ki-67 antigen (clone MIB-1).
Statistical analysis
Nonparametric tests were used for statistical analyses because the data were not normally distributed. All data are presented as the mean ± standard deviation (SD). The significance of differences in continuous variables between groups was assessed using the Mann-Whitney U test, Kruskal-Wallis test, or Jonckheere-Terpstra test. The significance of differences in categorical variables between groups was assessed using the chi-square test or Fisher’s exact test. Spearman correlation test was used to assess the significance of relationships between continuous variables. Survival was analyzed using Kaplan-Meier analysis, and the effects of variables on survival were assessed using a log-rank test. Overall survival was estimated from the date of surgery to the date of death, censoring or last follow-up. A Cox proportional hazards regression model (backward) was used to perform univariate and multivariate analyses of the associations between survival period and predictive parameters. Multiple linear regression (stepwise) was used to evaluate the relationship between prognostic parameters and tumor fluorodeoxyglucose (FDG) uptake. Data were analyzed using the Statistical Package of Social Sciences version 22.0 (SPSS, IBM Corp., Armonk, NY, USA). P values <0.05 were considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Uijeongbu St. Mary’s Hospital (No. UC21RISI0123) and individual consent for this retrospective analysis was waived.
Results
A consecutive 154 patients (male 74, female 80; mean age: 65.0±10.1 years) were included into the present study. All patients underwent a lobectomy with standard mediastinal lymph node dissection and R0 resection. There were 127 adenocarcinoma and 27 squamous cell carcinoma cases. Fifteen patients underwent neoadjuvant therapy. Final pathologic stages were IA1 (n=13), IA2 (n=30), IA3 (n=32), IB (n=40), IIA (n=9), IIB (n=18), and IIIA (n=12). Subtype analyses were performed in 124 of 127 patients with adenocarcinoma. Among the adenocarcinoma cases, the distribution of predominant subtypes as follows: lepidic (n=26), acinar (n=72), papillary (n=14), micropapillary (n=3), solid (n=7), and variant type (n=2). Mean ratios of adenocarcinoma subtypes in all subjects were lepidic (28.9%), acinar (47.1%), papillary (10.9%), micropapillary (3.7%), solid (7.6%), and variant type (1.8%). Mean observation period was 53.4 (±17.4) months. Overall clinico-histopathological characteristics of the patient population are presented in Table 1.
Table 1
| Characteristics | WON (n=139) | WN (n=15) | P value |
|---|---|---|---|
| Age (year) | 65.0 (±10.1) | 65.6 (±9.5) | 0.826 |
| Gender | 0.013 | ||
| Male | 62 | 12 | |
| Female | 77 | 3 | |
| Cell type | <0.001 | ||
| Adenocarcinoma | 121 | 6 | |
| Squamous cell carcinoma | 18 | 9 | |
| Tumor size (cm2) | 6.5 (±8.6) | 5.9 (±4.0) | 0.588 |
| Pathologic stage | 0.290 | ||
| IA1 | 12 | 1 | |
| IA2 | 28 | 2 | |
| IA3 | 29 | 3 | |
| IB | 37 | 3 | |
| IIA | 8 | 1 | |
| IIB | 17 | 1 | |
| IIIA | 8 | 4 | |
| R0 resection | NA | ||
| No | 0 | 0 | |
| Yes | 139 | 15 | |
| Predominant subtype of adenocarcinoma | 0.471 | ||
| Lepidic | 25 | 1 | |
| Acinar | 67 | 5 | |
| Papillary | 14 | 0 | |
| Micropapillary | 3 | 0 | |
| Solid | 7 | 0 | |
| Variant | 2 | 0 | |
| Pre-operative tumor SUVmax | 5.5 (±4.7) | 8.2 (±6.9) | 0.087 |
| Differentiation | 0.013 | ||
| Well | 30 | 0 | |
| Moderate | 71 | 6 | |
| Poor | 38 | 9 | |
| Mean ratio of tumour components (%) | |||
| Viable cancer cell | 71.6 (±20.2) | 39.8 (±26.1) | <0.001 |
| Necrosis | 5.0 (±12.8) | 16.9 (±24.4) | 0.005 |
| Calcification | 0.04 (±0.4) | 0.33 (±1.3) | 0.162 |
| Fibrosis | 23.3 (±18.3) | 42.3 (±28.1) | 0.010 |
WON, the group without neoadjuvant therapy; WN, the group with neoadjuvant therapy; SUVmax, the maximum standardized uptake value.
