
Effects of Kangdaxin on myocardial fibrosis in heart failure with preserved ejection fraction rats
Introduction
Myocardial fibrosis is a pathological process characterized by excessive deposition of extracellular matrix (ECM) and imbalance of collagen composition after myocardial injury (1,2). It is closely related to a variety of heart diseases [myocardial infarction, chronic heart failure (CHF), and atrial fibrillation]. Studies have shown that excessive physiological stress can lead to myocardial inflammation, fibrosis, and myocardial injury (3,4). A member of mitogen activated protein kinases (MAPKs) family, P38 MAPK, can activate nuclear factor-κB (NF-κB) by phosphorylating mitogen and stress activated protein kinase 1 (MSK1). As the main regulator of inflammatory response, NF-κB induces the expression of pro-inflammatory factors such as TGF-β1, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β).
Among the pro-inflammatory factors, TGF-β1 is the main inducer of muscle fibrosis (5,6). It is a kind of multi-effect cytokine, which can promote cell proliferation and differentiation (7). Additionally, it can promote collagen synthesis and inhibit collagen degradation. In myocardial fibrosis, TGF-β1 is considered the strongest fibrosis-promoting growth factor, which can promote the secretion of myocardial fibrosis cells, accumulation of ECM, and up-regulate the expression of nicotinamide adenine nucleotide phosphate (NADPH) oxidase, and mediate the production of inflammatory factor interleukin-6 (IL-6) (8). In these ways, TGF-β1 promotes the progression of myocardial fibrosis. In the TGF-β1 signal pathway, Smads protein is the main effector molecule in the middle and downstream of the signal pathway, The TGF-β1 receptor regulates the expression of related genes by phosphorylating Smad protein and promotes the development of fibrosis (9,10).
Heart failure with preserved ejection fraction (HFpEF), which represents more than 50% of total heart failure, is a clinical syndrome characterized by impaired diastolic function, the incidence of which is continually increasing (11). As a result, how to effectively slow down and prevent the progression of HFpEF has become a major challenge in the treatment of CHF (12). In this study, a model of rat with HFpEF was established to observe the protective effect of Kangdaxin oral liquid on myocardial fibrosis and diastolic function of rats with HFpEF.
In this study, we firstly demonstrated that Kangdaxin significantly inhibited the myocardial tissue injury through suppressing the protein expression of TGF-β1. This study might provide a novel therapeutic target for the prevention and treatment of myocardial fibrosis in heart failure. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-198/rc).
Methods
Specific-pathogen free (SPF) grade Sprague Dawley (SD) rats (Male, 5–6 weeks, 150–200 g) were provided by Experimental Animal Center of Fujian University of Traditional Chinese Medicine. All animal experiments followed animal ethics norms. Kangdaxin oral liquid, the main composition of which includes monkshood, milkvetch root, tansymustard seed, Chinese Angelica, and polyporus umbellatus, is preparation of the Affiliated People’s Hospital of Fujian University of Traditional Chinese Medicine. The first and second antibody of TGF-β1 were purchased from Cell Signaling Technology (CST; Beverly, MA, USA). AN-terminal pro b-type natriuretic peptide (NT-proBNP) detection kit was purchased from Vigene Biosciences (Rockville, MD, USA). Small animal ventilator (TKR-200C) was produced by Xiamen Xinrui Instrument Co., Ltd. (Xiamen, China). The small animal ultrasound imaging system was produced by Visual Sonics Company (Toronto, ON, Canada). All animal protocols have been approved by the Animal Ethical Committee of People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine (No. 2021-16), and all experiments were performed in accordance with the guidelines for the laboratory animal use.
HFpEF model
After anesthesia, the abdominal walls of rats were cut open, and the 2 abdominal walls were pulled apart with retractors made of paper clips. Then, the small intestine was removed with sterile cotton swabs to enable a view of the abdominal aorta area, and then the connective tissue covering the abdominal aorta was removed to expose the abdominal aorta. A segment of the abdominal aorta was captured with a hooked glass minute needle just above the right renal artery. The abdominal aorta was then ligated, and the syringe needle was retracted with the surgical line, causing coarctation of the abdominal aorta in its inner diameter at the ligation point (the inner diameter of the abdominal aorta after recanalization at the ligation point was the outside diameter of the needle). The small intestine was replaced, 2% penicillin/streptomycin solution was dropped into the abdominal cavity, and the abdomen was closed. In 12 weeks after surgery, HFpEF rat model was established.
