Acetaminophen for febrile patients with suspected infection: potential benefit and further directions
Fever, increased body temperature, is a physiological expression of the host’s response to an infective (1) or non-infective pathology (2-6). Non-infective fever is common in critically ill patients, which includes ones related with post-surgical reaction, acute myocardial infarction, cerebral infarction, cerebral hemorrhage, acute pancreatitis, malignant tumor, post-transfusion reaction, transplant rejection and drug fever. Fever is also common in infective patients. In multicenter observational study, among the patients who developed body temperature equal or more than 38.5 °C, approximately 63% of patients were diagnosed as sepsis (7).
Fever may have detrimental effects such as increasing the oxygen consumption and worsen the neurological outcomes (8-10). Thus, antipyretic treatments are frequently administered in critically ill patients. Among septic patients, at least one antipyretic therapy was prescribed in one-third of patients who developed body temperature between 38.5–39.4 °C, and more than half of patients that body temperature equal or more than 39.5 °C (7). However, high body temperature could be an optimal host response against infectious disease. Fever may result in reduced bacterial growth, promotion of the synthesis of antibodies, and activation of T cells, neutrophils and macrophages (11-13). In this regards, the antipyretics could be either friends or foes in patients with infection. It is unfortunate that the impact of antipyretics in infective patients has been unclear and there are no recommendations for body temperature control for febrile patients with infection (1,14).
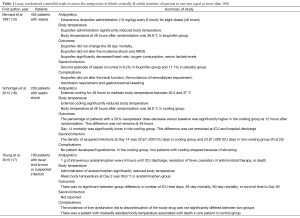
One randomized controlled study in 1997, ibuprofen administration (10 mg per kilogram of body weight) significantly decreases fever and oxygen consumption in septic patients. This study did not show any benefit of ibuprofen on the patients’ centered outcome including the incidence of the acute respiratory distress syndrome and mortality (15) (Table 1). In this study, 44% of the patients in the placebo arm were received acetaminophen administration and 22% of those in the ibuprofen arm. In this regards, the impact of ibuprofen as an antipyretics on the outcomes in septic patients might not be able to determine in this study (18). However, one may consider that this study might show that the reduction of body temperature to normothermic range (36.5–37.0 °C) may be safe in septic patients.
Full table
Another randomized controlled study was conducted to assess the effect of external cooling in 200 febrile adult patients with septic shock who were sedated, required mechanical ventilation and received vasopressor. External cooling for 48 hours was reduced body temperature in the normothermic range (36.5–37.0 °C). External cooling significantly reduced the vasopressor requirement and mortality at 14 days after randomization (16) (Table 1). This trial also showed that the acquired infections for 14 days was tended to be increase in cooling arm in compared with non-cooling arm (32.6/1,000 vs. 23.8/1,000 ICU days, P=0.25). Then, the mortality benefit observed at Day 14th did not remain at ICU or hospital discharge. The major concerns to apply external cooling in febrile patients were patient’s discomfort and potential shivering. To prevent shivering, sedative drugs may be required. We should note that they choose the septic patients who were sedated and required mechanical ventilation.
Although above RCTs reported the lack of adverse effect or potential benefit of lowering body temperature using ibuprofen and external cooling in septic patients, those of two may not be a major antipyretic used in critically ill patients. The administration of acetaminophen would be common antipyretic in critically ill patients. One retrospective study including 15,818 ICU patients had shown that 64% of study patients received at least 1 g of acetaminophen. And the administration of acetaminophen was independently associated with decreased mortality both in surgical and medical patients (19). However, antipyretic therapy may vary among countries. In a prospective observational study conducted in Korea and Japan including 1,425 critically ill patients had shown that acetaminophen was used in 10.4% of patients (7) and the administration of acetaminophen was independently associated with increased mortality in septic patients. This controversy seen in these two observational studies suggests that there may be major confounders on the association between the acetaminophen administration and mortality. Thus, the randomised controlled trial to assess the impact of acetaminophen in patients with infection was definitely necessary.
Acetaminophen for fever in critically ill patients with suspected infection
Recently, “the Permissive Hyperthermia through Avoidance of Acetaminophen in Known or Suspected Infection in the Intensive Care Unit (HEAT) trial” was published in New England Journal of Medicine (17) (Table 1). They included 700 patients with ≥38 °C of body temperature and known or suspected infection. Patients were randomly assigned to receive either 1 g of intravenous acetaminophen or placebo every 6 hours. The study drugs were stopped when body temperature was less than 37.5 °C for last 24 hours, antimicrobial treatment was stopped or patients were discharged from ICU discharge. They allowed using the physical cooling at body temperature equal or more than 39.5 °C. They also permit to use the open-label acetaminophen after the administration of study medication. They defined as the primary outcome as ICU-free day at 28 days after randomization.
