
Efficacy of dacomitinib in patients with non-small cell lung cancer carrying complex EGFR mutations: a real-world study
Introduction
The most common mutations on the epidermal growth factor receptor (EGFR) gene in non-small cell lung cancer (NSCLC) are called “common mutations”, and include exon 19 deletion (19Del) (49–72%) and L858R (28–43%) (1). They have shown a remarkable response to EGFR-tyrosine kinase inhibitors (EGFR-TKIs) which include first-generation TKI (gefitinib, erlotinib), second generation TKI (afatinib, dacomitinib), and third-generation TKI (osimertinib), while other mutations (10–20%) on EGFR are called “uncommon mutations” or “rare mutations”, with a significant heterogenic response to EGFR-TKIs (1-5). Some uncommon mutations, including G719X, S768I, and L861Q, are also called “major uncommon mutations” and are sensitive to different generations of TKIs, especially afatinib. In comparison, T790M and exon 20 insertion mutations are insensitive to most listed TKIs (except for osimertinib) (2,6-8). Compound mutations are defined as the coexistence of two or more EGFR mutations, regardless of these being common or uncommon mutations, and they are associated with poor prognosis in patients that carry them (9). With the improvement of molecular detection technologies, the proportion of the identified compound mutations has been increased significantly up to 26% (9,10). However, studies on compound mutations are limited due to the numerous permutations between common and uncommon mutations and the variety of uncommon mutation types. These studies are primarily retrospective and have divergent findings, thus leading to a lack of clinical data to confirm and help physicians make decisions for clinical practice (4,5).
As an irreversible, highly selective, second-generation EGFR-TKI, dacomitinib can inhibit signaling from all members of the human EGFR family. The ARCHER 1,050 study demonstrated significantly improved progression-free survival (PFS) [14.7 vs. 9.2 months, hazard ratio (HR) 0.59; 95% confidence interval (CI), 0.47–0.74; P<0.0001] of dacomitinib over gefitinib, leading to its approval as a new standard first-line treatment in patients with EGFR-positive NSCLC (11). In addition, a few published studies have indicated that dacomitinib has potential applications in patients harboring uncommon mutations (12,13), although no conclusive evidence has been presented thus far.
Therefore, to evaluate the efficacy of dacomitinib, we retrospectively analyzed 18 patients with NSCLC harboring complex EGFR mutations by focusing only on complex mutations that include a common mutation and an uncommon mutation. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1841/rc).
Methods
Patient eligibility and data collection
Patients were enrolled from the outpatient department of the Chinese PLA General Hospital and the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, and the Peking Union Medical College. Eligible patients met the following requirements: (I) cytologically or histologically confirmed diagnosis of NSCLC; (II) unresectable stage III patients who refused to receive chemoradiotherapy or stage IV patients; (III) harbored common EGFR mutations (19del or L858R, set as C group) or complex EGFR mutations (including a common mutation and an uncommon mutation, set as C+U group) excluding exon 20 insertion mutations and T790M; and (IV) tumor tissue or cell-free DNA were available from plasma, pleural effusion, or cerebrospinal fluid samples and were tested using next-generation sequencing (NGS) which was performed by qualified third-party genetic testing companies that had been accredited by the College of American Pathologists (CAP) before dacomitinib treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Boards of the Chinese PLA General Hospital and the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (No. 18-070 and 1648). The Research Ethics Boards waived the need for informed consent as this was a retrospective study.