TN and TV according to neoadjuvant therapy, cancer cell types, cell differentiation, involvement of mediastinal lymph nodes, and pathologic stage
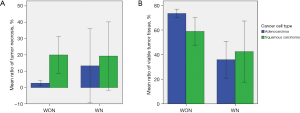
The mean ratios of viable cancer tissue, necrosis, calcifications, and fibrosis in the whole cohort were (68.5±22.8)%, (6.2±14.7)%, (0.1±0.6)%, and (25.1±20.1)%, respectively. All analyses were performed according to neoadjuvant therapy [group without neoadjuvant therapy (WON) vs. group with neoadjuvant therapy (WN)] because TN and TV are influenced by neoadjuvant therapy and the effect of neoadjuvant therapy on them is various.
Relationships of TN and TV with neoadjuvant therapy and cancer cell type
We analyzed the relationships of TN and TV and neoadjuvant therapy and cancer cell type (adenocarcinoma vs. squamous cell carcinoma). There was no statistical difference in tumor size, pT, or overall stage between WON and WN patients. Tumors from WN patients showed significantly greater necrosis (P=0.005) and less TV (P<0.001) than tumors from WON patients (Table 1). There was no statistical difference in tumor size, pT, or overall stage between adenocarcinoma and squamous cell carcinoma. In WON, squamous cell carcinoma cases showed significantly higher TN (P<0.001) and lower TV (P=0.006) than adenocarcinoma cases. In WN, there were no significant differences in TN or TV according to cancer cell type. These findings are illustrated in Figure 2.
Relationships of TV and TN with differentiation, vascular invasion, lymphatic invasion, and perineural invasion
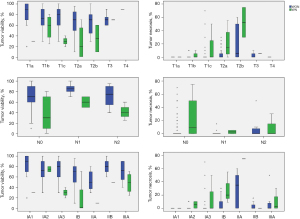
Prognosis of NSCLC is affected by tumor differentiation and extent of vascular, lymphatic, and perineural invasion (1,3,7). We investigated the relationships of TV and TN with vascular, lymphatic, and perineural invasion as well as differentiation. In WON, cases with vascular, lymphatic, and perineural invasion showed significantly lower TV and higher TN than cases without invasion (P=0.014, P=0.019, and P=0.012 for TV; P=0.001, P<0.001, and P<0.001 for TN, respectively). Poorer differentiation was associated with lower TV and more severe necrosis (P<0.001 and P<0.001, respectively). In WN, there were no relationship among these parameters except for a significant relationship between TN and differentiation (P=0.035). These findings are shown in Table 2.
Table 2
| Variables | TV (%) | P value | TN (%) | P value |
|---|---|---|---|---|
| WON | ||||
| Vascular invasion | 0.014 | 0.001 | ||
| No | 72.8±20.5 | 3.4±10.9 | ||
| Yes | 62.4±16.2 | 14.7±19.3 | ||
| Lymphatic invasion | 0.019 | <0.001 | ||
| No | 74.6±21.1 | 0.9±4.2 | ||
| Yes | 67.7±18.6 | 9.7±17.3 | ||
| Perineural invasion | 0.012 | <0.001 | ||
| No | 72.4±19.7 | 4.1±11.5 | ||
| Yes | 48.3±20.4 | 25.0±24.3 | ||
| Differentiation | <0.001 | <0.001 | ||
| Well | 81.7±19.3 | 0.0±0.0 | ||
| Moderate | 71.7±19.7 | 3.4±11.2 | ||
| Poor | 64.6±19.2 | 12.4±18.1 | ||
| WN | ||||
| Vascular invasion | 0.768 | 0.594 | ||
| No | 41.7±28.6 | 17.4±21.0 | ||
| Yes | 36.0±23.0 | 16.0±33.1 | ||
| Lymphatic invasion | 0.075 | 0.254 | ||
| No | 23.2±14.5 | 11.0±24.6 | ||
| Yes | 48.1±27.2 | 19.9±25.1 | ||
| Perineural invasion | 0.4 | 0.133 | ||
| No | 41.9±25.7 | 12.8±19.1 | ||
| Yes | NA | NA | ||
| Differentiation | 0.406 | 0.035 | ||
| Well | NA | NA | ||
| Moderate | 46.7±20.7 | 3.3±8.2 | ||
| Poor | 35.2±29.5 | 26.0±27.8 |
TV, tumor viability; TN, tumor necrosis; WON, the group without neoadjuvant therapy; WN, the group with neoadjuvant therapy.
