The outcome of lung transplantation for end-stage pulmonary diseases with pulmonary hypertension: a single-center experience
Introduction
Lung transplantation is over the half-century mark since the first was performed by James Hardy in 1963. Significant advances have been made in the field of lung transplantation over the last few decades (1). Lung transplantation in China has also struggled and explored like other places in the world and found the Chinese experience (2). Since Dr. Xin performed the first lung transplantation in 1979 (3), lung transplantation in China has been developing for more than 40 years. From January 1, 2015 to December 31, 2018, 1,053 lung transplantations were performed according to the China Lung Transplantation Registry records. With the increasing of transplant volume and experience, the gap between China and the world is narrowing (2).
Pulmonary hypertension (PH) is defined as mean pulmonary artery pressure (mPAP) of 25 mmHg or above at rest (4). The hemodynamic alterations of PH with right ventricular hypertrophy and relative left ventricular underfilling increases the risk of pulmonary edema and primary graft dysfunction (PGD) compared with other indications (4,5). Patients with PH who remain in World Health Organization functional class III or IV on maximal medical therapy should be referred as early as possible for lung transplantation (4). However, the best lung transplant procedures for patients with PH are still controversial (6-10).
The majority of related studies are based mainly on Western populations, but the Chinese population tends to differ substantially in population characteristics and culture. We therefore decided to analyze the outcome of patients with PH referred for lung transplantations that was performed at a single center. We investigated the relationship between single-lung transplantation (SLT) and double-lung transplantation (DLT), and the effect of age, mPAP, body mass index (BMI), and indication of transplantation on outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1738/rc).
Methods
All patients referred for lung transplantation in our center from March 2016 to December 2019 were retrospectively reviewed. The mPAP of 25 mmHg or above at rest on right heart catheterization was diagnosed as PH. For the current analysis, all consecutive PH patients who underwent lung transplantation were selected to collect detailed information and followed up. Heart-lung transplantation, re-transplantation, and age less than 18 years old were excluded. This report describes the experience by comparing different procedures, age, mPAP, BMI, and indication of transplantation.
The study was approved by the First Affiliated Hospital of Guangzhou Medical University Research Ethics Committee (2020-K-51) and informed consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Recipient selection
The selection of recipients for lung transplantation had a standard procedure that mainly referred to the International Society for Heart and Lung Transplantation (ISHLT) guidelines (11). Patients were selected when they had end-stage lung disease with a limited prognosis and displayed an inadequate response to treatment. These patients should be referral to lung transplant center early so that patients have enough time to consider lung transplantation, and transplant center can evaluate candidates in detail and optimize pre-transplant management. But in reality, these patients were often referred in a deteriorating disease state in which they couldn’t tolerate some necessary evaluation.
Procedure and donor selection
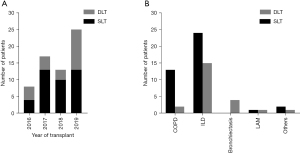
Underlying disease, condition of patients, the quality of donor, prognosis, and economy should be comprehensively considered to choose SLT or DLT. Bronchiectasis and other forms of suppurative lung disease were contraindications for SLT. Lung donors in China were relatively sufficient. Given the abundance of lung donors in China, disease and patient factors were more important than donor availability in choosing between SLT and DLT. We were more inclined to perform SLT in older, severely underweight, weak, and extremely ill recipients.
In the current study, donor lungs were obtained from brain-dead and circulatory-dead organ donors by organ procurement organization. Donor lung selection criteria and techniques for lung procurement referred to Toronto’s experience (12). Differently, all voluntary donors received intravenous methylprednisolone (500–1,000 mg) after announcing brain death. Celsior or low-potassium dextran (60 mL/kg, Perfadex, Vitrolife, Goteborg, Sweden) was used as the preservation solution. A retrograde flush with 1.5 L of Perfadex was added to the anterograde pulmonary artery flush while the lung was still inflated.
Surgical technique
On January 22nd, 2003, Professor Jianxing He successfully performed the first SLT in our center. SLT, DLT, the use of extracorporeal membrane oxygenation (ECMO), and heart-lung transplantation were been mature procedures in our center since 2015. Indication for intraoperative ECMO support included (I) hypoxemia and inability to tolerate single lung ventilation; (II) intraoperative hemodynamic instability, severe PH, or obviously increased PH after pulmonary artery clamping; (III) preoperative ECMO as a bridge to transplantation.
