Immune checkpoint inhibitor-related interstitial lung disease in patients with advanced non-small cell lung cancer: systematic review of characteristics, incidence, risk factors, and management
Introduction
Immune checkpoint inhibitors (ICIs) have shown clinical benefits in various cancers, both in locally advanced and metastatic states. Furthermore, ICIs are used in combination with other treatment modalities, such as platinum-based chemotherapy. Notable trials, including ICI as neoadjuvant and adjuvant therapy, are also ongoing, extending their usage (1).
However, immune-related adverse events (irAEs) have also been reported, with a prevalence of about 20–30% (2). IrAEs show various clinical manifestations, such as pneumonitis, skin reactions, endocrinologic diseases, colitis, hepatitis, and infusion reactions (3). Among the irAEs, pneumonitis, including interstitial lung disease (ILD), is clinically significant and can be life-threatening, which frequently requires immediate clinical attention. The incidence of ICI-related ILD is approximately 4% in patients treated with PD-1 inhibitors (such as nivolumab and pembrolizumab) and 2% in those treated with PD-L1 inhibitors (such as atezolizumab and durvalumab) (4). In a meta-analysis, the incidence of ICI-related ILD was 3.6% and 1.3% in the PD-1 and PD-L1 inhibitor-treated groups, respectively.
In this study, recent evidence on possible risk factors, clinical manifestations, and safety regarding ICI-related ILD, and in-depth studies on ICI-related ILD in patients with previously diagnosed ILD were reviewed. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-93/rc).
Methods
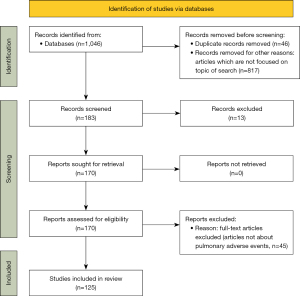
Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (5), an online search of the literature on pulmonary adverse events and immunotherapy was conducted. The National Center for Biotechnology Information (NCBI) PubMed, Cochrane Library, Google Scholar, and Embase databases were searched. All studies published in English between January 2016 and June 2021 were included. Various combinations of search words were tried using the following terms: (‘NSCLC’ OR ‘non-small cell lung’) AND (‘interstitial lung disease’ OR ‘pneumonitis’ OR ‘organizing pneumonia’) AND (‘nivolumab’ OR ‘pembrolizumab’ OR ‘atezolizumab’ OR ‘immune checkpoint inhibitor’ OR ‘immunotherapy’ OR ‘ipilimumab’) AND (‘idiopathic pulmonary fibrosis’ OR ‘connective tissue disease’). The search strategy is illustrated in Figure 1.
Pathophysiology
ICIs activate and enhance antitumor activities by inhibiting intrinsic down-regulators of immunity, such as CTLA-4, PD-1, and PD-L1. However, it can also stimulate the immune system resulting in irAEs. IrAEs frequently involve the gastrointestinal tract, endocrine system, liver, and skin. They less commonly involve the central nervous system, pulmonary, cardiovascular, musculoskeletal, and hematologic systems. IrAEs in the lungs can manifest as pneumonitis (6,7).
The pathophysiology of irAEs can be explained by four mechanisms. Firstly, irAEs may have increased T-cell activity against cross-antigens shared between tumor and normal tissues. The responses of cytotoxic antigen-directed T cells may lead to ICI-induced ILD. The second mechanism involves increased baseline levels of pre-existing autoantibodies that provoke irAEs, such as anti-thyroid peroxidase, antinuclear, anti-thyroglobulin, and anti-rheumatoid factor antibodies. However, the specific antibodies that are related to ICI-related ILD remain unknown. The third mechanism is the increased levels of inflammatory cytokines. A case report showed that C-reactive protein and interleukin-6 (IL-6) levels were higher than baseline levels in a patient with NSCLC who developed ICI-related ILD after atezolizumab treatment (8). Johnson et al. and Lim et al. reported that elevated inflammatory cytokines are also related to severe ICI toxicity (9,10). The fourth mechanism is increased inflammation mediated by the complement system due to direct binding of anti-CTLA-4 antibodies with CTLA-4 on normal tissue, such as the pituitary gland. This mechanism accounts for pituitary inflammation as a specific irAE of anti-CTLA-4 therapy (7,11,12).
In short, overstimulation of the host immune system and increased levels of preexisting autoantibodies may be the underlying mechanisms behind ICI-related ILD. Further studies are needed to clarify the pathophysiological background.
Prevalence of ICI-related ILD
ICI-related ILD has a prevalence of 3–5% in NSCLC trials. In phase III Checkmate 017 and Checkmate 057, the prevalence of nivolumab-induced ILD were 4.6% (6/131) and 3.5% (10/287), respectively (13-15). A meta-analysis reported that 4.2% of patients treated with nivolumab for NSCLC developed ILD (16). However, the incidence in the real-world setting remains higher. One retrospective study using real-world data showed a much higher incidence rate of 19% (17). Another multicenter prospective study of 138 patients with NSCLC showed that the overall incidence rate of ICI-related ILD was 14.5%. This is similar to the results of other retrospective studies, ranging from 14.6% to 19.0% (18,19).