Relationships of TN and TV with tumor size, pT stage, pN, and overall pathologic stage
In WON, there was a positive correlation between TN and size (P<0.001) and a negative correlation between TV and size (P=0.031). In WN, there was a positive correlation between TN and size (P=0.008) but no significant correlation between TV and size. In WON, there was a positive correlation between TN and pT stage (P<0.001) and a negative correlation between TV and pT stage (P=0.011). In WN, there was a positive correlation between TN and pT stage (P=0.031) but no significant correlation between TV and pT stage. Seven of 139 in WON and three of 15 in WN showed mediastinal lymph node involvement. There was no significant association of TN and TV with mediastinal lymph node involvement in either WON or WN. In WON, TN increased significantly as pathologic stage increased (P=0.001) and TV decreased significantly as pathologic stage increased (P=0.038). In WN, there was no relationship of TN and TV with overall pathologic stage. These findings are summarized in Figure 3.
Survival analyses according to TN and TV in pN0 cases
Because this study showed that TV and TN were associated with pT and overall stage except pN, and there were few cases with mediastinal lymph node involvement, we analyzed overall survival according to TN and TV in patients with pN0. These patients were divided into negative and positive TN groups (<5% vs. ≥5% necrosis) and low and high TV groups based on mean ratio values (72% in WON and 40% in WN). In WON, the group without necrosis showed significantly longer survival than the group with necrosis (P=0.016); however, there was no difference in survival according to the necrosis in WN (P=0.053) (Figure 4). There was no difference in survival according to TV in either WON (P=0.485) or WN (P=0.355). We performed a Cox proportional hazard model (backward) to investigate the presence of TN as an independent prognostic factor for overall survival. Age, sex, overall pathology stage, cancer cell type, neoadjuvant therapy, and presence of TN were included as covariates. Neoadjuvant therapy and pathologic stage were independent prognostic factors. In addition, we performed Cox proportional hazard regression (backward) to investigate if the presence of TN was an independent prognostic factor in N0 disease. Age, pT stage, cancer cell type, neoadjuvant therapy, and presence of TN were included as covariates in our model. Presence of TN and pT stage were independent prognostic factors of survival among patients with N0 stage cancer. These findings are summarized in Table 3.
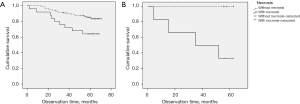
Table 3
| Covariates | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| A | |||
| Neoadjuvant therapy | 2.777 | 1.193–6.488 | 0.018 |
| Overall pathologic stage | 0.001 | ||
| IA1 | Reference | ||
| IA2 | 0.412 | 0.026–6.591 | 0.531 |
| IA3 | 1.973 | 0.230–16.896 | 0.535 |
| IB | 5.063 | 0.665–38.536 | 0.117 |
| IIA | 8.832 | 1.029–75.774 | 0.047 |
| IIB | 2.315 | 0.241–22.270 | 0.467 |
| IIIA | 7.509 | 0.899–62.700 | 0.063 |
| B | |||
| Necrosis | 2.340 | 1.050–5.212 | 0.037 |
| pT stage | 0.021 | ||
| T1a | Reference | ||
| T1b | 0.352 | 0.0228–5.665 | 0.462 |
| T1c | 1.585 | 0.182–13.799 | 0.676 |
| T2a | 3.255 | 0.409–25.894 | 0.265 |
| T2b | 52.152 | 0.555–47.793 | 0.149 |
| T3 | 2.060 | 0.181–23.381 | 0.560 |
| T4 | 27.055 | 1.640–446.129 | 0.021 |
Covariate A, in all subjects; Covariate B, in N0 disease.
TN and TV according to adenocarcinoma subtype and survival
Relationships of TN and TV with the predominant adenocarcinoma subtype
Adenocarcinoma is the most common histopathologic type of NSCLC, and prognosis varies according to adenocarcinoma subtype (16-18). Therefore, we investigated the relationships of TN and TV with the adenocarcinoma subtype. The predominant histologic subtypes in the entire cohort were lepidic (26 cases), acinar (72 cases), papillary (14 cases), variant (2 cases), solid (7 cases), and micropapillary (3 cases). There was no association of TN or TV with the predominant histologic subtype in either WON or WN (Table 1).