Single lung transplant was usually performed through an anterolateral thoracotomy. The patient was placed in the lateral position with the ipsilateral arm abducted. The lung chosen for replacement was resected routinely. Anastomosis was performed from posterior to anterior in the following sequence: bronchus, artery, and atrium. Bilateral lung transplantations were performed through a clamshell incision or bilateral sequential anterolateral thoracotomy. The inferior functional lung which was determined by preoperative ventilation/perfusion scans was resected and replaced first.
Postoperative care and follow-up
Routine empiric broad-spectrum antibiotics, antifungal agents, and antiviral agents were used. Subsequent antibiotic selection was based on the results of sputum culture. A standard immunosuppressive protocol consisting of tacrolimus, mycophenolate mofetil, and corticosteroids was employed. Additional drug therapy for PH was used after SLT. We routinely removed ECMO and extubated as soon as standard weaning criteria were met. Pulmonary function tests, echocardiography, and chest radiography were performed at follow-up visit or when clinically warranted. A flexible bronchoscope was performed when clinically indicated. The survival status at the last follow-up was recorded and the last follow-up time was January 2021.
Statistical analysis
Data were presented as mean value with standard deviation (SD) or median with interquartile range (IQR) for continuous variables, and percentages for categorical variables. Continuous variables were compared using t-test or Mann-Whitney U-test. Categorical variables were compared using the Pearson’s χ2 test or Fisher’s exact test. Survival curves were plotted by using the Kaplan-Meier method and compared by using the log-rank test. The survival time was estimated as days from surgery to death or end of available follow-up. The primary endpoint of the study was overall survival. All P values were bilaterally distributed, and P<0.05 was considered statistically significant. Statistical analysis was performed using Stata 16.0 (StataCorp, College Station, TX, USA) and GraphPad Prism 8.0.
Results
Preoperative characteristics
From March 2016 to December 2019, a total of 213 patients underwent lung transplantation and 63 patients with PH were finally included in the analysis. All included patients were secondary pulmonary artery hypertension. SLT was performed in 40 (63.5%) patients and DLT in 23 (36.5%) patients. The demographic and physiologic characteristics of these patients were presented in Table 1 and Figure 1. The mean age was 56.37 (SD, 11.76; range, 30–75) years, the mean BMI was 19.56 (SD, 19.56; range, 12.49–28.13) kg/m2, and the mPAP was 35.4 (SD, 8.54; IQR, 27–42) mmHg. The pulmonary function which included forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC, and carbon monoxide diffusion capacity (DLCO) were similar between SLT and DLT group. Overall, patients who underwent DLT had younger age, higher mPAP, higher proportion of infectious disease, and lower PaO2 and SO2. There were no significant differences in the donor characteristics between these two groups.
Table 1
| Variables | Total, N=63 | SLT, N=40 | DLT, N=23 | P value |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Age (years) | 56.37 (11.76) | 58.60 (9.77) | 52.48 (13.99) | 0.05 |