In a single-center retrospective study that included 98 patients with NSCLC, 19 patients developed immunotherapy-induced ILD. The median time to the development of ICI-related ILD was 97 days. Among the 19 patients, 10 patients had grade 1–2 and 9 patients had grade 3–4 pneumonitis (20).
The incidence of ICI-related ILD differs according to the type of ICI. According to a meta-analysis by Pillai et al., the incidence of ICI-related ILD was significantly higher in patients treated with PD-1 inhibitors, such as nivolumab and pembrolizumab than in those treated with PD-L1 inhibitors, such as atezolizumab and durvalumab (4% vs. 2%, P=0.001) (4). In another meta-analysis by Khunger et al., the incidence of ICI-related ILD was also higher in PD-1 than in PD-L1 inhibitors treated group, both for any grade (3.6% vs. 1.3%, P=0.001) and grade 3 or higher (1.1% vs. 0.4%, P=0.002). In the PD-1 inhibitor group, there was no significant difference in the incidence of ICI-related ILD between nivolumab and pembrolizumab (21).
Risk factor for ICI-related ILD
Several studies investigated the potential risk factors for ICI-related ILD. These include baseline patient characteristics, pre-existing diseases, disease features, and treatment modalities.
In a study by Cho et al., patients aged >70 years old were more common in the ICI-related ILD group (54.5% vs. 30.3%, P=0.025) (22). In another retrospective study, however, the rates of immunotherapy-related toxicities did not differ by age group (23). The prevalence of ICI-related ILD was higher in females than in males, with no statistically significant difference (17).
Pre-existing lung diseases can be risk factors for ICI-related ILD. Asthma and COPD were mentioned as possible contributing factors of ICI-related ILD. However, the association requires validation. In an FDA approval summary of pembrolizumab for metastatic NSCLC, patients with asthma or COPD more commonly developed ICI-related ILD than in those without, though there was no confirmation of statistically significant difference (5.4% vs. 3.1%) (24). Other studies have not reported association between prior asthma and development of ICI-related ILD. In one study, prior asthma history was associated with higher grade of ICI-related ILD if it occurs, however the size of cohort was small (25). In a retrospective study of 216 NSCLC patients who were treated with nivolumab, total and severe nivolumab-related ILD occurred more frequently in patients with pre-existing ILD than in those without (31% vs. 12%, P=0.014 and 19% vs. 5%, P=0.022, respectively) (26). Pre-existing abnormal lung image findings, such as fibrosis or inflammation, are also considered as risk factors. In a study of nivolumab-induced ILD in NSCLC patients, some patients had radiologic findings of inflammation before ICI treatment, which may have resulted from past radiation pneumonitis or bacterial pneumonia (13). In a retrospective study, pre-existing pulmonary fibrosis was associated with an increased risk of ICI-related ILD (19). Another study by Atchley et al. reported that a history of ILD (OR =15.7, 95% CI: 2.52–98.20), obstructive lung disease (OR =3.13, 95% CI: 1.24–7.88), including COPD (OR 2.42, 95% CI: 1.12–5.22), and pre-existing fibrosis on baseline chest CT (OR =7.06, 95% CI: 2.76–18.0) were associated with an increased risk of ICI-related ILD (27). In a multicenter prospective study, pulmonary function parameters such as FVC and FEV1, and dyspnea symptoms graded by modified Medical Research Council (mMRC) were related to the incidence of ICI-related ILD. Here, FVC showed significant association after multivariate analysis (HR =0.734, 95% CI: 0.891–0.979, P=0.0044) (18).
Prior thoracic radiotherapy has been suggested as a potential risk factor. In an FDA approval summary of pembrolizumab for metastatic NSCLC patients, the incidence of ICI-related ILD was higher in patients with prior thoracic radiation history than in patients without (6.0% vs. 2.6%) (24).
Tumor histologic type may also be a risk factor for ICI-related ILD. In a retrospective study of 205 NSCLC patients, squamous carcinoma showed a higher incidence rate of ICI-related ILD (IRR =2.29, 95% CI: 1.08–4.83) than other histologic types (IRR =4.32, 95% CI: 1.24–12.19) (17). However, Atchley et al. reported that the tumor histologic type did not show a significant association (27).
Combination ICI therapy increases the risk of ICI-related ILD. A meta-analysis of 4,496 patients with NSCLC, melanoma, and renal cell carcinoma (RCC) by Nishino et al. reported that the incidence of ICI-related ILD in melanoma was higher in the combination treatment than in the single ICI regimen (6.6% vs. 1.6%, P<0.001). Furthermore, the combination regimen showed a significantly higher association than monotherapy for ICI-related ILD (OR =2.04, 95% CI: 1.69–2.50, P<0.001) (28). Cui et al. showed that combination therapy was associated with an increased risk of ICI-related ILD (OR =3.42, 95% CI: 1.65–7.09, P=0.001) (29). Suresh et al. also suggested combination ICI therapy as a risk factor for ICI-related ILD, although it was not statistically significant (17).