Relationships of TN and TV with subtype score
The prognosis according to subtype from best to worst is as follows: lepidic, papillary, acinar, variant, solid, and micropapillary (14,16,18,19). Adenocarcinoma usually comprises a mixture of adenocarcinoma subtypes (12,19). Therefore, we designed a new scoring system to reflect the prognosis of the subtypes of adenocarcinoma. We assigned points for each adenocarcinoma subtypes as follows: 1 for lepidic, 2 for papillary, 3 for acinar, 4 for variant, 5 for solid, and 6 points of micropapillary. The subtype score of each tumor of adenocarcinoma was defined as the sum of the points for each subtype in a sample multiplied by the ratio of the adenocarcinoma subtypes. In WON, there was a positive correlation between necrosis ratio and subtype score (P<0.001) and a negative correlation between TV ratio and the subtype score (P=0.007). The group with necrosis showed a significantly higher subtype score than the group without necrosis (3.3±0.8 vs. 2.5±0.8; P<0.001). The low viability group showed a significantly higher subtype score than the high viability group (2.7±0.7 vs. 2.5±0.9, P=0.030). However, there was no relationship of TN or TV with subtype score in WN.
Survival according to subtype score
We divided the cohort into two groups based on mean subtype score (2.6±0.9 in WON, and 2.8±0.9 in WN) and compared survival between these. The low subtype score group showed significantly longer survival than the high subtype score group in WON (P=0.020); however, there was no difference of survival according to subtype score in WN (Figure 5).
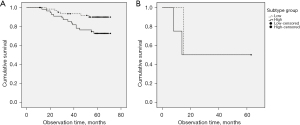
Multivariate analysis for survival in adenocarcinoma
We also performed a Cox proportional hazard model (backward) to investigate the subtype score as an independent prognostic factor for overall survival in cases with adenocarcinoma. Age, overall pathology stage, differentiation, subtype score, neoadjuvant therapy, and presence of TN were included as covariates. Neoadjuvant therapy and subtype score were independent prognostic factors of survival among patients with adenocarcinoma (Table 4).
Table 4
| Covariates | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Neoadjuvant therapy | 3.882 | 1.148–13.119 | 0.029 |
| Subtype score group | 2.600 | 1.073–6.298 | 0.021 |
Relationships of adenocarcinoma subtype, TN, and TV with tumor FDG uptake
Relationships of TN and TV and adenocarcinoma subtype with tumor FDG uptake
PET-CT is an essential tool for staging as well as surveilling lung cancer (20,21). In addition, many studies have shown that tumor FDG uptake is associated with prognosis (20,21). We assumed that TN and TV and adenocarcinoma subtype would be associated with tumor FDG uptake because these factors were associated with prognosis (21). We analyzed tumor FDG uptake using the maximum standardized uptake value (SUVmax) of the tumor. In WON, there was a significant positive correlation between TN and tumor FDG uptake (P<0.001). However, there was no relationship between viability and tumor FDG uptake in WON or between TN or TV and tumor FDG uptake in WN. There was a significant positive correlation between subtype score and tumor FDG uptake in WON (P=0.007) but no relationship between TN or TV and subtype score and tumor FDG uptake in WN.
Prediction of tumor FDG uptake based on tumor biology
Tumor FDG uptake is associated with tumor size, differentiation, and cell type (6,20,21). We found that TN and adenocarcinoma subtype were associated with tumor FDG uptake in WON. We performed a multiple linear regression test (stepwise) to identify independent predictive factors of tumor FDG uptake in WON. Tumor size, differentiation, cell type, and TN and TV were included as covariates. Linear regression analysis showed that tumor FDG uptake was associated with tumor size, differentiation, cell type, and TN. In addition, we investigated predictive factors in adenocarcinoma cases in WON. Tumor size, differentiation, subtype score, TN, and TV were included as covariates. Linear regression analysis showed that tumor FDG uptake was significantly associated with tumor size, differentiation, and TN. These findings are summarized in Table 5.