In HEAT study, study medication was used 8 times in acetaminophen group and 9 times is placebo group. Open-label acetaminophen was administered approximately 30% of patients in each groups. The difference of mean daily peak body temperature in the ICU was −0.25 °C (P<0.001). They found that there was trend to increase the ICU-free day at 28 days after randomization in acetaminophen group (median of 23 vs. 22 days, P=0.07). They also found that acetaminophen administration increased length of ICU stay in non-survivors and decreased it in survivors. There was no significant difference of mortality and length of stay both in ICU and hospital. The incidence of liver dysfunction was comparable between two groups.
The HEAT trial asked clinically relevant question and is largest randomized trial in this issue. This trial had planned well (20,21) and performed with excellent concealment and follow up. HEAT trial also had several limitations including high incidence of protocol violation and the use of open-label acetaminophen. Additionally the difference of body temperature between two groups was relatively small, which was maximized at Day 1 (about 0.5 °C difference between two groups), then disappeared after Day 3. This might be due to their protocol for the stop of study drug (they stopped it when patients body temperature was less than 37.5 °C for last 24 hours).
HEAT trial should be a mile stone study on the body temperature control in febrile critically ill patients. However, it is not the end of the story. Future study is necessary to address how long we should use the acetaminophen, how lower we should control body temperature, and what type of patients we should use acetaminophen.
HEAT trial tells us that the use of acetaminophen in infective critically ill patients is safe, but not affect to patients centered outcome. It might not be necessary to treat fever in ALL patients with suspected infection. We afraid that it might be reasonable to use acetaminophen in patients with fever related distress, as such a tachycardia and tachypnea. However, it is also acceptable not to use acetaminophen in patients that fever does not cause any stress response.
Acknowledgements
None.
Footnote
Provenance: This is an invited article commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua 321000, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- O'Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 2008;36:1330-49. [PubMed]
- Badillo AT, Sarani B, Evans SR. Optimizing the use of blood cultures in the febrile postoperative patient. J Am Coll Surg 2002;194:477-87. [PubMed]
- Kennedy LD, Case LD, Hurd DD, et al. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion 2008;48:2285-91. [PubMed]
- Roush MK, Nelson KM. Understanding drug-induced febrile reactions. Am Pharm 1993;NS33:39-42. [PubMed]
- Hawksworth JS, Leeser D, Jindal RM, et al. New directions for induction immunosuppression strategy in solid organ transplantation. Am J Surg 2009;197:515-24. [PubMed]
- Egi M, Morita K. Fever in non-neurological critically ill patients: A systematic review of observational studies. J Crit Care 2012;27:428-33. [PubMed]
- Lee BH, Inui D, Suh GY, et al. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care 2012;16:R33. [PubMed]
- Manthous CA, Hall JB, Olson D, et al. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med 1995;151:10-4. [PubMed]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549-56. [PubMed]
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557-63. [PubMed]
- Ryan AJ, Flanagan SW, Moseley PL, et al. Acute heat stress protects rats against endotoxin shock. J Appl Physiol (1985) 1992;73:1517-22. [PubMed]
- Villar J, Ribeiro SP, Mullen JB, et al. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med 1994;22:914-21. [PubMed]
- Kluger MJ, Kozak W, Conn CA, et al. The adaptive value of fever. Infect Dis Clin North Am 1996;10:1-20. [PubMed]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327. [PubMed]
- Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med 1997;336:912-8. [PubMed]
- Schortgen F, Clabault K, Katsahian S, et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med 2012;185:1088-95. [PubMed]
- Young P, Saxena M, Bellomo R, et al. Acetaminophen for Fever in Critically Ill Patients with Suspected Infection. N Engl J Med 2015;373:2215-24. [PubMed]
- Hasday JD, Garrison A. Antipyretic therapy in patients with sepsis. Clin Infect Dis 2000;31 Suppl 5:S234-41. [PubMed]
- Suzuki S, Eastwood GM, Bailey M, et al. Paracetamol therapy and outcome of critically ill patients: a multicenter retrospective observational study. Crit Care 2015;19:162. [PubMed]
- Young PJ, Weatherall M, Saxena MK, et al. Statistical analysis plan for the HEAT trial: a multicentre randomised placebo-controlled trial of intravenous paracetamol in intensive care unit patients with fever and infection. Crit Care Resusc 2013;15:279-86. [PubMed]
- Young PJ, Saxena MK, Bellomo R, et al. The HEAT trial: a protocol for a multicentre randomised placebo-controlled trial of IV paracetamol in ICU patients with fever and infection. Crit Care Resusc 2012;14:290-6. [PubMed]