| Gender, n (%) | ||||
| Female | 13 (20.6) | 9 (22.5) | 4 (17.4) | 0.63 |
| FVC, %predicted | 49.96 (13.45) | 49.91 (16.08) | 50.03 (8.54) | 0.98 |
| FEV1, %predicted | 44.31 (20.38) | 42.18 (22.98) | 47.60 (15.90) | 0.46 |
| FEV1/FVC% | 84.64 (28.12) | 79.50 (31.19) | 92.56 (21.35) | 0.20 |
| DLCO, %predicted | 27.64 (12.11) | 27.10 (8.77) | 28.34 (15.97) | 0.81 |
| mPAP (mmHg) | 35.40 (8.54) | 33.80 (7.23) | 38.17 (10.02) | 0.05 |
| BMI (kg/m2) | 19.56 (3.32) | 19.71 (2.98) | 19.30 (3.89) | 0.64 |
| History of surgery, n (%) | 0.69 | |||
| Thoracic surgery | 5 (7.9) | 4 (10.0) | 1 (4.3) | |
| Other surgery | 17 (27.0) | 10 (25.0) | 7 (30.4) | |
| Smoking index (pack-year) | 20.68 (29.90) | 25.08 (33.16) | 13.04 (21.78) | 0.06 |
| LVEF% | 71.52 (5.94) | 70.78 (5.53) | 72.83 (6.52) | 0.19 |
| proBNP (pg/mL) | 176.60 (64.03, 597.00) | 155.25 (58.75, 513.60) | 303.00 (111.20, 796.20) | 0.13 |
| PaO2 (mmHg) | 103.80 (82.00, 131.40) | 121.00 (88.60, 135.10) | 87.60 (71.70, 103.40) | <0.01 |
| PaCO2 (mmHg) | 49.60 (43.90, 59.70) | 48.35 (44.15, 56.65) | 51.50 (42.80, 64.90) | 0.66 |
| SO2% | 97.20 (94.50, 98.30) | 97.80 (95.70, 98.35) | 96.00 (93.40, 97.20) | 0.02 |
| Assisted ventilation, n (%) | 0.20 | |||
| Low-flow | 33 (52.4) | 24 (60.0) | 9 (39.1) | |
| High-flow | 8 (12.7) | 4 (10.0) | 4 (17.4) | |
| NMV | 12 (19.0) | 8 (20.0) | 4 (17.4) | |
| IMV | 8 (12.7) | 4 (10.0) | 4 (17.4) | |
| ECMO | 2 (3.2) | 0 (0.0) | 2 (8.7) | |
| Scr (μmol/L) | 62.29 (19.19) | 64.07 (17.97) | 59.19 (21.21) | 0.34 |
| BUN (mmol/L) | 4.50 (3.20, 7.20) | 4.95 (3.30, 7.15) | 4.30 (2.90, 7.60) | 0.77 |
| Donor characteristics | ||||
| Age (years) | 33.37 (10.55) | 33.08 (9.11) | 33.87 (12.90) | 0.78 |
| BMI (kg/m2) | 22.35 (2.28) | 22.46 (2.13) | 22.18 (2.54) | 0.68 |
| PaO2 (100%, mmHg) | 427.21 (78.58) | 422.94 (71.05) | 433.95 (90.85) | 0.64 |
| IMV (days) | 4.15 (2.63) | 4.59 (2.99) | 3.36 (1.62) | 0.08 |
SLT, single-lung transplantation; DLT, double-lung transplantation; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, carbon monoxide diffusion capacity; mPAP, mean pulmonary artery pressure; BMI, body mass index; LVEF, left ventricular ejection fraction; NMV, noninvasive mechanical ventilation; IMV, Invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation; Scr, serum creatinine; BUN, blood urea nitrogen.
Intraoperative outcomes
There were no intraoperative deaths. The intraoperative outcomes of these patients were described in Table 2. The mean operative time was 387.06 min. A majority of lung transplantations (69.84%) were performed through an anterolateral thoracotomy and 28 (44.4%) patients required ECMO support. Compared to SLT, patients in DLT group had longer operative time, higher frequency of use of ECMO, more bleeding, and more blood transfusion. The time for left bronchus anastomosis was significantly shorter (P=0.02) in DLT group, but there was no significant difference (P=0.05) between anterolateral incision and clamshell incision. Time for anastomosis was shorter for right lung than for left lung. Three patients suffered from atrial fibrillation in total, two after clamping the pulmonary artery and one after clamping the left atrial wall.