Treatment and efficacy/toxicity evaluation
All patients were treated with dacomitinib alone in a multi-line setting. The physician determined the starting dose based on the patient’s condition. In general, the starting dose was 45 mg for patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 and weight ≥60 kg; 30 mg for patients with a PS of 1 and weight ≤60 kg; 30 mg for patients with a PS of 1 and weight ≥60 kg, with an increase to 45 mg if the patient had a good tolerance; and 15 mg for patients with PS ≥2. Imaging evaluation included chest computed tomography (CT) scans and brain magnetic resonance imaging (MRI), performed every 1–2 months after drug administration. Objective tumor response was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) guidelines (14). The objective response was divided into two categories: complete response (CR) and partial response (PR), while disease control included CR, PR, and stable disease (SD) combined. Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Categorical variables were reported as numbers and percentages. The chi-square test was used for comparison between different groups. The data cut-off date was September 21, 2021, when the disease status of the patients was confirmed. PFS was defined as the time from dacomitinib administration to disease progression or death from any cause. Overall survival (OS) was defined as the time from dacomitinib administration to death from any cause. Patients who were lost to follow-up were judged to be censored and the last determinable time of survival was used as the time of termination of follow-up. The relationship between various variables and survival was evaluated using the Kaplan-Meier method. Differences between survival curves were tested for statistical significance using log-rank tests. To reduce the effect of confounding factors, stratified analyses of clinical characteristics that differed significantly between the two groups were conducted. Significant prognostic predictors for patients identified by univariate analyses were further assessed by multivariate analyses using the Cox proportional hazards regression model. Statistical analyses were performed, and analytic graphs were created using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). An α value of 0.05 was used as the examination standard.
Results
Baseline characteristics
In total, 72 patients with NSCLC harboring complex EGFR mutations (C+U group, n=18) or common EGFR mutations (C group, n=54), who were treated with dacomitinib, were enrolled in two medical centers between August 2019 and August 2021. The baseline characteristics of the two cohorts are displayed in Table 1. For the C+U group, there were 12 women (66.7%) and 6 men (33.3%), with a median age of 68 years (range, 50–81 years). More than 70% of the patients were non-smokers, and adenocarcinoma was the histologic type detected in all patients. More than half of the patients (55.6%, 10/18) received dacomitinib as the first-line treatment, and nearly 70% of patients received it at a dosage of 30 mg. The L858R was the most frequent mutation (88.9%, 16/18) in complex EGFR mutations (Table 1). The ECOG PS of the 18 patients ranged from 0 to 2, and most patients (77.8%, 14/18) had PS ≤1. Except for the application line of dacomitinib, no significant statistical differences between the C and C+U groups were found in other characteristics, including age, sex, smoking history, histology, disease stage, and common mutation status (Table 1). More patients in the C+U group received dacomitinib as the first-line treatment than those in the C group (55.6% vs. 25.9%, P=0.039).
Table 1
| Characteristics | C group (n=54) | C+U group (n=18) | P value# |
|---|---|---|---|
| Age (years), n (%) | 0.586 | ||
| ≤60 | 28 (51.9) | 8 (44.4) | |
| >60 | 26 (48.1) | 10 (55.6) | |
| Gender, n (%) | 0.219 | ||
| Female | 27 (50.0) | 12 (66.7) | |
| Male | 27 (50.0) | 6 (33.3) | |
| Smoking history, n (%) | 0.248 | ||
| Yes | 20 (37.0) | 4 (22.2) | |