Sham operation model
After anesthesia, the abdominal walls of Sham group rats were cut along the median line to expose the abdominal aorta, but the abdominal aorta was not ligated.
After weighing, 30 SD rats were randomly divided into 3 groups: Sham operation group (Sham), HFpEFgroup (HFpEF), and HFpEF with drug intervention group (HFpEF + I). Rats in HFpEF + I group were given Kangdaxin oral liquid at a dose of 2.7 mL/(kg·d), the dose of Kangdaxin was determined based on our pre-experiments; rats in the Sham and HFpEF groups were given equal doses of normal saline. The 3 groups of rats were given the drug by gavage for 12 weeks.
Evaluation of diastolic function
After 12 weeks, color Doppler echocardiography was used to measure the value of E/A and E/e' in each group of rats to evaluate cardiac diastolic function. The NT-proBNP level of rats in each group were determined by enzyme-linked immunosorbent assay (ELISA).
Measurement of Hw/W and LVw/W
Rat was sacrificed after weighing, followed by immediate removal of the heart. After weighing the heart and left ventricle separately, the index of heart weight/body weight (Hw/W) and left ventricular weight/body weight index (LVw/W) were calculated.
Assessment of myocardial fibrosis
Cardiac tissue was taken out at a size of approximately 1 mm3 and fixed in 10% formalin solution immediately. After 24 h, paraffin-embedded tissue sections were subjected to hematoxylin and eosin (HE) staining, and the contents and distribution of collagen fibers in myocardial tissue were observed to determine the degree of myocardial fibrosis.
Western blot analysis
Total protein was extracted from the heart tissues of rats in each group, and the protein concentration was measured by bicinchoninic acid (BCA), followed by blocking for 1 h in Tris-buffered saline and Tween-20 (TBST) containing 5% bovine serum albumin (BSA). The membranes were washed in TBST and probed with the following primary antibodies overnight at 4 ℃: rabbit polyclonal antibody to TGF-β1, goat polyclonal antibody to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The membranes were then incubated with the secondary antibody and visualized by enhanced chemiluminescence (ECL) according to the manufacturer’s instructions. We used GAPDH as the internal loading control. For quantification, the Image-ProPlus 6.0 software (Media Cybernetics, Rockville, MD, USA) was used to measure the integrated optical density (IOD) of the bands. The relative protein levels were expressed as the ratio to the levels of GAPDH. The protein expression levels of TGF-β 1 in the heart tissues of rats in each group were detected.
Statistical analysis
The software SPSS19.0 (IBM Corp., Armonk, NY, USA) was used to analyze the experimental results, and the data were expressed as mean ± standard deviation. Statistical analysis of data was performed with one-way analysis of variance (ANOVA). A P value <0.05 was considered statistically significant.
Results
Kangdaxin oral liquid improved diastolic function and reduces NT-proBNP level
Compared with the Sham group, the value of E/A in the HFpEF group was significantly decreased (1.16±0.05 vs. 0.69±0.07, P<0.05), but the value of E/e' was remarkably promoted (7.44±0.30 vs. 11.43±0.52, P<0.05) (Figure 1). However, compared with the HFpEF group, the value of E/A in the HFpEF + I group was significantly increased (0.69±0.07 vs. 0.94±0.05, P<0.05), but the value of E/e' (11.43±0.52 vs. 9.34±0.60, P<0.05) was markedly inhibited (Figure 1).

In addition, the level of NT-proBNP was significantly increased (5.36±0.18 vs. 55.14±1.93, P<0.05) compared with group HFpEF, but the level of NT-proBNP was significantly decreased (55.14±1.93 vs. 33.98±1.34, P<0.05) in the group HFpEF + I (Figure 2).
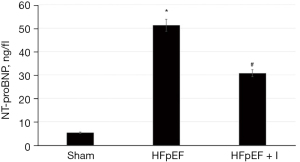
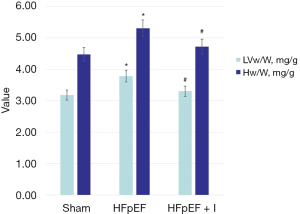
Effect of kangdaxin oral liquid on heart weight of rat with HFpEF
Compared with the Sham group, Hw/W and LVw/W values in the HFpEF group were significantly increased (4.46±0.20 vs. 5.36±0.33, P<0.05; and 3.17±0.22 vs. 3.78±0.16, P<0.05). Compared with the HFpEF group, Hw/W and LVw/W values in the HFpEF + I group were significantly decreased (5.36±0.33 vs. 4.72±0.12, P<0.05; and 3.78±0.16 vs. 3.33±0.09, P<0.05) (Figure 3).