There are studies that evaluated risk of ILD development in patients under combination of platinum-based chemotherapy. In the KEYNOTE-189 trial, which was phase 3 trial of 616 patients with metastatic non-squamous NSCLC who received pemetrexed, platinum-based drug and pembrolizumab or placebo, the incidence of any grade and grade 3 or higher pneumonitis was 4.4% (18/405) and 2.7% (1/405), respectively. It was higher than the placebo combination group, which was 2.5% (5/202) and 2.0% (4/202), though there was no confirmation of statistically significant difference (30). In real-world data shown in a multicenter, retrospective cohort study of advanced NSCLC patients who received platinum, pemetrexed, and pembrolizumab combination therapy, the incidence of all-grade and grade 3 or higher pneumonitis was 12.4% and 3.3%, respectively, suggesting relatively high incidence (31).
Regarding the risk factors for severe (grade 3 or higher) ICI-related ILD, Tone et al. reported that Eastern Cooperative Oncology Group (ECOG) performance status (PS) score 2 or higher and pre-existing ILD were significantly associated with development of severe ICI-related ILD. ECOG PS score was higher in the patients with severe ICI-related ILD than in those without (ECOG PS 2–3; 54.5% vs. 18.3%, P=0.01), and more patients were complicated with pre-existing ILD in severe ICI-related ILD group (27.3% vs. 6.7%, P=0.035) (32). In a meta-analysis of studies about melanoma, NSCLC, and RCC, the incidence of grade 3 or higher ICI-related ILD was more frequent in NSCLC than melanoma (1.8% vs. 0.2%, P<0.001). Also, the combination therapy group showed higher incidence of severe ICI-related ILD than the monotherapy group (1.5% vs. 0.2%, P<0.001) (28).
Considering these results, patients who have poor lung function, prior lung diseases, or radiologic evidence of lung inflammation or fibrosis before administration of ICI, and who will be treated with combination ICI therapy should be closely monitored for ICI-related ILD (Table 1).
Table 1
| Authors | Year | Cancer | Regimen | Design | Number | Incidence rate of ICI-related ILD |
Risk factors |
|---|---|---|---|---|---|---|---|
| Sul et al. (24) | 2016 | NSCLC | Pembrolizumab | Prospective | 550 | 3.5% (19 patients) | History of asthma/COPD; history of prior thoracic radiation |
| Khunger et al. (21) | 2017 | NSCLC | Nivolumab; pembrolizumab; atezolizumab; durvalumab; avelumab | Meta-analysis | 5,038 | 2.8% (140 patients) | PD-1 inhibitors (when compared with PD-L1 inhibitors) |
| Kato et al. (13) | 2017 | NSCLC | Nivolumab | Case series | 111 | 7.2% (8 patients) | Presence of inflammation in the lungs at baseline |
| Saverdian et al. (33) | 2017 | NSCLC | Pembrolizumab | Retrospective | 97 | 4.1% (4 patients) | Previous treatment with radiotherapy |
| Tamiya et al. (34) | 2017 | NSCLC | Nivolumab | Retrospective | 201 | 11.9% (24 patients) | History of radiation pneumonitis |
| Suresh et al. (17) | 2018 | NSCLC | Nivolumab; pembrolizumab; durvalumab | Retrospective | 205 | 19% (39 patients) | Female; tumor histologic type; combination ICI therapy |
| Cho et al. (22) | 2018 | NSCLC | Nivolumab; pembrolizumab; durvalumab; nivolumab + ipilimumab | Retrospective | 167 | 13.2% (22 patients) | Pre-existing interstitial lung disease |
| Yamaguchi et al. (19) | 2018 | NSCLC | Nivolumab; pembrolizumab | Retrospective | 123 | 14.6% (18 patients) | Pre-existing pulmonary fibrosis |
| Kanai et al. (26) | 2018 | NSCLC | Nivolumab | Retrospective | 216 | 13.9% (30 patients) | Pre-existing ILD |
| Voong et al. (35) | 2019 | NSCLC | Nivolumab; pembrolizumab; durvalumab | Prospective | 188 | 19% (36 patients) | Curative-intent chest radiotherapy (when compared to palliative-intent) |
| Shibaki et al. (36) | 2020 | NSCLC | Nivolumab; pembrolizumab | Retrospective | 331 | 11% (36 patients) | Pre-existing ILD |
| Suzuki et al. (18) | 2020 | NSCLC | Nivolumab; pembrolizumab | Prospective Cohort study |
138 | 14.5% (20 patients) | Impaired spirometry; dyspnea defined by mMRC |
| Tasaka et al. (37) | 2021 | NSCLC | Nivolumab; pembrolizumab | Retrospective | 461 | 11.7% (54 patients) | Pre-existing ILD |
| Isono et al. (38) | 2021 | NSCLC | Nivolumab; pembrolizumab; atezolizumab | Retrospective | 119 | 22.7% (27 patients) | Pre-existing ILD |
| Atchley et al. (27) | 2021 | NSCLC SCLC |
Nivolumab; pembrolizumab; nivolumab + ipilimumab | Retrospective; multicenter | 315 | 9.5% (30 patients) | Presence of baseline fibrosis on chest CT scan; composite measure of obstructive lung disease; treatment with pembrolizumab |
ICI, immune checkpoint inhibitor; ILD, interstitial lung disease; NSCLC, non-small cell lung cancer; COPD, chronic obstructive pulmonary disease; PD-1, programmed cell death 1; PD-L1, programmed cell death-ligand 1; mMRC, Modified Medical Research Council; SCLC, small cell lung cancer.