| No | 34 (63.0) | 14 (77.8) | |
| Histology, n (%) | 1.000 | ||
| AC | 52 (96.3) | 18 (100) | |
| Others | 2 (3.7) | 0 (0) | |
| Disease stage, n (%) | 0.942 | ||
| III | 3 (5.6) | 1 (5.6) | |
| IV | 41 (75.9) | 13 (72.2) | |
| Recurrence | 10 (18.5) | 4 (22.2) | |
| Common EGFR mutation status, n (%) | 0.402 | ||
| 19del | 13 (24.1) | 2 (11.1) | |
| L858R | 41 (75.9) | 16 (88.9) | |
| Brain metastases, n (%) | 0.584 | ||
| Yes | 25 (46.3) | 7 (38.9) | |
| No | 29 (53.7) | 11 (61.1) | |
| Tumor burden, n (%) | 0.715 | ||
| ≥3 metastatic organs | 8 (14.8) | 4 (22.2) | |
| <3 metastatic organs | 46 (85.2) | 14 (77.8) | |
| Therapies given prior to dacomitinib | <0.001 | ||
| None | 14 (25.9) | 10 (55.6) | |
| Targeted therapy only | 17 (31.5) | 6 (33.3) | |
| Chemotherapy only | 0 (0) | 2 (11.1) | |
| Targeted therapy/chemotherapy | 23 (42.6) | 0 (0) | |
| Dacomitinib application line, n (%) | 0.039 | ||
| 1 | 14 (25.9) | 10 (55.6) | |
| 2 | 11 (20.4) | 4 (22.2) | |
| ≥3 | 29 (53.7) | 4 (22.2) | |
| Dacomitinib dosage, n (%)* | 0.797 | ||
| 15 mg | 9 (16.7) | 3 (16.7) | |
| 30 mg | 29 (53.7) | 12 (66.7) | |
| 45 mg | 16 (29.6) | 3 (16.7) | |
| ECOG PS, n (%)* | 0.304 | ||
| 0 | 9 (16.7) | 5 (27.8) | |
| 1 | 40 (74.1) | 9 (50.0) | |
| ≥2 | 5 (9.3) | 4 (22.2) |
*, percentages might add up to more than 100% due to rounding; #, the chi-square test was used for the comparison. C group, common mutations group; C+U group, common mutations combined with uncommon mutations group; AC, adenocarcinoma; others, including adenosquamous carcinoma and squamous cell carcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status.
Genetic profiling
Tumor tissue or cell-free DNA from plasma, pleural effusion, or cerebrospinal fluid samples before dacomitinib treatment were tested using the NGS method in all patients. Figure 1 shows the three-dimensional distribution of uncommon mutations in complex mutations and the corresponding number of cases of the C+U group. Figure 2 shows the details of uncommon mutations, common mutations, and accompanying mutations. Sixteen patients (88.9%) harbored L858R co-existing with rare mutations located in exon 18 to exon 25 of EGFR (mostly E709X in exon 18), and two harbored 19del co-existing with G724S or K754E. The most common concomitant mutations were detected in TP53 (tumor protein p53), CDK4/6 (cyclin-dependent kinase 4/6), and STK11 (serine/threonine kinase 11) genes (Figure 2). In the C group, the most common mutation was also L858R (75.9%, 41/54), and the most common accompanying mutations were TP53, PI3KCA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha) point mutations, MET (MET proto-oncogene, receptor tyrosine kinase), and EGFR amplification (Figure S1).

Efficacy/toxicity evaluation
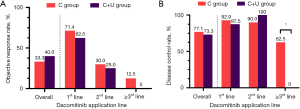
Among the 15 evaluable patients (3 patients could not be evaluated due to lack of target lesions) in the C+U group, 6 (40%), 5 (33.3%) and 4 (26.7%) had PR, SD, and de novo resistance to dacomitinib with PD as the best response, respectively. The objective response rate (ORR) was 40% (6/15) and the disease control rate (DCR) was 73.3% (11/15) (Figure 2A). At the data cut-off date, the median PFS (mPFS) was 7.5 months (95% CI, 4.4–10.6 months), and the median follow-up duration was 8.6 months (95% CI, 3.4–13.8 months) in the C+U group. The PFS was mature in 11 (61.1%) patients, and the tumors of seven patients were still under control (Figure 2B). In contrast, among the 48 evaluable patients (6 patients could not be evaluated due to lack of target lesions) in the C group, the ORR was 33.3% (16/48), and the DCR was 77.1% (37/48) (Figure S1). At the data cut-off date, the mPFS was 6.1 months (95% CI, 4.3–7.9 months), and the median follow-up duration was 10.5 months (95% CI, 8.1–12.9 months) in the C group. However, there were no significant differences in ORR (P=0.636) (Figure 3), DCR (P=0.765) (Figure 3), PFS (P=0.889) (Figure 4), and OS (P=0.703) (Figure 4) between the C and C+U groups.

