
Underlying mechanisms of saphenous vein graft stenosis after coronary artery bypass caused by clipping of the side branches: an experimental study
Introduction
Saphenous veins are regular bypass conduits selected in non-left anterior descending artery (LAD) coronary artery bypass graft (CABG) surgery. Graft stenosis of the saphenous vein may be associated with poor outcomes for CABG. Saphenous vein graft (SVG) occlusion occurs at a rate of approximately of 2–3% per year, and at a rate of approximately 4% 6–10 years after CABG (1). The mechanism of SVG atherosclerosis after CABG has been well documented (2,3). Despite the technical errors, acute thrombosis, intimal hyperplasia during the first year of CABG failure and the SVG arteriosclerosis during the late failure, the mechanism of clipping related late SVG stenosis is unique. The clinical and Intravascular ultrasound (IVUS) views of SVG stenosis caused by clipping of side-branches have rarely been reported. This study was conducted to investigate the clinical and IVUS characteristics of SVG stenosis caused by clipping of side-branches. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-235/rc).
Methods
Research design
This is an experimental study included the in vivo and in vitro. In vivo, we collected 41 patients who underwent coronary angiography (CA) after CABG in the Department of Cardiology, Beijing Anzhen Hospital, from January 2020 to September 2021. There were six cases of SVG stenosis caused by the clipping of the side branches. Furthermore, we built an in vitro model to verify the identical IVUS pattern of metal clip in the cases above. While imitating the regular operation of SVG anastomosis, we performed traditional harvesting method with a single ligation and a metal clip.
Clinical characteristics were collected using the computerized patient record system at our hospital. The SVGs used in the in vitro model were collected from residual saphenous veins after grafts had been conducted according to routine surgical processes. All the grafts were collected with written informed consent from the Department of Cardiac Surgery, Beijing Anzhen Hospital. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki (as revised in 2013) and approved by the Clinical Research Ethics Board of Beijing Anzhen Hospital, Capital Medical University (No. 2021010X).
IVUS inspection: an IVUS system (Boston Scientific OptiCrossTM; Marlborough, MA, USA) was performed with a frequency of 60 MHz. An intracoronary nitroglycerin was administered before IVUS was drawn back. The ultrasound catheter was retracted at a constant speed (at 0.5–1 mm per second).
Statistical analysis
The baseline characteristics of patients such as the postoperative time of CABG were presented as mean ± SD for continuous variables. Categorical variables were presented as percentage. Statistical analyses were performed with SPSS statistics, version 20.0 (IBM Corporation, NY, USA). The clinical manifestations, medical history and treatment processes of each patient were documented in the Table 1.
Table 1
| No. | Gender | Age | Hypertension | Dyslipidemia | Diabetes | Antiplatelet drugs | Statin | History of CABG (y) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 75 | √ | √ | √ | √ | √ | 5 |
| 2 | F | 67 | √ | √ | √ | √ | 6 | |
| 3 | F | 64 | √ | √ | √ | √ | 11 | |
| 4 | M | 82 | √ | √ | √ | √ | √ | 18 |
| 5 | M | 67 | √ | √ | √ | 10 | ||
| 6 | F | 64 | √ | √ | √ | √ | 4 |
CABG, coronary angiography bypass graft.
Results
Clinical and imaging materials of six typical cases (14.6%) of diagnosed SVG stenosis caused by metal clipping were analyzed in this study. The average age of the patients was 69.83±7.195 years. The average postoperative time of CABG was 9±5.215 years. Their clinical manifestations, past medical history, and treatment processes are described in Table 1.