Radiotherapy
Radiotherapy is one of the treatment options for NSCLC and is frequently used before or after immunotherapy. Although it can improve the survival outcomes of patients, a previous history of thoracic radiotherapy can increase the risk of ICI-related ILD. In a secondary analysis of KEYNOTE-001, they assessed the association of previous radiotherapy with the clinical activity and toxicity of pembrolizumab in NSCLC. Patients with previous thoracic radiotherapy showed a higher incidence of lung toxicity than those without in all grades [13% (3/24 patients) vs. 1% (1/73 patients), P=0.016] (33). In a case-control study by Cui et al., prior thoracic radiotherapy was significantly associated with the development of ICI-related ILD (OR =3.33, 95% CI: 1.39–7.97, P=0.007) (29). Regarding the purpose of radiotherapy, a retrospective study of 188 NSCLC patients showed that ICI-related ILD more frequently occurred in patients receiving curative radiotherapy as compared to palliative radiotherapy [89% (17/19 patients) vs. 11% (2/19 patients), P=0.051) (35). The treatment timing can also be important. However, a retrospective study of 79 patients with primary lung cancer or lung metastatic lesions showed no significant difference between sequential and concurrent treatment in association with ICI-related ILD occurrence (39).
Another reason for the possible increased risk of ICI-related ILD is that radiotherapy can cause radiation-induced pneumonitis. In a study on the correlation between prior radiation pneumonitis and nivolumab-related ILD, the incidence of nivolumab-induced ILD was higher in patients with a history of radiation pneumonitis than in those without (26.5% vs. 9.6%, P=0.018). However, the median progression-free survival (PFS) was longer in the patients with radiation pneumonitis history (3.6 vs. 2.3 months). Furthermore, a history of radiation pneumonitis significantly associated with a longer PFS (HR =0.59, 95% CI: 0.35–0.93, P=0.023) (34).
After the resolution of radiation-induced pneumonitis, radiation recall pneumonitis, an acute inflammatory reaction in a previously irradiated area of the lung triggered by other anticancer drugs can occur (40). In a case report by Itamura et al., the patient developed radiation recall pneumonitis one month after initiating pembrolizumab, who had previously recovered from radiation pneumonitis (41).
Administering ICI with concurrent chemoradiation (CCRT) may induce higher pulmonary toxicity than monotherapy. In phase 2 KEYNOTE-799—a non-randomized trial of pembrolizumab administration with CCRT in stage III NSCLC—the incidence of grade 3 or higher ICI-related ILD was less than 10% (42). Other studies on ICI combined with CCRT have shown similar results. Grade 3–5 pneumonitis occurred in 10% of patients receiving pembrolizumab with CCRT (43), 6% in pembrolizumab consolidation after CCRT (44), 12% in nivolumab with CCRT (45), 3% in atezolizumab with CCRT (46), and 4% in durvalumab after CCRT, which is known as the PACIFIC trial (47).
In patients with previous radiation therapy or radiation-induced pneumonitis, clinicians should be more cautious regarding the risk of developing ICI-related ILD.
Radiographic manifestations of ICI related ILD
ICI-related ILD manifests in various radiological forms. They can be classified into multiple patterns according to the ATS/ERS international multidisciplinary classification of interstitial pneumonia. These include cryptogenic organizing pneumonia (COP), hypersensitivity pneumonitis (HP), acute interstitial pneumonia (AIP)/diffuse alveolar damage (DAD), and nonspecific interstitial pneumonia (NSIP)-like patterns. Among them, the COP-like pattern is the most common pattern in previous studies. In a study of NSCLC patients with nivolumab-related ILD, the main radiologic finding was COP-like (53.4%), followed by HP-like (20.2%), DAD-like (10.9%), and NSIP-like (6.3%) pattern (48). Similarly, in a study by Baba et al., the most common pattern of ICI-related ILD was COP-like (47.2%), followed by HP-like (24.3%), AIP/DAD-like (13.2%), NSIP-like pattern (8.3%), and others (6.9%) (49). In patients with pre-existing ILD, Shibaki et al. reported that the most common radiologic finding was DAD-like (40%), followed by organizing pneumonia (OP)-like, HP-like, and other patterns (20% each, respectively) (36).
AIP-like and DAD-like manifestations are considered as acute stage patterns, followed by organizing stage and fibrotic stage patterns such as NSIP (11). In a study of two nivolumab phase II trials, most of the patients (7/8) whose ILD improved previously showed radiologic patterns of OP or NSIP without traction bronchiectasis (13).
Regarding the severity of ILD, most of the patients with OP or NSIP pattern had grade 1 or 2, and all patients with a DAD pattern had grade 3 or higher. In a retrospective study, patients with long-term disease control tended to show OP patterns at the onset of ICI-related ILD (2). Saito et al. reported that a DAD-like pattern is associated with a worse prognosis and a higher mortality rate (48).