All patients had grade 1–2 adverse effects (AEs), but no grade 4–5 treatment-emergent AEs occurred. No significant differences were found in grade 1–3 AEs between the C and C+U groups (Table 2). In the C+U group, only one patient required a dosage reduction from 45 to 15 mg due to intolerable grade 3 rash, and one patient had a dosage increase from 30 to 45 mg due to good tolerance. In the C group, 2 patients required a dosage reduction from 45 to 15 mg due to intolerable grade 3 diarrhea or rash, and 3 patients had a dosage increase from 30 to 45 mg due to good tolerance.
Table 2
| AEs | G1 | G2 | G3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C group | C+U group | P value# | C group | C+U group | P value# | C group | C+U group | P value# | |||
| Diarrhea | 23 (42.6%) | 11 (61.1%) | 0.831 | 7 (13.0%) | 4 (22.2%) | 0.668 | 1 (1.9%) | 0 | 0.736 | ||
| Rash | 22 (40.7%) | 7 (38.9%) | 11 (20.4%) | 5 (27.8%) | 4 (7.4%) | 2 (11.1%) | |||||
| Oral mucositis | 15 (27.8%) | 4 (22.2%) | 7 (13.0%) | 2 (11.1%) | 1 (1.9%) | 1 (5.6%) | |||||
| Dry skin | 12 (22.2%) | 4 (22.2%) | 4 (7.4%) | 3 (16.7%) | 0 | 0 | |||||
| Paronychia | 9 (16.7%) | 5 (27.8%) | 11 (20.4%) | 2 (11.1%) | 1 (1.9%) | 0 | |||||
Data are n (%). There were no grade 4–5 treatment-emergent AEs. #, the chi-square test was employed for the comparative analysis. AEs, adverse events; C group, common mutations group; C+U group, common mutations combined with uncommon mutations group.
Survival analysis
Stratified analyses
Given the significant differences of baseline characteristics in the application line of dacomitinib between the C and C+U groups, we conducted stratified analyses on ORR, DCR, PFS, and OS based on different application lines. The total ORR (33.3% vs. 40.0%, P=0.636) and the DCR (77.1% vs. 73.3%, P=0.089) of the C group were not significantly different compared with those of the C+U group (Figure 3). However, stratified results showed that the ORR gradually decreased as the application line moved back. The C+U group showed a worse treatment response than the C group in all application line subgroups (Figure 3A), although the difference was not statistically significant. Patients in the C+U group receiving third-line dacomitinib had a significantly lower DCR than those in the C group (P=0.040) (Figure 3B). In the survival analysis, both the PFS (P=0.889) (Figure 4A) and OS (P=0.703) (Figure 4B) of the C group were not significantly different compared with those of the C+U group. However, stratified analyses demonstrated that the PFS of the C+U group was worse than that of the C group when receiving first-line (P=0.047) (Figure 4) or ≥ third-line (P=0.018) (Figure 4) dacomitinib treatment, and the OS of the C+U group was worse than that of the C group when receiving ≥ third-line treatment (P=0.003) (Figure 4).
Univariate and multivariate analyses
To determine the effect of the compound mutation status on prognosis, we conducted univariate and multivariate analyses (Table 3) for the whole cohort (C group plus C+U group). In the univariate analysis, age (P=0.037), smoking status (P=0.033), total tumor burden (P=0.011), and administration of dacomitinib (P<0.001) were all statistically significant prognostic factors for PFS, while only the application line of dacomitinib (P<0.001) was a statistically significant prognostic factor for OS. In the multivariate analysis, smoking status (HR =2.541, 95% CI: 1.069–6.040; P=0.035), brain metastases (HR =0.467, 95% CI: 0.232–0.946; P=0.035), and application line of dacomitinib (HR =5.049, 95% CI: 1.694–15.050; P=0.004) were independent predictors of PFS, whereas only smoking status (HR =5.971, 95% CI: 1.118–31.888; P=0.037) and compound mutation status (HR =5.405, 95% CI: 1.096–26.316; P=0.038) were independent predictors of OS (Table 3).