Table 2
| Variables | All, N=63 | SLT, N=40 | DLT, N=23 | P value |
|---|---|---|---|---|
| Incision | <0.01 | |||
| Anterolateral | 44 (69.84%) | 39 (97.5%) | 5 (21.74%) | |
| Clamshell | 19 (30.2%) | 1 (2.5%) | 18 (78.3%) | |
| Ischemic time (min) | ||||
| First lung | 300.35 (103.44) | 294.43 (95.75) | 312.80 (122.64) | 0.65 |
| Second lung | 498.56 (98.76) | 0 | 498.56 (98.76) | |
| Operative time (min) | 387.06 (138.37) | 303.03 (79.47) | 527.11 (94.51) | <0.01 |
| Intraoperative ECMO | 28 (44.4%) | 10 (25.0%) | 18 (78.3%) | <0.01 |
| Intraoperative bleeding (mL) | 1,200.00 (400.00, 2,500.00) | 675.00 (400.00, 1,250.00) | 2,700.00 (1,500.00, 4,500.00) | <0.01 |
| Atrial fibrillation | 3 (4.8%) | 3 (7.5%) | 0 (0.0%) | |
| Lung volume reduction | 2 (3.2%) | 2 (5.0%) | 0 (0.0%) | |
| Blood products | ||||
| Red blood cells (units) | 11.28 (8.62) | 8.07 (4.82) | 16.00 (10.74) | <0.01 |
| Fresh frozen plasma (mL) | 1,283.67 (863.40) | 1,006.90 (574.10) | 1,685.00 (1,054.08) | <0.01 |
| Platelet concentrate (units) | 5.14 (5.25) | 9.50 (5.69) | 3.40 (4.14) | 0.04 |
| Cryoprecipitate (units) | 10.88 (3.56) | 9.90 (1.60) | 11.50 (4.38) | 0.45 |
| Time for anastomosis (min) | ||||
| Left pneumonectomy | 86.08 (41.33) | 89.71 (43.73) | 81.83 (39.17) | 0.56 |
| Right pneumonectomy | 74.55 (49.00) | 76.42 (52.08) | 73.24 (48.29) | 0.87 |
| Left bronchus | 21.95 (12.45) | 26.10 (14.66) | 17.11 (6.90) | 0.02 |
| Right bronchus | 20.41 (7.54) | 21.50 (10.19) | 19.53 (4.70) | 0.51 |
| Left pulmonary artery | 17.13 (7.58) | 18.29 (8.79) | 15.78 (5.83) | 0.31 |
| Right pulmonary artery | 16.34 (5.72) | 17.92 (7.20) | 15.24 (4.29) | 0.22 |
| Left atrial | 17.77 (8.08) | 18.00 (7.03) | 17.50 (9.37) | 0.85 |
| Right atrial | 16.55 (7.09) | 18.50 (6.88) | 15.18 (7.12) | 0.22 |
SLT, single-lung transplantation; DLT, double-lung transplantation; ECMO, extracorporeal membrane oxygenation.
Postoperative outcomes
The summary of early postoperative outcomes appeared in Table 3. Five patients died within 30 days after lung transplantation, one from severe pneumonia, two from disseminated intravascular coagulation, one from multisystem organ failure, and one from bronchial anastomotic fistula. The mean time for ECMO support, invasive mechanical ventilation, ICU stay, and hospital stay were 2.17±1.1, 9.19±10.1, 19.08±21.96, and 41.38±29.57 days respectively. There were no significant differences on the early postoperative outcomes between SLT and DLT group. Three patients required re-thoracotomy, two for active bleeding and one for oversize donor. Bronchial anastomotic fistula occurred in three patients on postoperative days 16, 28, 30, respectively.
Table 3
| Variables | Total, N=63 | SLT, N=40 | DLT, N=23 | P value |
|---|---|---|---|---|
| IMV (days) | 9.19 (10.10) | 7.70 (9.40) | 11.78 (10.93) | 0.12 |
| IABP | 4 (6.3%) | 3 (7.5%) | 1 (4.3%) | 0.62 |
| Time for ECMO support (days) | 2.17 (1.10) (n=29) | 1.82 (0.87) (n=11) | 2.39 (1.20) (n=18) | 0.18 |
| Re-thoracotomy | 3 (4.8%) | 0 (0.0%) | 3 (13.0%) | |
| Bronchial anastomotic fistula | 3 (4.8%) | 1 (2.5%) | 2 (8.7%) | 0.55 |
| Re-intubation | 11 (17.5%) | 5 (12.5%) | 6 (26.1%) | 0.17 |
| Length of ICU stay (days) | 19.08 (21.96) | 17.48 (24.50) | 21.87 (16.80) | 0.45 |
| CRRT | 12 (19.0%) | 5 (12.5%) | 7 (30.4%) | 0.08 |
| Renal failure | 17 (27.0%) | 8 (20.0%) | 9 (39.1%) | 0.10 |
| Length of hospital stay (days) | 41.38 (29.57) | 40.63 (29.00) | 42.70 (31.17) | 0.79 |
| Mortality within 30 days | 5 (7.94%) | 3 (7.5%) | 2 (8.70%) | 0.87 |
| Cause of death | 0.33 | |||
| Infection | 9 (14.3%) | 6 (15.0%) | 3 (13.0%) | |
| Heart failure | 3 (4.8%) | 2 (5.0%) | 1 (4.3%) | |
| DIC | 3 (4.8%) | 3 (7.5%) | 0 (0.0%) | |
| Multisystem organ failure | 3 (4.8%) | 2 (5.0%) | 1 (4.3%) | |
| Hemorrhage | 2 (3.2%) | 0 (0.0%) | 2 (8.7%) | |
| Bronchial anastomotic fistula | 3 (4.8%) | 1 (2.5%) | 2 (8.7%) | |
| Tension pneumothorax | 1 (1.6%) | 0 (0.0%) | 1 (4.3%) | |
| Alive | 39 (61.9%) | 26 (65.0%) | 13 (56.5%) |
SLT, single-lung transplantation; DLT, double-lung transplantation; IMV, Invasive mechanical ventilation; IABP, intra-aortic ballon pump; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; DIC, disseminated intravascular coagulation.
