The efficacy and safety of Wulingsan modified formulas for chronic heart failure patients: a systematic review and meta-analysis
Introduction
Heart failure (HF) is the chronic phase of cardiovascular disease, which has high rates of incidence and mortality. Previous studies have indicated that approximately 64 million people suffer from HF (1,2), and the 5-year mortality rate exceeds 50% (3-5). Chronic heart failure (CHF), a persistent HF state, is an important geriatric syndrome. It also has a high rate of morbidity, mortality, and re-hospitalization (6,7) and results in a considerable economic burden on patients (8). Furthermore, its main symptoms, including fluid retention, dyspnoea, fatigue, and poor exercise tolerance, seriously impact the quality of life of patients (9). Despite the development of medical and cardiac resynchronization therapies, the clinical outcomes of CHF patients remain poor (10), and the costs will further exacerbate the economic burden on patients (8).
Wulingsan originates from “Treatise on Febrile Diseases”, which is a traditional Chinese formula composed of polyporus umbellatus, poria cocos, alisma orientalis, atractylodes macrocephala, and cinnamon twig (11,12). It has been reported that Wulingsan exerts protective and fluid balance effects in disorders such as CHF (13), obesity (14), vomiting and diarrhea (15) and dysuria (16). Wulingsan modified formulas (WMF) are made by adding and subtracting herbs from Wulingsan according to the clinical experience, with Wulingsan being the foundation of WMF. Studies have shown that WMF can significantly improve the heart function of patients with CHF and relieve clinical symptoms (17,18).
Due to the high incidence of CHF and its poor therapeutic effect, WMF is expected to become an adjuvant drug for CHF patients. It may relief the huge physical and mental pressure from the chronic illness. However, the efficacy and safety of WMF remain uncertain although some researchers report the positive curative effects (19,20). The clinical application of WMF for CHF is still lacking evidence-based medical analysis. Our study will be the first meta-analysis to assess the efficacy and safety of WMF for CHF patients, in order to provide clinical decision-making recommendations. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-261/rc).
Methods
Search strategy
We performed a literature search of the PubMed, EMBASE, Cochrane library, Web of Science, Medline (Ovid), China National Knowledge Infrastructure (CNKI), WanFang, China Science and Technology Journal, and SinoMed databases for articles about the relationship between CHF and the Wulingsan published from the date of inception of the database to the 1st November, 2021.
The specific retrieval strategies applied for PubMed were as follows:
- #1 chronic heart failure /exp
- #2 ((heart* or cardiac* or myocard*) adj2 (fail* or insuff*)).tw.
- #3 (heart* adj2 decomp*).tw.
- #4 (chf or hf).tw.
- #5 or/1-4
- #6 wulingsan /exp
- #7 wulingsan*.tw.
- #8 or/6-7
- #9 #5 and #8
Inclusion criteria
The inclusion criteria were as follows: (I) all patients were diagnosed as CHF; (II) randomized controlled trial (RCT); and (III) studies involving WMF as a main treatment intervention. Articles involving the combination of WMF and other regular treatments compared with those same other regular treatments alone were also included. Regular treatments were included Oxygen inhalation, captopril, furosemide, metoprolol and etc.
Exclusion criteria
The exclusion criteria were as follows: (I) duplicate articles; (II) animal experiments, conference summaries, case reports, and reviews; (III) studies from which no data could be extracted; and (IV) articles lacking sufficient information on baseline or primary/secondary outcome data.
Primary outcome
Efficacy of WMF in the treatment of CHF.
Secondary outcomes
- Any brain natriuretic peptide (BNP) changes;
- Any left ventricular ejection fractions (LVEF) changes; and
- Any changes to the patients’ condition.
- Any reports about the adverse events.
Data extraction
Two reviewers (ZL and LR) independently evaluated all of the retrieved documents and analyzed the data according to the inclusion/exclusion criteria. In cases of disagreement, the final results were discussed between the two researchers. In addition, the third reviewer (CZ) also helped to resolve any differences. The contents of data extraction included the basic characteristics of the included studies.
Bias risk assessment
Two authors (ZL and LR) independently assessed the risk of bias using the Cochrane Handbook Risk of Bias Assessment Tool (21). Disagreements between the authors were resolved by the third author through consensus.
Statistical analysis
Statistical analyses were conducted using the software RevMan 5.3 (Cochrane Collaboration, Oxford, UK) (22) and STATA version 15.0 (Stata Corp, College Station, TX, USA). The risk ratio (RR) was applied to evaluate efficiency, and the weighted mean difference (WMD) and 95% confidence interval (CI) were used to merge the continuous variables. The I2 statistic was used to assess the heterogeneity between the included studies; the random effect model was used when I2>50% (23). Sensitivity analysis was conducted to assess whether a single article result affected the overall conclusion.
Results
Literature search
In total, 669 related studies were retrieved in the initial search. According to the inclusion and exclusion criteria, 29 studies were included for full-text consideration. Finally, 19 studies were included for meta-analysis (Figure 1).
Characteristics of the study
Nineteen articles were included in this meta-analysis, and their characteristics are displayed below (Table 1).