Table 3
| Variables | n | Univariate analysis* | Multivariate analysis# | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS (months) | OS (months) | PFS (months) | OS (months) | |||||||||||
| Median | P value | Average | P value | HR | 95% CI | P value | HR | 95% CI | P value | |||||
| Complex mutation status | ||||||||||||||
| C group/C+U group | 54/18 | 7.5/6.1 | 0.889 | 18.8/15.3 | 0.702 | 5.405 | 1.096–26.316 | 0.038 | ||||||
| Age | ||||||||||||||
| ≤60/>60 years | 36/36 | 8.2/5.4 | 0.037 | 20.8/14.4 | 0.152 | |||||||||
| Gender | ||||||||||||||
| Male/female | 33/39 | 6.0/7.5 | 0.230 | 19.2/15.5 | 0.927 | |||||||||
| Smoking | ||||||||||||||
| No/yes | 48/24 | 7.5/5.4 | 0.033 | 20.2/14.9 | 0.076 | 2.541 | 1.069–6.040 | 0.035 | 5.971 | 1.118–31.888 | 0.037 | |||
| ECOG PS | ||||||||||||||
| 0–1/2–4 | 63/9 | 6.8/6.0 | 0.118 | 19.2/9.8 | 0.144 | |||||||||
| Disease stage | ||||||||||||||
| III+IV/recurrence | 58/14 | 6.0/9.8 | 0.121 | 18.5/16.9 | 0.460 | |||||||||
| Tumor burden | ||||||||||||||
| <3/≥3 metastatic organs | 60/12 | 7.5/3.7 | 0.011 | 19.4/9.9 | 0.142 | |||||||||
| EGFR mutation subtypes | ||||||||||||||
| 19del/L858R | 15/57 | 6.4/6.8 | 0.412 | 19.5/17.4 | 0.850 | |||||||||
| Brain metastases | 2.141 | 1.057–4.329 | 0.035 | |||||||||||
| No/yes | 40/32 | 8.5/5.6 | 0.206 | 17.8/17.8 | 0.459 | |||||||||
| Initial dosage of dacomitinib | 0.719 | 0.906 | ||||||||||||
| 15 mg | 12 | 4.2 | 13.9 | |||||||||||
| 30 mg | 44 | 7.5 | 19.2 | |||||||||||
| 45 mg | 16 | 6.1 | 18.0 | |||||||||||
| Treatment line of dacomitinib | <0.001 | <0.001 | 0.004 | |||||||||||
| 1st line | 24 | 9.8 | 11.3 | – | – | – | ||||||||
| 2nd line | 15 | 9.4 | 12.5 | 1.336 | 0.427–4.182 | 0.618 | ||||||||
| ≥3rd line | 33 | 3.8 | 10.7 | 5.049 | 1.694–15.050 | 0.004 | ||||||||
All variables were included in the multivariate analysis, but only statistically significant results were demonstrated. Set variables before the “/” as reference. *, the log-rank test was employed for the comparative analysis. #, the cox’s proportional hazards regression model was used to analyze the influencing factors of PFS and OS. C group, common mutations group; C+U group, common mutations combined with uncommon mutations group; PFS, progression-free survival; OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status.
Discussion
According to previous studies, the incidence of complex EGFR mutations can be as high as 26%, which may be attributed to the progressive improvements in detection technology (4,9,10). Based on the combination mode and the incidence of different types of uncommon mutations, complex EGFR mutations can be roughly divided into the following four main types: (I) a combination of uncommon and common mutations (such as E709K + L858R) (C+U group in our study), (II) double major uncommon mutations (such as G719X + S768I), (III) major uncommon mutations combined with non-major uncommon mutations (such as G719X + R108K), and (IV) double common mutations (such as 19del + L858R) (1,3,15-18). In our study, we focused on the first type of complex EGFR mutations. To the best of our knowledge, there are only a few reports on the efficacy of dacomitinib on treating complex EGFR mutations, and the influence of complex mutations on prognosis remains elusive. Our study revealed that patients with NSCLC harboring uncommon mutations combined with common mutations (C+U group) were less responsive to treatment with dacomitinib than those harboring common mutations (C group).