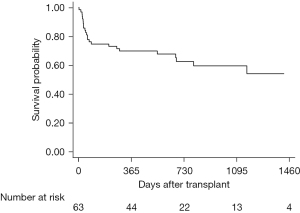
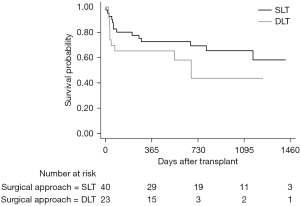
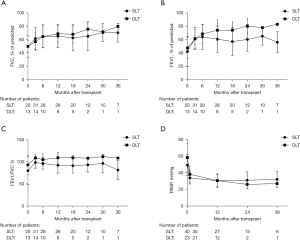
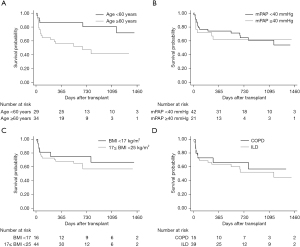
Median follow-up after lung transplantation was 845 days, with a maximum follow-up of 1,753 days. A total of 24 (38.1%) patients died during the follow-up period. Most of these patients (14.3%) died from infection. Among patients after lung transplantation, the overall 1-, 2-, and 3-year survival was 70%, 63%, and 60%, respectively (Figure 2). Survival rate at 1, 2, and 3 years were 73%, 69%, 65% in SLT group, and 65%, 43%, 43% in DLT group (P=0.23) (Figure 3). Compared to SLT group, percentage predicted FEV1 (P=0.02) and FEV1/FVC (P<0.01) was significant higher in the DLT group (Figure 4). There was no significant difference (P=0.93) on PASP between these two groups (Figure 4D). Patients younger than 60 years of age had better survival (P=0.01) and the survival for mPAP <40 vs. ≥40 mmHg (P=0.66), BMI <17 vs. ≥17 BMI <25 kg/m2 (P=0.52), and obstructive disease vs. restrictive disease (P=0.64) were similar (Figure 5).
Discussion
Lung transplantation is the final option for patients with severe lung disease that have failed standard medical and other surgical treatment (13). To the best of our knowledge, the present study is the first to analyze the outcomes of lung transplantation for end-stage pulmonary diseases with PH in China. Unlike in Western countries, a large part of patients in China is older, underweight, and lacking of financial support and in relatively later timing of transplantation (2,14). Our results highlight that, in our experience, there were no significant differences on the short- and medium-term survival outcomes of SLT and DLT but FEV1 and FEV1/FVC were better in DLT. Patients older than 60 years of age had worse survival.
The most recent ISHLT Registry data show an obvious long-term survival advantage to DLT over SLT in all lung transplantation recipients. However, the ISHLT Registry report in 2011 still recommended that survival advantage according to procedure type are impacted by clinical factors, including age, recipient comorbidities, underlying diagnosis, and preference of the transplant center (15). DLT results in fewer ventilation/perfusion mismatches and eliminates all diseased lung parenchyma and potentially related complications such as infection and residual PH (16). DLT provides more implanted allograft tissue and thereby patients have better pulmonary function. In addition, DLT recipients are more resistant to rejection and bronchiolitis obliterans syndrome and more likely to have better long-term survival (17). SLT has easier technical procedure, better intraoperative outcomes, and less early morbidity and mortality compared with DLT. SLT omits the transverse sternotomy to keep the chest wall stable and accelerate recovery. However, SLT recipients with PH are more at risk for developing severe postoperative pulmonary edema (18).