Radiologic patterns are also classified into typical or atypical patterns of drug-induced pneumonitis. Typical patterns include ground glass opacity (GGO) or consolidation with unilateral/bilateral nonsegmental distribution. These are relatively frequent findings during conventional chemotherapy or targeted therapy. Other atypical findings include peritumoral GGO, exacerbation of radiation fibrosis, and abnormal ipsilateral lung opacities (49). In a study of NSCLC patients with nivolumab-related ILD, most patients (87.8%) did not show peritumoral infiltration while 11.3% of patients showed peritumoral infiltration (48).
Safety in pre-existing ILD patients
Pre-existing ILD as a risk factor for development of ICI-related ILD
In several studies, pre-existing ILD was associated with an increased risk of ICI-related ILD. In a study on the prevalence of nivolumab-related ILD in NSCLC, head and neck cancer, and gastric cancer, pre-existing ILD was an independent predictor of ICI-related ILD (OR =5.92, 95% CI: 2.07–18.54, P<0.05) (50). In another retrospective study on the association between pre-existing ILD and anti-PD-L1 antibody-related ILD in NSCLC, the incidence of ICI-related ILD was higher in patients with pre-existing ILD than in those without ILD (29% vs. 10%, P=0.027) (36). On the other hand, a study by Yamaguchi et al. showed that the frequency of ICI-related ILD was not different between the pre-existing ILD group and those without it in patients receiving pembrolizumab (51).
In addition to pre-existing ILD, interstitial lung abnormalities (ILA) can be a risk factor for ICI-related ILD. ILA is defined as incidentally found increased lung density in CT without definite diagnosis of ILD. Radiologic features of ILA include ground-glass attenuation (GGA), reticular abnormalities, traction bronchiectasis, honeycombing, diffuse centrilobular nodularity, and non-emphysematous cysts (52). ILA is shown in 14% of treatment-naïve advanced NSCLC patients (53). Nakanishi et al. reported that the patients with pre-existing ILA showed higher incidence of ICI-related ILD than those without [6/14 (42.9%) vs. 7/69 (10.1%), P=0.007], and patients with GGA specifically, showed significantly higher incidence rate [6/14 (42.9%) vs. 1/69 (1.4%), P<0.001] (52). In another retrospective cohort study of patients with non-lung cancers (head and neck cancer, malignant melanoma, oral cavity cancer, urological cancer, and gastrointestinal cancer) who received anti-PD-1 antibody therapy, pre-existing ILA (OR =6.42, 95% CI: 1.96–21.03, P=0.002) and GGA (OR =4.05, 95% CI: 1.29–12.71, P=0.01) were independent risk factors for ICI-related ILD in each multivariate analysis model (54).
Since pre-existing ILD and ILA can be risk factors for ICI-related ILD, patients with prior ILD and abnormal lung density in baseline chest CT, especially GGA, should be carefully monitored for the development of ICI-related ILD.
Pre-existing ILD in association with prognosis
In a retrospective study by Tasaka et al., clinical outcomes such as response rate (RR) and disease control rate (DCR) were not inferior in patients with pre-existing ILD compared to those without ILD (RR: 49.0% vs. 30.1%, P<0.01; DCR: 69.4% vs. 51.2%, P=0.016, respectively). Patients with pre-existing ILD also showed non-inferior median PFS and OS than those without ILD (5.9 vs. 3.5 months, P=0.14, and 27.8 vs. 25.2 months, P=0.74, respectively) (37). In another retrospective study, Isono et al. reported similar results (38). Though pre-existing idiopathic interstitial pneumonia was associated with an increased risk of ICI-induced ILD (HR =4.350, 95% CI: 1.225–15.440, P=0.023) among other pre-existing respiratory diseases, it was not associated with poor outcomes such as low objective response rate (ORR) or shorter OS (38). In a systemic review and meta-analysis of patients with NSCLC and pre-existing ILD by Zhang et al., the incidence rates of any grade and grade 3 or higher ICI-related ILD were more frequent in patients with pre-existing ILD than in those without (OR =3.23, 95% CI: 2.06–5.06, and OR =2.91, 95% CI: 1.47–5.74, respectively). The pooled ORR in patients with pre-existing ILD was better compared to those without ILD (OR =1.99, 95% CI: 1.31–3.00). The DCR and PFS were also not inferior in patients with pre-existing ILD (pooled OR =1.46, 95% CI: 0.94–2.25 for DCR). This study suggests that ICI treatment is effective in prognosis of NSCLC patients with pre-existing ILD, even though more frequent development of ICI-related ILD is observed (55).
Management of ICI related ILD
The primary treatment for ICI-related ILD is steroid therapy. For patients with steroid-resistant ICI-related ILD, several treatment options such as intravenous immunoglobulin (IVIG), IL-6 receptor inhibitors, TNF-inhibitors, or immunosuppressants can be considered. However, no standard treatment is currently established (56,57).