Currently, EGFR-TKIs have the best therapeutic outcomes for common mutations, with the outcomes for uncommon mutations being generally inferior to common mutations (19,20), except for some sensitive major uncommon mutations (including G719X, L861Q, and S768I) (21). Compound mutations imply the presence of heterogeneous clones (both primary and secondary) within the tumor and heterogeneity in the therapeutic response. It is taken for granted that the primary clone determines the outcome of treatment, but according to previous studies (22,23), the treatment response does not depend exclusively on the primary clone but mainly on the concomitant mutations (secondary clone). When a common or major uncommon mutation is combined with a sensitive uncommon mutation (such as L858R + G719C, G719C + S768I), the therapeutic effect is not necessarily affected (19). However, when it is combined with a resistant uncommon mutation (such as L858R + Q787R, L858R + H870R, and G719C + E709K), the therapeutic effect is generally diminished (22).
Keam et al. (19) found no significant difference in ORR (74.8% vs. 68.8%) and mPFS (11.9 vs. 8.1 months) between the common mutations group (n=16) and the group of combined uncommon and common mutations (n=16) who received either gefitinib or erlotinib. However, Hata et al. (24) revealed that patients who mainly received gefitinib harboring 19del and L858R had a better ORR (86%, 6/7) than those that carried uncommon mutations combined with common mutations (40%, 2/5). No statistical significance was detected (P=0.222), although the mPFS was longer in the former group (16.5 vs. 3.8 months) (P=0.046). By comparing NSCLC patients harboring common EGFR mutations (n=97), Tan et al. (3) revealed that treatment outcomes of patients harboring uncommon mutations combined with common mutations (n=52, similar to the first complex mutations type defined in our study), complex uncommon mutations (n=22, as the combination of the second and third complex mutations types defined in our study), and uncommon mutations treated with first-line gefitinib/erlotinib/icotinib or afatinib were significantly different (ORR: 76.3%, 61.5%, 54.5%, and 50.0%, P=0.023; mPFS: 13.3, 14.7, 8.1, and 6.0 months, P=0.004). We speculate that these conflicting results may arise from the study size and treatment context (e.g., number of lines treated) in the different studies.
In our study, contrary to the results of Tan et al. (3), patients in the C group harboring 19del or L858R had a better ORR (71.4%, 10/14) than those in the C+U group (62.5%, 5/8), although not statistically significant (P=0.665). In addition, the mPFS was also longer in the C group (not reached vs. 7.5 months; P=0.047). This suggests that the treatment disadvantages caused by uncommon mutations in the C+U group cannot be reversed by second-generation TKI dacomitinib. Besides, an in vitro study by Nishino et al. showed that, several uncommon EGFR mutations (including L718Q, L718V, L792H, and L792F) combined with L858R could lead to various responses when dacomitinib were administrated on Ba/F3 cells, and most combinations had worse responses than the single L858R did (25). Nevertheless, previous studies suggested that the second-generation TKI, afatinib, showed superior efficacy over gefitinib/erlotinib/icotinib in patients harboring uncommon mutations combined with common mutations (ORR: 100% vs. 54.5%, P=0.017; mPFS: not reached vs. 13.6 months, P=0.032) (1,3,17). Another point we should mention is that, in addition to second-generation TKI, limited evidence showed that the third-generation TKI osimertinib also had promising efficacy for EGFR uncommon mutations (2). However, the efficacy of osimertinib for compound EGFR mutations warrants more data to confirm.