Most studies have shown a long-term survival advantage to DLT over SLT (15,17,19,20). However, the superiority of DLT can be offset by perioperative complications in older, weak, and extremely ill recipients (7). A study by Nasir et al. (6) showed that patients with elevated pulmonary artery pressure undergoing DLT have similar survival of patients with lower mPAP. Thabut et al. (21) concluded that SLT confers short-term survival benefit but long-term harm, DLT confers short-harm but long-term survival benefit. The balance between short-term risk and long-term benefit is a crucial principle that decides SLT or DLT. In our analysis, the short-term and mid-term survival between SLT and DLT was similar for selected patients with PH. SLT was not inferior to DLT in improving PASP and postoperative pulmonary function was better in DLT.
ISHLT guidelines from 2006 recommend age greater than 65 years to be a relative contraindication for lung transplantation (11). A Web-based survey of all the United Network for Organ Sharing (UNOS) lung transplant centers by Levine et al. show that some centers refused to perform lung transplantation in patients older than 60 years, and more than 20% of centers considered age >60 years to be an absolute contraindication for DLT (22). A recent UNOS data revealed higher mortality at 1 and 2 years after lung transplantation for patients older than 60 years and the ISHLT data showed that older patients had less optimal survival (15,23). Our data also displayed patients older than 60 years had significantly worse survival.
The latest data for the ISHLT Registry shows a 5-year survival for PH patients of ~50% following lung transplantation. And PH increases 0.52-fold risk of both early and late mortality (15). But a recent UNOS data analysis concluded that there is no strong evidence suggesting that PH significantly alters the risk of death in IPF patients after transplantation (9). This is probably because of the ECMO used and the selection of procedure. The cardiac output can overflow single implanted lung or the first implanted lung during off-bypass DLT in PH recipients. Our center tended to axillary artery-percutaneous femoral vein cannulation for V/A ECMO. Axillary cannulation could be performed with no need for clamshell incision, competently decompress the left ventricle, reduce pulmonary edema, extend ECMO used conveniently, avoid upper limb and brain hypoperfusion (24). Salman et al. demonstrated that extended V/A ECMO used in patients with severe PH undergoing lung transplantation decreased postoperative mortality (25). A mPAP greater than 40 mmHg was associated with worse survival (7), and DLT may be superior to SLT in patients with mPAP over 40 mmHg (6). In our analysis, the majority of DLT used ECMO support and mPAP over 40 mmHg did not have worse survival. According to the ISHLT guidelines for selection of Lung Transplant Candidates, BMI exceeding 30 has been designated as a relative contraindication for lung transplantation, but underweight isn’t mentioned (11). Being severely underweight is related to worse capacity to tolerate surgery, underlying malnutrition, and skeletal muscle loss. A study from Japan displayed that Patients with BMI <17.0 kg/m2 had a worse prognosis than those with 17.0≤ BMI <18.5 kg/m2 after transplantation (26). But our data show that patients with BMI <17.0 kg/m2 did not have a worse prognosis. In our center, we tended to choose SLT and performed jejunostomy actively for severe underweight patients.
There are several limitations to our study. This was a retrospective with small sample size from a single center, including inherent selection bias, and the potential for missing data. Lung allocation score and the incidence of PGD were missing.