Table 1
| Study | Experimental group | Control group | Research designs | |||||
|---|---|---|---|---|---|---|---|---|
| Average age | No. | Treatment method | Average age | No. | Treatment method | |||
| Wang 2010 (19) | 62.3±12.58 | 35 | WMF + regular treatment (oxygen inhalation, captopril, furosemide, metoprolol) | 63.6±11.37 | 35 | Regular treatment (oxygen inhalation, captopril, furosemide, metoprolol) | RCT | |
| Wang 2011 (20) | N/A | 44 | WMF + regular treatment (oxygen inhalation, dexamethasone, cedilanid, metoprolol) | N/A | 42 | Regular treatment (oxygen inhalation, dexamethasone, cedilanid, metoprolol) | RCT | |
| Ning 2012 (24) | 61.36±11.65 | 70 | WMF + regular treatment (Isosorbide mononitrate, spironolactone, digoxin) | 63.33±7.16 | 70 | Regular treatment (Isosorbide mononitrate, spironolactone, digoxin) | RCT | |
| Huang 2013 (25) | 63.51±6.21 | 48 | regular treatment (oxygen inhalation, hydrochlorothiazide, cedilanid or digoxin) | 62.83±6.52 | 48 | Regular treatment (oxygen inhalation, hydrochlorothiazide, cedilanid or digoxin) | RCT | |
| Shen 2013 (26) | 63±15.2 | 12 | WMF + regular treatment (dobutamine, dobutamine, paracetamol) | 62±4.51 | 12 | Regular treatment (dobutamine, dobutamine, paracetamol) | RCT | |
| Zhou 2014 (27) | 63.08±5.74 | 38 | WMF + regular treatment | 64.83±6.07 | 38 | Regular treatment | RCT | |
| Yang 2014 (28) | 62.1+5.9 | 35 | WMF + regular treatment (comprehensive treatment of angiotensin converting enzyme inhibitors and B receptor blockers) | 62.7±6.1 | 35 | Regular treatment (comprehensive treatment of angiotensin converting enzyme inhibitors and B receptor blockers) | RCT | |
| Qing 2015 (29) | 54.5 | 41 | WMF + regular treatment (digoxin, furosemide) | 53.9 | 37 | Regular treatment (digoxin, furosemide) | RCT | |
| Cao 2016 (30) | 66.87±9.89 | 26 | WMF + regular treatment (captopril, metoprolol) | 67.98±10.32 | 26 | Regular treatment (captopril, metoprolol) | RCT | |
| Liu 2017 (31) | 69.2 | 60 | WMF + regular treatment (valsartan hydrochlorothiazide, antiplatelet drugs) | 66.2 | 60 | Regular treatment (valsartan hydrochlorothiazide, antiplatelet drugs) | RCT | |
| Yi 2017 (32) | 79.2±7.9 | 46 | WMF + regular treatment | 75.8±8.5 | 46 | Regular treatment | RCT | |
| Su 2017 (33) | N/A | 30 | WMF + regular treatment | N/A | 30 | Regular treatment | RCT | |
| Hong 2018 (34) | 68.2±6.6 | 41 | WMF + regular treatment (betaloc, candesartan) | 68.1±7.0 | 41 | Regular treatment (betaloc, candesartan) | RCT | |
| Chen 2019 (35) | 62.7±0.4 | 49 | WMF + regular treatment (benazepril, spironolactone, furosemide, metoprolol, trimetazidine) | 62.5±0.6 | 49 | Regular treatment (benazepril, spironolactone, furosemide, metoprolol, trimetazidine) | RCT | |
| Peng 2019 (36) | 62.1±5.8 | 90 | WMF + regular treatment (furosemide) | 62.7±6.1 | 90 | Regular treatment (furosemide) | RCT | |
| Tang 2020 (37) | 71.5 | 5l | WMF + regular treatment (valsartan) | 70.3 | 5l | Regular treatment (valsartan) | RCT | |
| Wang 2020 (38) | 57.29±4.33 | 34 | WMF + regular treatment (spironolactone, isosorbide mononitrate, digoxin) | 57.31±4.11 | 34 | Regular treatment (spironolactone, isosorbide mononitrate, digoxin) | RCT | |
| Hu 2021 (39) | 60.27±5.03 | 46 | WMF + regular treatment (trimetazidine, spironolactone, furosemide, irbesartan, isosorbide mononitrate, metoprolol) | 60.91±4.82 | 46 | Regular treatment (trimetazidine, spironolactone, furosemide, irbesartan, isosorbide mononitrate, metoprolol) | RCT | |
| Li 2021 (40) | 61.71±3.17 | 44 | WMF + regular treatment (Isosorbide mononitrate, spironolactone, digoxin) | 61.59±2.86 | 43 | Regular treatment (Isosorbide mononitrate, spironolactone, digoxin) | RCT | |
WMF, Wulingsan modified formulas; RCT, randomized controlled trial.