Recently, several studies have shown that dacomitinib is potentially effective in EGFR-positive NSCLC with central nervous system metastasis, with the ORR ranging from 87.5–92.9% and DCR of 100% (26-29). Peng et al. demonstrated that a patient with brain metastases harboring G719A achieved an objective response, indicating a potential therapeutic effect in NSCLC patients harboring uncommon mutations with brain metastases (26). It is worth mentioning that, in our study, we did not observe any significant differences in the ORR (40% vs. 42.9%, P=0.893), DCR (90% vs. 71.4%, P=0.234), or mPFS (10.2 vs. 6.8 months, P=0.721) in NSCLC patients with brain metastases between the C and C+U groups. This shows that dacomitinib still has a good intracranial control ability in a population harboring a combination of common and uncommon mutations. To our knowledge, this is the first study to report the benefits of dacomitinib in this subset of patients.
An interesting finding in our study is that in the C+U group, the proportion of L858R is significantly higher than that of 19del. In fact, the previous study conducted by Hong et al. suggested that, compared with patients with 19del, those with L858R were more likely to incorporate other concomitant mutations and had worse survival (30). Wu et al. (1) reported that L858R was the predominant subtype contained in the complex EGFR mutation, which could possibly explain the phenomenon in our study. Besides, the TP53 mutation rate was 18.5% as we calculated in the C group, which was indeed relatively lower than that reported by other scholars (~ more than 30%) (31), we thought that this may contributed to the heterogeneity of the gene testing panels and the relatively small number of the study population.
The wide application of highly sensitive NGS technology and the liquid-based mutation detection analysis in clinical practice can identify a broader spectrum of uncommon EGFR mutations. Determining the precise treatment based on these mutations is the next challenge we must embrace.
A limitation of this study is the small sample size of the C+U group, which may make the comparison with the C group not strongly convincing. Second, as this is not a multi-center study, selection bias cannot be avoided. Therefore, our data should be interpreted with caution. Furthermore, the resistance mechanisms of dacomitinib have not been investigated thus far.
In conclusion, through limited cases, this real-world study revealed a worse response and prognosis of patients with NSCLC harboring complex EGFR mutations than those harboring common EGFR mutations when treated with dacomitinib. Nevertheless, according to the limited available evidence and our study results, second-generation EGFR-TKI dacomitinib is optional for this subset of patients, especially for those with brain metastases.
Acknowledgments
We would also like to thank Editage (www.editage.cn) for English language editing.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 82072590) and the Beijing Health Promotion Association (Grant No. 2021-053-ZZ).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1841/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1841/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1841/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Boards of the Chinese PLA General Hospital and the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (No. 18-070 and 1648). The Research Ethics Boards waived the need for informed consent as this was a retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu SG, Yu CJ, Yang JC, et al. The effectiveness of afatinib in patients with lung adenocarcinoma harboring complex epidermal growth factor receptor mutation. Ther Adv Med Oncol 2020;12:1758835920946156. [Crossref] [PubMed]
- Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020;38:488-95. [Crossref] [PubMed]
- Tan J, Hu C, Deng P, et al. The Predictive Values of Advanced Non-Small Cell Lung Cancer Patients Harboring Uncommon EGFR Mutations-The Mutation Patterns, Use of Different Generations of EGFR-TKIs, and Concurrent Genetic Alterations. Front Oncol 2021;11:646577. [Crossref] [PubMed]