Effect of Kangdaxin oral liquid on cardiac fibrosis of rat with HFpEF
The HE staining results were observed under the microscope. Compared with the Sham group, obvious myocardial fibrosis was shown in the HFpEF group, and in the HFpEF + I group, obvious improvement of myocardial fibrosis was observed compared with the HFpEF group (Figure 4A).

Compared with the Sham group, the expression of TGF-β1 protein in heart tissue of HFpEF group rats was significantly increased (1.07±0.45 vs. 6.22±0.13, P<0.05). Compared with the HFpEF group, the expression of TGF-β1 protein in the HFpEF + I group was significantly decreased (6.22±0.13 vs. 2.43±0.11, P<0.05) (Figure 4B,4C).
Discussion
Myocardial fibrosis, resulting in a decline in myocardial compliance, is an important pathophysiological mechanism of HFpEF. Especially in its early stage, myocardial fibrosis could exert more influence on the diastolic function (13). The abnormal increase and excessive deposition of ECM play a key role in the development of myocardial fibrosis (14). Among the many regulatory factors, the role of TGF-β1 in promoting myocardial fibrosis has been increasingly confirmed. The TGF-β1 promotes the occurrence and progress of myocardial fibrosis and HFpEF by regulating the function of cardiac fibroblasts and the metabolic balance of ECM (15).
According to the theory of traditional Chinese medicine (TCM), HFpEF belongs to the category of “dyspnea”, “edema due to dysfunction of heart”, and “chest discomfort”, the pathogenesis of which is based on root deficiency and branch excess. The heart is the monarch of all the organs, which governs the blood and vessels. Insufficiency of heart Yang might lead to stagnant blockade of heart blood and retention of phlegm-fluid. phlegm-fluid is a pathogen of Yin nature which can easily damage the body’s Yang Qi and lead to Yang deficiency of heart and kidney. In the case of Yang deficiency of the heart and kidney, body fluids cannot be transported normally, resulting in water pathogen insulting heart and lung, which produces symptoms such as shortness of breath, cough, and expectoration. Furthermore, these symptoms are often aggravated by several factors such as exogenous, internal injuries, and fatigue. Interestingly, our previous research found that the majority of HFpEF patients exhibited the syndrome of deficiency of heart Yang, blood stasis, and accumulation of phlegm-fluid (16). Kangdaxin oral liquid is an in-hospital preparation based on many years’ experience of the Affiliated People’s Hospital of Fujian University of Traditional Chinese Medicine, the preparation of which uses monkshood and milkvetch as monarch drugs to invigorate kidney and heart Yang, invigorate the spleen, and replenish Qi. Tansy mustard seed and polyporus umbellatus are used as official drugs to relieve asthma and promote diuresis. Chinese Angelica is used as an adjuvant drug to dredge blood vessels and promote blood circulation. Our previous studies had confirmed that Kangdaxin oral liquid could have significant effects in the treatment of CHF. Its potential mechanisms might include: reducing serum matrix metalloproteinase (MMP)-9 level in CHF patients, enhancing myocardial contractility, increasing cardiac output (17), inhibiting apoptosis, reducing TNF-a and IL-6 levels, improving cardiac remodeling, and so on (18). Based on the principle of “warming Yang and benefiting Qi, removing blood stasis for diuresis”, in the previous study, we applied Kangdaxin oral liquid to patients with HFpEF. The results demonstrated that on the basis of standard treatment, combined application of Kangdaxin oral liquid can reduce E/e', increase E/A value and significantly reduce serum level of NT-proBNP in HFpEF patients (19).
Conclusions
In this study, we established an HFpEF rat model and confirmed in vivo that Kangdaxin oral liquid could reduce the E/e' value, increase the E/A value of HFpEF rats, and significantly reduce the serum NT-proBNP level. Furthermore, we found that Kangdaxin oral liquid could reduce the expression of TGF-β1 in the heart tissue of HFpEF rats, which may be a possible mechanism for Kangdaxin oral liquid to inhibit myocardial fibrosis and improve cardiac diastolic function. Further study will be conducted to investigate the effect of Kangdaxin oral liquid on the signal pathway related to TGF-β1 in myocardial tissue, and to reveal the specific mechanism of Kangdaxin oral liquid in the treatment of HFpEF.