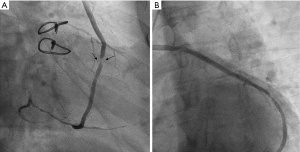
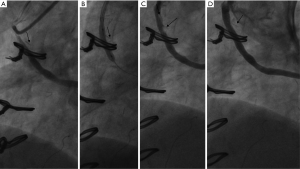
Case 1, a 75-year-old Chinese male (with hypertension, dyslipidemia, type-2 diabetes, and a history of inferior myocardial infarction) presented with recurrent exertional chest pain for 5 years following CABG surgery. The patient’s grafts included: left internal mammary artery (LIMA) → LAD, and aorta → SVG → diagonal branch → right coronary artery (RCA). His regular medication included aspirin, metoprolol, rosuvastatin, and metformin after the CABG. The CA showed stenosis at the mid-portion of the SVG (see Figure 1). An IVUS (Boston Scientific OptiCrossTM; Marlborough, MA, USA) was performed at the SVG stenosis and the clipping of side-branches were observed at the side of stenosis (see Figure 2). Due to the rare signs of neo-arteriosclerosis and the unsatisfied immediate effect after pre-dilatation with non-compliant balloon (Boston Scientific Quantum 3.5 mm × 15 mm; Boston Scientific, USA), a drug-coated balloon (DCB; Bingo, 3.5 mm × 15 mm, Yinyi Biotech, Dalian, China) was used instead of a drug eluting stent (DES) without the distal protection device (Figure 3).
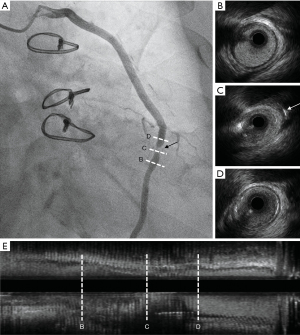
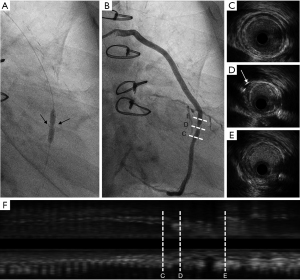
Case 2, a 67-year-old female with hypertension and dyslipidemia was diagnosed with stable angina at 6 years from CABG. The CA showed acceptable patency in the LIMA and a stenosis at the proximal segment of SVG to the left circumflex coronary (Figure 4). The IVUS showed neo-arteriosclerosis at the proximal segment of the SVG (Figure 4C) and different signs of stenosis caused by clipping of side-branches in the middle segment of the SVG (Figure 4B). A percutaneous coronary intervention (PCI) was performed using a distal protection device, and a drug-eluting stent (TIVOLI 3.0 mm × 15 mm, Essen Technology Beijing Co. Ltd., Beijing, China) was implanted (Figure 5).
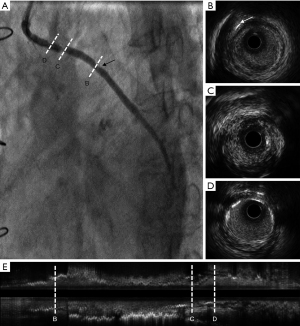
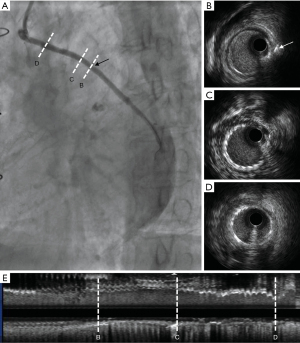
Case 3, a 64-year-old female with hypertension and dyslipidemia was diagnosed with unstable angina 1 week after CABG. The patient’s grafts included: LIMA → LAD, aorta → SVG → diagonal branch → obtuse marginal branch → posterior descending branch. Aspirin, clopidogrel, and atorvastatin were regularly prescribed. The CA showed the stenosis in SVG from the diagonal branch to the obtuse marginal branch was partly caused by 2 metal clippings. When the instruments such as the distal protection device and the drug-eluting stent (Medtronic Resolute Integrity 3.0 mm × 18 mm, Medtronic, Dublin, Ireland) were pushed through the stenosis caused by metal clipping, rough resistance could be felt (Figure 6).