- Passaro A, Mok T, Peters S, et al. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J Thorac Oncol 2021;16:764-73. [Crossref] [PubMed]
- Gristina V, Malapelle U, Galvano A, et al. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat Rev 2020;85:101994. [Crossref] [PubMed]
- Remon J, Hendriks LEL, Cardona AF, et al. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev 2020;90:102105. [Crossref] [PubMed]
- Huang CH, Ju JS, Chiu TH, et al. Afatinib treatment in a large real-world cohort of nonsmall cell lung cancer patients with common and uncommon epidermal growth factor receptor mutation. Int J Cancer 2022;150:626-35. [Crossref] [PubMed]
- Piotrowska Z, Wang Y, Sequist L, et al. ECOG-ACRIN 5162: A phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. J Clin Oncol 2020;38:9513. [Crossref]
- Kim EY, Cho EN, Park HS, et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 2016;17:237-45. [Crossref] [PubMed]
- Attili I, Passaro A, Pisapia P, et al. Uncommon EGFR Compound Mutations in Non-Small Cell Lung Cancer (NSCLC): A Systematic Review of Available Evidence. Curr Oncol 2022;29:255-66. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Jänne PA, Boss DS, Camidge DR, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 2011;17:1131-9. [Crossref] [PubMed]
- Reckamp KL, Giaccone G, Camidge DR, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer 2014;120:1145-54. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Brindel A, Althakfi W, Barritault M, et al. Uncommon EGFR mutations in lung adenocarcinoma: features and response to tyrosine kinase inhibitors. J Thorac Dis 2020;12:4643-50. [Crossref] [PubMed]
- Chiu CH, Yang CT, Shih JY, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Jung HA, Park S, Sun JM, et al. Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations? Biology (Basel) 2020;9:326. [Crossref] [PubMed]
- Tsai MJ, Hung JY, Lee MH, et al. Better Progression-Free Survival in Elderly Patients with Stage IV Lung Adenocarcinoma Harboring Uncommon Epidermal Growth Factor Receptor Mutations Treated with the First-line Tyrosine Kinase Inhibitors. Cancers (Basel) 2018;10:434. [Crossref] [PubMed]
- Keam B, Kim DW, Park JH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol 2014;19:594-600. [Crossref] [PubMed]
- Baek JH, Sun JM, Min YJ, et al. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: a retrospective analysis in Korea. Lung Cancer 2015;87:148-54. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Tam IY, Leung EL, Tin VP, et al. Double EGFR mutants containing rare EGFR mutant types show reduced in vitro response to gefitinib compared with common activating missense mutations. Mol Cancer Ther 2009;8:2142-51. [Crossref] [PubMed]
- Shen YC, Tseng GC, Tu CY, et al. Comparing the effects of afatinib with gefitinib or Erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer 2017;110:56-62. [Crossref] [PubMed]
- Hata A, Yoshioka H, Fujita S, et al. Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol 2010;5:1524-8. [Crossref] [PubMed]
- Nishino M, Suda K, Kobayashi Y, et al. Effects of secondary EGFR mutations on resistance against upfront osimertinib in cells with EGFR-activating mutations in vitro. Lung Cancer 2018;126:149-55. [Crossref] [PubMed]
- Peng W, Pu X, Jiang M, et al. Dacomitinib induces objective responses in metastatic brain lesions of patients with EGFR-mutant non-small-cell lung cancer: A brief report. Lung Cancer 2021;152:66-70. [Crossref] [PubMed]
- Zhang J, Wang Y, Liu Z, et al. Efficacy of dacomitinib in patients with EGFR-mutated NSCLC and brain metastases. Thorac Cancer 2021;12:3407-15. [Crossref] [PubMed]
- Mizusaki S, Otsubo K, Ninomiya T, et al. Remarkable response to dacomitinib in a patient with leptomeningeal carcinomatosis due to EGFR-mutant non-small cell lung cancer. Thorac Cancer 2021;12:114-6. [Crossref] [PubMed]
- Kudo K, Kawakado K, Kawajiri T, et al. Dramatic Response of Brain Metastasis from EGFR-mutation-positive NSCLC to Dacomitinib. Intern Med 2020;59:1739-40. [Crossref] [PubMed]
- Hong S, Gao F, Fu S, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:739-42. [Crossref] [PubMed]
- Yu HA, Suzawa K, Jordan E, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res 2018;24:3108-18. [Crossref] [PubMed]