Table 5
| Variables | Coefficient (β) | t | P value | 95% CI for β |
|---|---|---|---|---|
| A | ||||
| Differentiation | 2.459 | 4.897 | <0.001 | 1.463–3.454 |
| Cancer cell type | 2.752 | 2.521 | 0.013 | 0.588–4.916 |
| Tumor size | 0.107 | 2.982 | 0.004 | 0.036–0.178 |
| TN | 2.770 | 2.992 | 0.003 | 0.935–4.605 |
| B | ||||
| Differentiation | 2.338 | 4.578 | <0.001 | 1.324–3.352 |
| Tumor size | 0.244 | 3.291 | 0.001 | 0.097–0.391 |
| TN | 2.584 | 2.598 | 0.011 | 0.609–4.558 |
TN, tumor necrosis; FDG, fluorodeoxyglucose; Variables A, all cases; Variables B, cases with adenocarcinoma.
Relationships of TN with EGFR and ALK mutations and Ki-67 antigen level in adenocarcinoma
Finally, we analyzed the relationships of TN with EGFR and ALK mutations and Ki-67 antigen level in adenocarcinoma within WON. Of 121 cases with adenocarcinoma in WON, we performed tests of EGFR mutation in 99 cases, ALK mutation in 73 cases, and Ki-76 antigen in 68 cases. There was a positive correlation between TN with Ki-67 level (P=0.027). However, there was no relationship between TN and gene mutations in EGFR or ALK.
Discussion
A better understanding of tumor biology would help to identify additional prognostic parameters that could facilitate better management of cancers (10,11,14). We investigated if TN and TV affected NSCLC prognosis and showed that TN was associated with various prognostic parameters including neoadjuvant therapy; cancer cell type; vascular, lymphatic, and perineural invasion; differentiation; cancer stage; subtype of adenocarcinoma; tumor FDG uptake; and Ki-67 antigen level, as well as overall survival (3,6,14). TN can be used as an auxiliary parameter along with the TNM staging system in NSCLC as follows (1). First, NSCLC with squamous cell carcinoma has been reported to have a worse prognosis than adenocarcinoma, which can be explained somewhat by the greater necrosis in squamous cell carcinomas (20). Second, the decision to perform sublobar resection usually depends on tumor size (14,22). However, TN also can affect the extent of resection. Hence, pathologists should evaluate TN to guide thoracic surgeons in cases where there is an option for sublobar resection vs. lobectomy because the impact of TN on prognosis can be reduced by lobectomy compared to sublobar resection (22). In addition, the presence of TN in cases with early-stage NSCLC leads to further studies or consideration of alternate therapies, such as stereotactic body radiation therapy or other ablative therapies, based on the findings of this study. Third, the presence of TN was an independent predictor of a worse prognosis in N0 disease. The presence of TN could allow better prognosis stratification and serve as an indicator for the need for adjuvant therapy even in early-stage lung cancer as well as follow-up strategies. Furthermore, preoperative identification of the presence of TN should merit consideration of the potential benefits of neoadjuvant therapy. Fourth, FDG uptake refers to the amount of radiotracer uptake by tumor and is determined by hypoxia, angiogenesis, and glucose metabolism of the tumor (6). Many studies have been suggested that tumor FDG uptake is associated with prognosis, with a higher tumor SUVmax predicting a worse prognosis (6,20). We demonstrated that FDG uptake was associated with TN but not TV. Previous studies reported that FDG uptake was significantly higher in patients with squamous cell carcinoma than those with adenocarcinoma, which was explained by TN (6,20). We found that adenocarcinoma subtype was related to tumor FDG uptake; however, it was not an independent predictor of tumor FDG uptake in multivariate analysis. Fifth, we found that prognoses differed according to adenocarcinoma subtype, which could be explained partially by varying amounts of necrosis in the various adenocarcinoma subtypes.
Previous studies have reported that TN is related to poor outcomes in patients with NSCLC, consistent with our findings (1). Some aspects of our study are novel. First, we developed a novel subtype score reflecting the mixture of adenocarcinoma subtypes and investigated the association between this subtype score and prognosis (19). Second, we investigated TV as well as TN as factors affecting prognosis and demonstrated that that presence of TN rather than viability is associated with various prognostic factors and has a negative effect on survival after complete resection for NSCLC. Interestingly, TV itself was not associated with prognosis in NSCLC before anticancer treatment. Numerous studies have assessed TV after anticancer treatment and suggested that the greater is the decrease in TV after anticancer treatment, the better is the prognosis (2,8,23). In the present study, we showed that the association between cancer viability and prognosis differed according to anticancer treatment. TV was not associated with prognosis before anticancer treatment, while a decrease in TV rather than TV itself was associated with prognosis after anticancer treatment. Third, most studies have investigated TN in early lung cancer cases, such as tumor size <2 cm or T1 stage (1,7). However, we included patients with stage II and III cancer in our study to better reflect real-world practice.