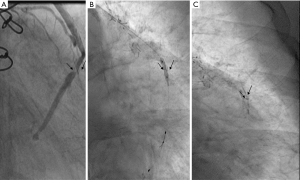
Case 4, an 82-year-old male with hypertension, type 2 diabetes, and dyslipidemia was diagnosed with stable angina for 2 years following 18-year CABG. The patient’s grafts were as follows: LIMA → LAD, aorta → SVG → obtuse marginal branch, aorta → SVG → posterior descending branch. The patient had continually taken aspirin and rosuvastatin since the CABG 18 years ago. The CA showed 3 metal clipping in the stenosis of SVG from aorta to posterior descending branch (Figure 7).
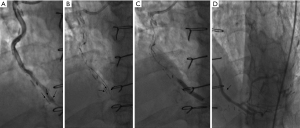
Case 5, a 67-year-old male (with hypertension, dyslipidemia, and a history of inferior myocardial infarction) experienced atypical chest pain after 10 years CABG. The patient had regularly taken statin. The patient’s grafts were as follows: LIMA → LAD, aorta → SVG → OM → PDA. The CA and the coronary computed scan showed mild stenosis at the SVG which was caused by the metal clipping (Figure 8).
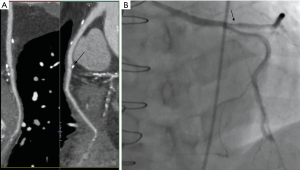
Case 6, a 64-year-old female with type 2 diabetes, dyslipidemia, and a history of inferior myocardial infarction was diagnosed with unstable angina 4 years after CABG. She had regularly taken dual antiplatelet drugs and rosuvastatin. The patient’s grafts were as follows: LIMA → LAD, aorta → SVG → diagonal branch → obtuse marginal branch → posterior descending branch. The CA showed the stenosis at the proximal of the SVG caused by the metal clipping. Vascular dissection occurred after non-compliant balloon pre-dilatation (Boston Scientific Quantum Maverick 3.5 mm × 20 mm); therefore, a drug-eluting stent (Medtronic Resolute Integrity 4.0 mm × 22 mm) was implanted (Figure 9).
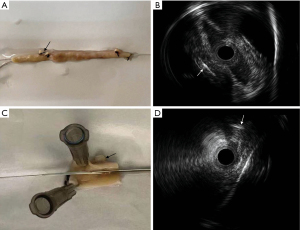
IVUS image of SVGs in vitro
Images of IVUS performed in both intact and longitudinally incised SVG were demonstrated (Figure 10). Similar patterns of IVUS images in the in vitro model showed the SVG failures in the cases above were mainly caused by the metal clip.
Discussion
In our research, we found that 6 of 41 patients occurred metal-clipping-related late SVG failure. The stenosis of SVG caused by metal clipping mostly occurred at the corner and multipole clipping points. In this situation, great resistance could be felt when pushing the instruments through the stenosis and crystallized cholesterol was rarely caught by the distal protection device.
The number of CABG operated has been increasing at a rate of 10% annually in China, reaching 45,455 in 2017 (4). Although internal thoracic artery, radial artery, and gastroepiploic artery have become optional graft locations, the SVG remains the most widely used non-LAD bypass conduit due to its accessibility, although without satisfactory longevity and durability (5). Previous studies have indicated that 10–20% of SVGs fail in 1 year after the procedures (6,7). An additional 5–10% of SVGs fail in the 1–5 years after CABG and the proportion reaches 20–25% in 6–10 years (8). Patency at 10 years is only about 60% for SVGs (9).
The mechanism of SVG failure after CABG has been extensively discussed. Technical errors, acute thrombosis, and intimal hyperplasia are the main cause of SVG failure during the first year after CABG (10). Furthermore, late failure occurs due to SVG arteriosclerosis (10). Advanced endovascular imaging technology sheds light on the potential mechanisms and physiology of SVG failure progression, such as twisted SVG leading to acute mechanical stenosis (11), and “upside down” venous valve leading to late SVG stenosis following 12-year-CABG (12). The cases above demonstrated another mechanism that led to the SVG failure. This report is the first IVUS documentation of the late SVG stenosis caused by clipping of side-branches. Metal-clipping-related SVGs failure may improve the understanding of the pathophysiology of venous graft failure. Surgical manipulation of preparation and technology of harvesting may play a critical role in long-term SVG patency (13). Mechanical force by either clipping or ligation may lead to iatrogenic injury of SVG structure. A previous study showed that mechanical shrinkage of clipped side branches affects the layers of adjacent SVG segments (14). Sanisoglu et al. reported that compared to ligation, clipping the side-branches of the SVG during its harvesting for coronary bypass grafting is associated with decreased vein damage (14). Not only the external stent (15), but also the “no-touch” technique (16) is associated with reduced SVG patency.