The guideline for managing irAEs in patients with ICI therapy by the American Society of Clinical Oncology recommends that the treatment should differ according to the toxicity grade. In grade 1 (mild) asymptomatic patients, close observation is needed unless there is evidence of progression or no improvement. Discontinuing ICI and corticosteroid treatment are not necessary in these patients. In grade 2 (moderate) patients, ICI treatment should be immediately stopped, and prednisolone 1–2 mg/kg/day should be administered with tapering by 5–10 mg/week over 4–6 weeks. ICI can be rechallenged after ILD improves. In grade 3 to 4 (severe) patients who need oxygen therapy, ICI treatment should be immediately withheld, and empirical antibiotics and (methyl)prednisolone IV 1–2 mg/kg/day should be administered. Other drugs such as infliximab 5 mg/kg, mycophenolate mofetil IV 1 g twice a day, IVIG for 5 days, or cyclophosphamide can be added if there is no improvement after 48 h. They recommended that ICI should be permanently discontinued in these patients (58,59). In a retrospective study of patients with NSCLC treated using nivolumab in Japan, corticosteroids were administered in more than 80% of the study patients to treat nivolumab-induced ILD. Most patients responded well to corticosteroids, even in relapse cases. Starting doses of corticosteroids varied from 0.5 to 2.0 mg/kg/day to pulse therapy. Better outcomes were observed in patients receiving more than 28 days of corticosteroid treatment (60).
Steroid-resistant cases
In some cases, steroids can show poor response, and other treatment modalities should be considered. A case report described a patient with newly-developed ILD after initiating pembrolizumab improved after five days of corticosteroid and nintedanib treatment (150 mg twice daily) (61). In another case report of steroid-refractory ICI-related ILD, a patient with nivolumab-induced ILD after receiving concurrent chemoradiotherapy and lobectomy showed improvement with initial steroid treatment (2 mg/kg intravenous methylprednisolone for 3 days, followed by 1 mg/kg/day oral prednisolone). However, ILD relapsed after conversion to oral prednisolone. Thus, the patient received one dose of 5 mg/kg intravenous infliximab and kept on 1 mg/kg/day of oral prednisolone. ILD resolved both clinically and radiologically after one month (62).
In another case of steroid-resistant ICI-related ILD, the patient was initially treated with methylprednisolone (100 mg/12 h), immunoglobulin, and antibiotics. However, the symptoms were aggravated. There was no significant improvement despite tocilizumab (8 mg/kg, iv), and tacrolimus treatment, as well as tocilizumab readministration. However, adding pirfenidone as antifibrotic treatment significantly reduced the patient’s symptoms and oxygen demand (56).
One case suggested that triple combination therapy, consisting of high-dose corticosteroids, tacrolimus, and cyclophosphamide, is effective in a patient who developed ICI-related ILD after receiving pembrolizumab (63).
In a case series of 12 patients with steroid-refractory ICI-related ILD, patients were treated with IVIG (n=7), infliximab (n=2), or combination of IVIG and infliximab (n=3). The group treated with IVIG showed lower mechanical ventilation requirement rates (25%), while infliximab group and a combination therapy group showed rates of 53% and 80%, respectively. The mortality of IVIG group was lower (43%) than infliximab alone group (100%) (64).
Rechallenge
In a retrospective study of cancer patients with ICI-related ILD, ICI was rechallenged in 10 (17.2%) patients including 9 patients with NSCLC. Among them, ICI-related ILD did not recur in 7 patients. Three patients (2 patients with grade 2 and 1 with grade 1) who developed a second event of ICI-related ILD also recovered after drug discontinuation and corticosteroid treatment (65).
Shibaki et al. reported 2 cases (1 patient with grade 1 and 1 with grade 2) who restarted anti-PD-1 antibody, and neither patient showed relapse of ICI-related ILD (36).
In a retrospective study by Naidoo et al., among 43 patients (including 9 patients with NSCLC) who developed ILD after anti-PD-1/PD-L1 treatment, 12 patients (9 patients with grade 1 and 3 with grade 2) were retreated with immunotherapy. Three patients (1 patient with grade 1 and 2 with grade 2) developed recurrent ICI-related ILD, and all patients recovered after drug discontinuation and corticosteroid therapy (66).
Sato et al. retrospectively analyzed the outcomes of subsequent systemic cancer therapy in NSCLC patients who developed ICI-related ILD. Among 32 (14%) patients who experienced ICI-related ILD due to anti-PD-1 therapy, 16 (50%) patients received subsequent systemic cancer treatment including chemotherapy and ICI. The median OS was longer in the patients with systemic cancer therapy than those without, but it was not statistically significant (22.2 vs. 4.5 months, P=0.067). The ICI-related ILD recurred in 50% of systemic cancer therapy group, and median OS was shorter in patients with recurrent ICI-related ILD, though there was no statistically significant difference (22.2 vs. 7.0 months, P=0.3154) (67).
As there is no definite consensus on rechallenging ICI in patients who experienced ICI-related ILD, ICI rechallenge needs careful consideration based on the severity and conditions of each patient (68). Corticosteroid treatment with immediate discontinuation of ICIs is essential for grade 2 or higher (moderate to severe) ICI-related ILD. ICI can be restarted in grade 2 after a sign of improvement, while ICI rechallenge is usually not recommended in grade 3 (severe) or higher-grade ICI-related ILD. Subsequent systemic cancer therapy after resolution of ICI-related ILD might improve survival outcomes, however, recurrence of ICI-related ILD should be monitored. In cases of steroid-refractory ICI-related ILD, administration of other immunosuppressants can be considered.