TN is caused by tumor overgrowth, which reflects tumor aggressiveness and affects treatment (7). Ki-67 level reflects tumor behavior and aggressiveness (7). We found a positive correlation between TN and Ki-67 level. In addition, necrosis attenuates the effects of radiotherapy and chemotherapy (8). Radiotherapy is less effective at treating hypoxic tumors than well oxygenated tumors because the amount of reactive oxygen species generated is low in hypoxic tumors (8). If a tumor grows rapidly, blood vessels can be remote from the tumor, making it difficult for drugs to reach the tumor (8). Furthermore, any drugs that reach tumor cells can be lost in areas of necrosis (8).
Clinical implication of our findings is that TN should be utilized routinely as an additional prognostic factor when pathologically evaluating resected specimens from patients with NSCLC, especially in cases without lymph node involvement. TN should be evaluated in intraoperative frozen sections to determine the extent of resection (4). Despite recent advances in our understanding of the genetics of NSCLC, histopathologic assessment remains essential for diagnosis, prognosis, and determining the type of resection (23). Diverse clinical outcomes in different histologic subtypes are likely due to different genetic profiles (11,12,24). Our results may provide a theoretical background for exploring the association between functional phenotypes and underlying genotypes in NSCLC (24-26). Assessment of TN is simple, can be incorporated into existing staging systems, and might help identify cases at high risk for recurrence, predict prognosis, and develop individualized therapies (11,23). TN should be considered when designing clinical trials of adjuvant therapy and interpreting the findings (26).
If assessment of TN is possible prior to surgery, TN should be investigated whether or not it is used as a parameter to plan neoadjuvant therapy. However, exact histological necrosis assessment is only possible in resected tumor specimens. Further development and clinical adoption of imaging tools are required to solve this problem (8,27).
There are certain limitations of our study that should be taken into consideration. Our sample size was relatively small, and this was a retrospective study. Most cases were early-stage cases and data were non-parametric. Genetic data were not available in many cases because gene studies are not part of routine exams and are usually performed in later stage cancers. Because the effects of neoadjuvant therapy effects on NSCLC vary widely, further large-scale studies in WN patients are needed to validate the findings in the present study. We were unable to analyze patients according to postoperative adjuvant therapy. However, all cases included in this study underwent a lobectomy and mediastinal lymph node dissection with no limit to the extent of resection.
Conclusions
We demonstrated that TN is significantly associated with overall survival and various prognostic factors after pulmonary resection for NSCLC. Thus, TN appears to play an important role in the progression of NSCLC, especially in N0 disease. Its use as a diagnostic or prognostic tool will facilitate the development of more appropriate management strategies for NSCLC. TN might allow for more personalized treatment in addition to better selection and stratification of patients in future clinical trials. Future genomic and gene expression profiling studies that focus on TN in the setting of NSCLC will provide further insight into the biology of this prevalent tumor type.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1597/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1597/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1597/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Uijeongbu St. Mary’s Hospital (No. UC21RISI0123) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kiliçgün A, Turna A, Sayar A, et al. Very important histopathological factors in patients with resected non-small cell lung cancer: necrosis and perineural invasion. Thorac Cardiovasc Surg 2010;58:93-7. [Crossref] [PubMed]
- Badovinac S, Korsic M, Mursic D, et al. Cancer-related inflammation as predicting tool for treatment outcome in locally advanced and metastatic non-small cell lung cancer. J Thorac Dis 2016;8:1497-503. [Crossref] [PubMed]
- Samejima J, Yokose T, Ito H, et al. Prognostic significance of blood and lymphatic vessel invasion in pathological stage IA lung adenocarcinoma in the 8th edition of the TNM classification. Lung Cancer 2019;137:144-8.