Limitations
Unfortunately, research documenting the phenomenon of metal-clipping-related late SVG failure is scarce. In our study we reported that 6 of 41 patients occurred metal-clipping-related late SVG failure. But the limitation of study is that the sample of study is too small to assess the incidence rate of metal-clipping-related late SVG failure. Large-scale clinical research for further exploring was required.
Conclusions
Our study indicated that IVUS is a very useful modality for clarification of the SVG stenosis caused by clipping of side-branches, as it sheds lights on the potential mechanisms and physiology of late SVG progression failure.
Acknowledgments
Funding: This study was supported by grants from the Beijing Hospitals Authority Incubating Program (No. PZ2021007), Beijing Hospitals Authority Youth Program (No. QML20200604), and the National Natural Science Foundation of China (No. 81770412). The sponsors had no role in the data collection and analysis, decision to publish, or manuscript preparation.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-235/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-235/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-235/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki (as revised in 2013) and approved by the Clinical Research Ethics Board of Beijing Anzhen Hospital, Capital Medical University (No. 2021010X) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chesebro JH, Fuster V, Elveback LR, et al. Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations. N Engl J Med 1984;310:209-14. [Crossref] [PubMed]
- Raza S, Chang C, Deo SV, et al. Current role of saphenous vein graft in coronary artery bypass grafting. Indian J Thorac Cardiovasc Surg 2018;34:245-50. [Crossref] [PubMed]
- Wadey K, Lopes J, Bendeck M, et al. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc Res 2018;114:601-10. [Crossref] [PubMed]
- Ma LY, Chen WW, Gao RL, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol 2020;17:1-8. [PubMed]
- Martínez-González B, Reyes-Hernández CG, Quiroga-Garza A, et al. Conduits Used in Coronary Artery Bypass Grafting: A Review of Morphological Studies. Ann Thorac Cardiovasc Surg 2017;23:55-65. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Sabik JF 3rd, Lytle BW, Blackstone EH, et al. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg 2005;79:544-51; discussion 544-51. [Crossref] [PubMed]
- Bourassa MG, Fisher LD, Campeau L, et al. Long-term fate of bypass grafts: the Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation 1985;72:V71-8. [PubMed]
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. [Crossref] [PubMed]
- de Vries MR, Simons KH, Jukema JW, et al. Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol 2016;13:451-70. [Crossref] [PubMed]
- Ichimoto E, Horie S, Hasegawa A, et al. Subacute mechanical stenosis due to twisted saphenous vein graft identified by intravascular ultrasound. Cardiovasc Interv Ther 2018;33:95-6. [Crossref] [PubMed]
- Koeda Y, Itoh T, Fusazaki T, et al. A unique stenosis in saphenous vein graft visualized by optical coherence tomography. Heart Vessels 2014;29:278-81. [Crossref] [PubMed]
- Schanzer A, Hevelone N, Owens CD, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg 2007;46:1180-90; discussion 1190. [Crossref] [PubMed]
- Sanisoglu I, Caynak B, Onan B, et al. Comparison of clipping versus ligation of side-branches during saphenous vein graft harvesting: which method is superior? Ann Vasc Surg 2011;25:669-74. [Crossref] [PubMed]
- Taggart DP, Webb CM, Desouza A, et al. Long-term performance of an external stent for saphenous vein grafts: the VEST IV trial. J Cardiothorac Surg 2018;13:117. [Crossref] [PubMed]
- Samano N, Geijer H, Liden M, et al. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J Thorac Cardiovasc Surg 2015;150:880-8. [Crossref] [PubMed]
(English Language Editor: J. Jones)