Conclusions
ICI-related ILD is relatively common in the real world and can be fatal. Patients with possible risk factors such as pre-existing pulmonary diseases, prior pneumonitis, and poor lung function should be closely monitored for the development of ICI-related ILD. If the patient manifests with ICI-related ILD, prompt management is necessary.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-93/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-93/coif). JUL serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2021 to August 2023. SK has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim JU, Yeo CD. Update on adjuvant therapy in completely resected NSCLC patients. Thorac Cancer 2022;13:277-83. [Crossref] [PubMed]
- Yamagata A, Yokoyama T, Fukuda Y, et al. Impact of interstitial lung disease associated with immune checkpoint inhibitors on prognosis in patients with non-small-cell lung cancer. Cancer Chemother Pharmacol 2021;87:251-8. [Crossref] [PubMed]
- Ono K, Ono H, Toi Y, et al. Association of immune-related pneumonitis with clinical benefit of anti-programmed cell death-1 monotherapy in advanced non-small cell lung cancer. Cancer Med 2021;10:4796-804. [Crossref] [PubMed]
- Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271-7. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Akella P, Loganathan S, Jindal V, et al. Anti PD-1 immunotherapy related interstitial lung disease presenting as respiratory failure - A review with case series. Respir Med Case Rep 2019;26:17-22. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Naqash AR, Yang LV, Sanderlin EJ, et al. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol 2018;57:705-8. [Crossref] [PubMed]
- Johnson DB, Balko JM. Biomarkers for Immunotherapy Toxicity: Are Cytokines the Answer? Clin Cancer Res 2019;25:1452-4. [Crossref] [PubMed]
- Lim SY, Lee JH, Gide TN, et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin Cancer Res 2019;25:1557-63. [Crossref] [PubMed]
- Zhang Q, Tang L, Zhou Y, et al. Immune Checkpoint Inhibitor-Associated Pneumonitis in Non-Small Cell Lung Cancer: Current Understanding in Characteristics, Diagnosis, and Management. Front Immunol 2021;12:663986. [Crossref] [PubMed]
- Zhai X, Zhang J, Tian Y, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med 2020;17:599-611. [Crossref] [PubMed]
- Kato T, Masuda N, Nakanishi Y, et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer 2017;104:111-8. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Wu J, Hong D, Zhang X, et al. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Sci Rep 2017;7:44173. [Crossref] [PubMed]
- Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol 2018;13:1930-9. [Crossref] [PubMed]
- Suzuki Y, Karayama M, Uto T, et al. Assessment of Immune-Related Interstitial Lung Disease in Patients With NSCLC Treated with Immune Checkpoint Inhibitors: A Multicenter Prospective Study. J Thorac Oncol 2020;15:1317-27. [Crossref] [PubMed]
- Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer 2018;125:212-7. [Crossref] [PubMed]
- Sun Z, Wang S, Du H, et al. Immunotherapy-induced pneumonitis in non-small cell lung cancer patients: current concern in treatment with immune-check-point inhibitors. Invest New Drugs 2021;39:891-8. [Crossref] [PubMed]
- Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271-81. [Crossref] [PubMed]
- Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018;125:150-6. [Crossref] [PubMed]
- Lichtenstein MRL, Nipp RD, Muzikansky A, et al. Impact of Age on Outcomes with Immunotherapy in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:547-52. [Crossref] [PubMed]
- Sul J, Blumenthal GM, Jiang X, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 2016;21:643-50. [Crossref] [PubMed]
- Galant-Swafford J, Troesch A, Tran L, et al. Landscape of Immune-Related Pneumonitis in Cancer Patients with Asthma Being Treated with Immune Checkpoint Blockade. Oncology 2020;98:123-30. [Crossref] [PubMed]
- Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9:847-55. [Crossref] [PubMed]
- Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina. Chest 2021;160:731-42. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Cui P, Liu Z, Wang G, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: A case-control study. Cancer Med 2018;7:4115-20. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Fujimoto D, Miura S, Yoshimura K, et al. Pembrolizumab plus chemotherapy-induced pneumonitis in chemo-naive patients with non-squamous non-small cell lung cancer: A multicentre, retrospective cohort study. Eur J Cancer 2021;150:63-72. [Crossref] [PubMed]
- Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer 2019;10:2006-12. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Tamiya A, Tamiya M, Nakahama K, et al. Correlation of Radiation Pneumonitis History Before Nivolumab with Onset of Interstitial Lung Disease and Progression-free Survival of Patients with Pre-treated Advanced Non-small Cell Lung Cancer. Anticancer Res 2017;37:5199-205. [PubMed]
- Voong KR, Hazell SZ, Fu W, et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer 2019;20:e470-9. [Crossref] [PubMed]
- Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother 2020;69:15-22. [Crossref] [PubMed]