- Atanasov G, Schierle K, Hau HM, et al. Prognostic Significance of Tumor Necrosis in Hilar Cholangiocarcinoma. Ann Surg Oncol 2017;24:518-25. [Crossref] [PubMed]
- Richards CH, Mohammed Z, Qayyum T, et al. The prognostic value of histological tumor necrosis in solid organ malignant disease: a systematic review. Future Oncol 2011;7:1223-35. [Crossref] [PubMed]
- Koh YW, Lee SJ, Park SY. 18F-fluorodeoxyglucose positron emission tomography is correlated with the pathological necrosis and decreased microvessel density in lung adenocarcinomas. Ann Nucl Med 2019;33:93-102. [Crossref] [PubMed]
- Park SY, Lee HS, Jang HJ, et al. Tumor necrosis as a prognostic factor for stage IA non-small cell lung cancer. Ann Thorac Surg 2011;91:1668-73. [Crossref] [PubMed]
- Sobic-Saranovic D, Pavlovic S, Jovanovic D, et al. Assessment of non-small cell lung cancer viability and necrosis with three radiopharmaceuticals. Hell J Nucl Med 2008;11:16-20. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Ng KS, King Sun C, Boom Ting K, et al. Prognostic factors of EGFR-mutated metastatic adenocarcinoma of lung. Eur J Radiol 2020;123:108780. [Crossref] [PubMed]
- Hutchinson BD, Shroff GS, Truong MT, et al. Spectrum of Lung Adenocarcinoma. Semin Ultrasound CT MR 2019;40:255-64. [Crossref] [PubMed]
- Shioya S, Haida M, Ono Y, et al. Lung cancer: differentiation of tumor, necrosis, and atelectasis by means of T1 and T2 values measured in vitro. Radiology 1988;167:105-9. [Crossref] [PubMed]
- Kim M, Chung YS, Kim KA, et al. Prognostic factors of acinar- or papillary-predominant adenocarcinoma of the lung. Lung Cancer 2019;137:129-35. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Casteillo F, Guy JB, Dal-Col P, et al. Pathologic Subtypes of Lung Adenocarcinoma Brain Metastasis Is a Strong Predictor of Survival After Resection. Am J Surg Pathol 2018;42:1701-7. [Crossref] [PubMed]
- Miyazawa T, Marushima H, Saji H, et al. PD-L1 Expression in Non-Small-Cell Lung Cancer Including Various Adenocarcinoma Subtypes. Ann Thorac Cardiovasc Surg 2019;25:1-9. [Crossref] [PubMed]
- Urer HN, Kocaturk CI, Gunluoglu MZ, et al. Relationship between lung adenocarcinoma histological subtype and patient prognosis. Ann Thorac Cardiovasc Surg 2014;20:12-8. [Crossref] [PubMed]
- Park JK, Kim JJ, Moon SW, et al. Lymph node involvement according to lung adenocarcinoma subtypes: lymph node involvement is influenced by lung adenocarcinoma subtypes. J Thorac Dis 2017;9:3903-10. [Crossref] [PubMed]
- Karam MB, Doroudinia A, Behzadi B, et al. Correlation of quantified metabolic activity in nonsmall cell lung cancer with tumor size and tumor pathological characteristics. Medicine (Baltimore) 2018;97:e11628. [Crossref] [PubMed]
- Shao X, Niu R, Jiang Z, et al. Role of PET/CT in Management of Early Lung Adenocarcinoma. AJR Am J Roentgenol 2020;214:437-45. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Reiniger L, Téglási V, Pipek O, et al. Tumor necrosis correlates with PD-L1 and PD-1 expression in lung adenocarcinoma. Acta Oncol 2019;58:1087-94. [Crossref] [PubMed]
- Dong ZY, Zhang C, Li YF, et al. Genetic and Immune Profiles of Solid Predominant Lung Adenocarcinoma Reveal Potential Immunotherapeutic Strategies. J Thorac Oncol 2018;13:85-96. [Crossref] [PubMed]
- Biton J, Mansuet-Lupo A, Pécuchet N, et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clin Cancer Res 2018;24:5710-23. [Crossref] [PubMed]
- Zhang C, Zhang J, Xu FP, et al. Genomic Landscape and Immune Microenvironment Features of Preinvasive and Early Invasive Lung Adenocarcinoma. J Thorac Oncol 2019;14:1912-23. [Crossref] [PubMed]
- Henzler T, Konstandin S, Schmid-Bindert G, et al. Imaging of tumor viability in lung cancer: initial results using 23Na-MRI. Rofo 2012;184:340-4. [Crossref] [PubMed]