Conclusions
In conclusion, our data show that lung transplantation for end-stage pulmonary diseases with PH is suitable and the mortality within 30 days is less than 8%. The short- and medium-term survival outcomes between SLT and DLT are similar in selected patients with PH. Postoperative pulmonary function is better in DLT but the improvement of postoperative PH is similar. Patients have worse survival in age >60 years. Patients with mPAP ≥40 mmHg, BMI <17 kg/m2, and restrictive disease did not have inferior survival.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1738/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1738/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1738/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1738/coif). JH serves as an Executive Editor-in-Chief of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the First Affiliated Hospital of Guangzhou Medical University Research Ethics Committee (2020-K-51) and informed consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young KA, Dilling DF. The Future of Lung Transplantation. Chest 2019;155:465-73. [Crossref] [PubMed]
- Wu B, Hu C, Chen W, et al. China lung transplantation developing: past, present and future. Ann Transl Med 2020;8:41. [Crossref] [PubMed]
- Xin YL. A case of human lung transplantation (author's transl). Zhonghua Wai Ke Za Zhi 1979;17:328-32. [PubMed]
- Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018;360:j5492. [Crossref] [PubMed]
- Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant 2009;23:819-30. [Crossref] [PubMed]
- Nasir BS, Mulvihill MS, Barac YD, et al. Single lung transplantation in patients with severe secondary pulmonary hypertension. J Heart Lung Transplant 2019;38:939-48. [Crossref] [PubMed]
- Villavicencio MA, Axtell AL, Osho A, et al. Single- Versus Double-Lung Transplantation in Pulmonary Fibrosis: Impact of Age and Pulmonary Hypertension. Ann Thorac Surg 2018;106:856-63. [Crossref] [PubMed]
- de Perrot M, Granton JT, McRae K, et al. Outcome of patients with pulmonary arterial hypertension referred for lung transplantation: a 14-year single-center experience. J Thorac Cardiovasc Surg 2012;143:910-8. [Crossref] [PubMed]
- Hayes D Jr, Higgins RS, Black SM, et al. Effect of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis after lung transplantation: an analysis of the United Network of Organ Sharing registry. J Heart Lung Transplant 2015;34:430-7. [Crossref] [PubMed]
- Fadel E, Mercier O, Mussot S, et al. Long-term outcome of double-lung and heart-lung transplantation for pulmonary hypertension: a comparative retrospective study of 219 patients. Eur J Cardiothorac Surg 2010;38:277-84. [Crossref] [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [Crossref] [PubMed]
- Sundaresan S, Trachiotis GD, Aoe M, et al. Donor lung procurement: assessment and operative technique. Ann Thorac Surg 1993;56:1409-13. [Crossref] [PubMed]
- Goldberg AB, Mazur W, Kalra DK. Pulmonary hypertension: diagnosis, imaging techniques, and novel therapies. Cardiovasc Diagn Ther 2017;7:405-17. [Crossref] [PubMed]
- Hu CX, Chen WH, He JX, et al. Lung transplantation in China between 2015 and 2018. Chin Med J (Engl) 2019;132:2783-9. [Crossref] [PubMed]
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant 2011;30:1078-94. [Crossref] [PubMed]
- Gottlieb J. Lung transplantation for interstitial lung diseases and pulmonary hypertension. Semin Respir Crit Care Med 2013;34:281-7. [Crossref] [PubMed]
- Hadjiliadis D, Chaparro C, Gutierrez C, et al. Impact of lung transplant operation on bronchiolitis obliterans syndrome in patients with chronic obstructive pulmonary disease. Am J Transplant 2006;6:183-9. [Crossref] [PubMed]
- Boujoukos AJ, Martich GD, Vega JD, et al. Reperfusion injury in single-lung transplant recipients with pulmonary hypertension and emphysema. J Heart Lung Transplant 1997;16:439-48. [PubMed]
- Force SD, Kilgo P, Neujahr DC, et al. Bilateral lung transplantation offers better long-term survival, compared with single-lung transplantation, for younger patients with idiopathic pulmonary fibrosis. Ann Thorac Surg 2011;91:244-9. [Crossref] [PubMed]
- Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: a retrospective analysis of registry data. Lancet 2008;371:744-51. [Crossref] [PubMed]
- Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med 2009;151:767-74. [Crossref] [PubMed]
- Levine SMTransplant/Immunology Network of the American College of Chest Physicians. A survey of clinical practice of lung transplantation in North America. Chest 2004;125:1224-38. [Crossref] [PubMed]
- Weiss ES, Merlo CA, Shah AS. Impact of advanced age in lung transplantation: an analysis of United Network for Organ Sharing data. J Am Coll Surg 2009;208:400-9. [Crossref] [PubMed]
- Yang C, Peng G, Xu X, et al. The technique of intraoperative axillary artery cannulation for extracorporeal membrane oxygenation in lung transplantation. J Thorac Dis 2019;11:2939-44. [Crossref] [PubMed]
- Salman J, Ius F, Sommer W, et al. Mid-term results of bilateral lung transplant with postoperatively extended intraoperative extracorporeal membrane oxygenation for severe pulmonary hypertension. Eur J Cardiothorac Surg 2017;52:163-70. [Crossref] [PubMed]
- Komatsu T, Chen-Yoshikawa TF, Oshima A, et al. Severe underweight decreases the survival rate in adult lung transplantation. Surg Today 2017;47:1243-8. [Crossref] [PubMed]