Acknowledgments
Funding: This study was funded by Natural Science Foundation of Fujian Province (No. 2018J01330); and Young and Middle-aged Scientific Researcher Training Project of Fujian Provincial Health and Family Planning Commission (No. 2017-ZQN-67).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-198/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-198/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-198/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal protocols have been approved by the Animal Ethical Committee of People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine (No. 2021-16), and all experiments were performed in accordance with the guidelines for the laboratory animal use.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yue Y, Meng K, Pu Y, et al. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract 2017;133:124-30. [Crossref] [PubMed]
- Wong CKS, Falkenham A, Myers T, et al. Connective tissue growth factor expression after angiotensin II exposure is dependent on transforming growth factor-β signaling via the canonical Smad-dependent pathway in hypertensive induced myocardial fibrosis. J Renin Angiotensin Aldosterone Syst 2018;19:1470320318759358. [Crossref] [PubMed]
- Wu Y, Chen S, Wen P, et al. PGAM1 deficiency ameliorates myocardial infarction remodeling by targeting TGF-β via the suppression of inflammation, apoptosis and fibrosis. Biochem Biophys Res Commun 2021;534:933-40. [Crossref] [PubMed]
- Blyszczuk P, Müller-Edenborn B, Valenta T, et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J 2017;38:1413-25. [PubMed]
- Khan SA, Dong H, Joyce J, et al. Fibulin-2 is essential for angiotensin II-induced myocardial fibrosis mediated by transforming growth factor (TGF)-β. Lab Invest 2016;96:773-83. [Crossref] [PubMed]
- Yao Y, Hu C, Song Q, et al. ADAMTS16 activates latent TGF-β, accentuating fibrosis and dysfunction of the pressure-overloaded heart. Cardiovasc Res 2020;116:956-69. [Crossref] [PubMed]
- Maity S, Muhamed J, Sarikhani M, et al. Sirtuin 6 deficiency transcriptionally up-regulates TGF-β signaling and induces fibrosis in mice. J Biol Chem 2020;295:415-34. [Crossref] [PubMed]
- Bhandary B, Meng Q, James J, et al. Cardiac Fibrosis in Proteotoxic Cardiac Disease is Dependent Upon Myofibroblast TGF -β Signaling. J Am Heart Assoc 2018;7:e010013. [Crossref] [PubMed]
- Khalil H, Kanisicak O, Prasad V, et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017;127:3770-83. [Crossref] [PubMed]
- Kong JC, Miao WQ, Wang Y, et al. ANO1 relieves pressure overload-induced myocardial fibrosis in mice by inhibiting TGF-β/Smad3 signaling pathway. Eur Rev Med Pharmacol Sci 2020;24:8493-501. [PubMed]
- Burrage MK, Hundertmark M, Valkovič L, et al. Energetic Basis for Exercise-Induced Pulmonary Congestion in Heart Failure With Preserved Ejection Fraction. Circulation 2021;144:1664-78. [Crossref] [PubMed]
- Yamamoto K, Origasa H, Suzuki Y, et al. Relation of risk factors with response to carvedilol in heart failure with preserved ejection fraction - a report from the Japanese Diastolic Heart Failure Study (J-DHF). J Cardiol 2014;63:424-31. [Crossref] [PubMed]
- Caetano F, Barra S, Faustino A, et al. Cardiorenal syndrome in acute heart failure: a vicious cycle? Rev Port Cardiol 2014;33:139-46. [Crossref] [PubMed]
- Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res 2009;19:47-57. [Crossref] [PubMed]
- Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 2009;81:474-81. [Crossref] [PubMed]
- Guo JJ, Qiao JF, Lin C, et al. Ameliorative Effects of Wenyanghuoxuelishui Method on the Cardiac Function and Myocardial Fibrosis of Ischemic Cardiomyopathy. Journal of Emergency in Traditional Chinese Medicine 2013;22:1309-11.
- Zheng F, Zheng BR, Lin C, et al. Changes of Matrix Metalloproteinase-9 in Patients with Chronic Heart Failure and the Effect of Kangdaxin. Chinese Journal of Geriatric Care 2012;10:57-9.
- Wu X, Xiong SQ, Zheng F, et al. Analysis on the effect of kangdaxin oral liquid on improving cardiac and renal function in patients with 2-type heart-kidney syndrome. Fujian Medical Journal 2016;38:88-90.
- Lin C, Zhan P, Li CY, et al. Intervention of warming yang and removing blood stasis for diuresis on Serum N-terminal Probrain Natriuretic Peptide and Left Ventricular Function in Heart Failure Patients with Normal Left Ventricular Ejection Fraction. Fujian Journal of Traditional Chinese Medicine 2016;47:11-2.
(English Language Editor: J. Jones)