- Tasaka Y, Honda T, Nishiyama N, et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer 2021;155:120-6. [Crossref] [PubMed]
- Isono T, Kagiyama N, Takano K, et al. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer 2021;12:153-64. [Crossref] [PubMed]
- von Reibnitz D, Chaft JE, Wu AJ, et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv Radiat Oncol 2018;3:391-8. [Crossref] [PubMed]
- Ding X, Ji W, Li J, et al. Radiation recall pneumonitis induced by chemotherapy after thoracic radiotherapy for lung cancer. Radiat Oncol 2011;6:24. [Crossref] [PubMed]
- Itamura H, Ohguri T, Yahara K, et al. Pembrolizumab-induced Radiation Recall Pneumonitis After the Resolution of Typical Asymptomatic Radiation Pneumonitis. J UOEH 2020;42:261-6. [Crossref] [PubMed]
- Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol 2021; Epub ahead of print. [Crossref] [PubMed]
- Jabbour SK, Berman AT, Decker RH, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol 2020;6:848-55. [Crossref] [PubMed]
- Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer 2020;126:4353-61. [Crossref] [PubMed]
- Peters S, Felip E, Dafni U, et al. Progression-Free and Overall Survival for Concurrent Nivolumab With Standard Concurrent Chemoradiotherapy in Locally Advanced Stage IIIA-B NSCLC: Results From the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14). J Thorac Oncol 2021;16:278-88. [Crossref] [PubMed]
- Lin SH, Lin Y, Yao L, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol 2020;15:248-57. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Saito Y, Sasaki S, Oikado K, et al. Radiographic features and poor prognostic factors of interstitial lung disease with nivolumab for non-small cell lung cancer. Cancer Sci 2021;112:1495-505. [Crossref] [PubMed]
- Baba T, Sakai F, Kato T, et al. Radiologic features of pneumonitis associated with nivolumab in non-small-cell lung cancer and malignant melanoma. Future Oncol 2019;15:1911-20. [Crossref] [PubMed]
- Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: a retrospective analysis. BMC Cancer 2021;21:924. [Crossref] [PubMed]
- Yamaguchi O, Kaira K, Shinomiya S, et al. Pre-existing interstitial lung disease does not affect prognosis in non-small cell lung cancer patients with PD-L1 expression ≥50% on first-line pembrolizumab. Thorac Cancer 2021;12:304-13. [Crossref] [PubMed]
- Nakanishi Y, Masuda T, Yamaguchi K, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investig 2019;57:451-9. [Crossref] [PubMed]
- Nishino M, Cardarella S, Dahlberg SE, et al. Interstitial lung abnormalities in treatment-naive advanced non-small-cell lung cancer patients are associated with shorter survival. Eur J Radiol 2015;84:998-1004. [Crossref] [PubMed]
- Shimoji K, Masuda T, Yamaguchi K, et al. Association of Preexisting Interstitial Lung Abnormalities With Immune Checkpoint Inhibitor-Induced Interstitial Lung Disease Among Patients With Nonlung Cancers. JAMA Netw Open 2020;3:e2022906. [Crossref] [PubMed]
- Zhang M, Fan Y, Nie L, et al. Clinical Outcomes of Immune Checkpoint Inhibitor Therapy in Patients With Advanced Non-Small Cell Lung Cancer and Preexisting Interstitial Lung Diseases: A Systematic Review and Meta-Analysis. Chest 2022; Epub ahead of print. [Crossref] [PubMed]
- Miao K, Xu Y, Xu W, et al. Treatment of steroid-resistant checkpoint inhibitor pneumonitis with pirfenidone: A case report. Thorac Cancer 2021;12:2214-6. [Crossref] [PubMed]
- Reuss JE, Suresh K, Naidoo J. Checkpoint Inhibitor Pneumonitis: Mechanisms, Characteristics, Management Strategies, and Beyond. Curr Oncol Rep 2020;22:56. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Sun Y, Shao C, Li S, et al. Programmed cell death 1 (PD-1)/PD-ligand 1(PD-L1) inhibitors-related pneumonitis in patients with advanced non-small cell lung cancer. Asia Pac J Clin Oncol 2020;16:299-304. [Crossref] [PubMed]
- Sata M, Sasaki S, Oikado K, et al. Treatment and relapse of interstitial lung disease in nivolumab-treated patients with non-small cell lung cancer. Cancer Sci 2021;112:1506-13. [Crossref] [PubMed]
- Xie XH, Deng HY, Lin XQ, et al. Case Report: Nintedanib for Pembrolizumab-Related Pneumonitis in a Patient With Non-Small Cell Lung Cancer. Front Oncol 2021;11:673877. [Crossref] [PubMed]
- Andruska N, Mahapatra L, Hebbard C, et al. Severe pneumonitis refractory to steroids following anti-PD-1 immunotherapy. BMJ Case Rep 2018;2018:bcr-2018-225937. [Crossref] [PubMed]
- Utsumi H, Araya J, Okuda K, et al. Successful treatment of steroid-refractory immune checkpoint inhibitor-related pneumonitis with triple combination therapy: a case report. Cancer Immunol Immunother 2020;69:2033-9. [Crossref] [PubMed]
- Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer 2021;9:e001731. [Crossref] [PubMed]
- Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50:1700050. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Sato Y, Watanabe S, Ota T, et al. Subsequent systemic therapy for non-small cell lung cancer patients with immune checkpoint inhibitor-related interstitial lung disease. Transl Lung Cancer Res 2021;10:3132-43. [Crossref] [PubMed]
- Delaunay M, Prévot G, Collot S, et al. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev 2019;28:190012. [Crossref] [PubMed]