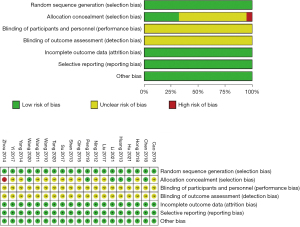
Risk of bias
The risk of bias assessment of the 19 included studies was summarized in Figure 2. All the studies described random sequence generation, attrition bias and reporting bias. None of the studies described performances or detection biases. One study (27) exhibited a high risk of bias allocation concealment.
Efficiency
Eighteen studies reported on the efficiency of WMF in the treatment of CHF. The meta-analysis results showed that WMF was effective in the treatment of CHF (RR =1.21, 95% CI: 1.15, 1.27, P<0.00001) and heterogeneity I2=3%. The funnel plot displayed bilateral asymmetry, which indicated potential publication bias (Figure 3A,3B).
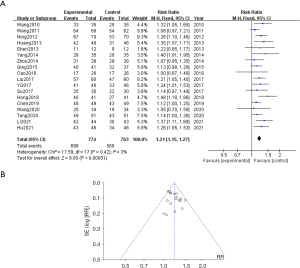
BNP
Seven studies reported on BNP. The meta-analysis results showed that WMF could decrease BNP in CHF patients (WMD =−269.14, 95% CI: −349.25, −189.04, P<0.00001) and heterogeneity I2=99%. Sensitivity analysis showed that no single article affected the overall analysis results, and all of the included studies were within the acceptable range (see Figure 4A,4B).
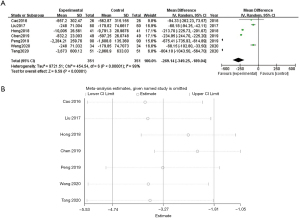
LVEF
Eight studies reported on the LVEF. The meta-analysis results showed that WMF could increase the LVEF in CHF patients (WMD =8.80, 95% CI: 5.93, 11.68; P<0.00001) and heterogeneity I2=96%, and the data was statistically significant. Sensitivity analysis showed that no single article affected the overall analysis results, and all of the included studies were within the acceptable range (see Figure 5A,5B).
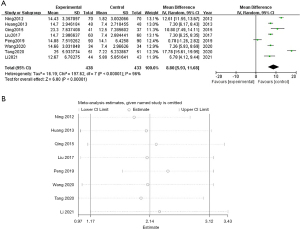
Adverse events
Two studies reported on adverse events. The meta-analysis results showed that (RR =0.57, 95% CI: 0.27, 1.17; P=0.13) and heterogeneity I2=52%, and the data was not statistically significant. This indicated that there was no difference in the incidence of adverse events between the experimental and control groups, suggesting that WMF was safe for CHF patients (Figure 6).
Discussion
Summary of the main findings
In this study, efficacy, BNP, LVEF, and adverse events were included to assess the effectiveness and safety of WMF. The meta-analysis results were as follows: efficiency, RR =1.21, 95% CI: 1.15, 1.27; BNP, WMD =−269.14, 95% CI: −349.25, −189.04; and LVEF, WMD =8.80, 95% CI: 5.93, 11.68. All of these findings were statistically significant. We also found that there was no statistically significant difference in the incidence of adverse events between the experimental and control groups (RR =0.57, 95% CI: 0.27, 1.17; P=0.13). The above results indicate that WMF is a safe and effective treatment for CHF.
Implications for clinical practice and further research
Despite the continuous development of anti-CHF drugs in recent years, the hospitalization and mortality rates of CHF patients remain high. Therefore, there is a pressing need to identify new therapeutic targets, so as to improve the prognosis of CHF patients. There is increasing evidence that impaired reduction of BNP levels is associated with a reduced risk of hospitalization due to worsening CHF (41).
Wulingsan, a classic prescription of traditional Chinese medicine, has the effect of warming yang to eliminate wetnessevil and removing dampness. Studies have demonstrated that WMF can protect myocytes and reduce cardiac preload by reducing the level of endothelin and BNP in patients with CHF (42). It can also improve the LVEF and left ventricular end diastolic volume in patients with CHF (43). However, individual studies have not provided sufficient evidence, and the role of WMF for CHF patients remains controversial. This meta-analysis found that WMF was effective for improving heart function, reducing BNP levels, and increasing the LVEF. Our study is the first meta-analysis to assess the effectiveness and safety of WMF in the treatment of CHF, which can provide clinical decision-making recommendations for the application of WMF. In the future, more large-scale and high-quality RCTs need to be conducted to obtain more accurate analysis results.
Limitations
This study had some limitations that should be taken into consideration. Firstly, the strength of evidence in this study was limited due to the risk of bias in the included studies. Secondly, most of the studies included in this review involved WMF instead of Wulingsan, which results in considerable clinical heterogeneity. Third, considering with the risk of bias in this study, clinical implications should be made according to the actual medical conditions. Finally, we conducted subgroup analysis according to age, course of disease, etc., which did not resolve the significant heterogeneity between the included studies. Although we adopted the random effects model, the heterogeneity may have still affected the quality of the evidence.
Conclusions
WMF is a reasonable and relatively safe adjuvant therapy for the treatment of CHF. However, more RCTs are needed to evaluate whether WMF is effective in the treatment of CHF.
Acknowledgments
Funding: This project was supported in part by the National Key Research and Development Program of China (No.2019YFC1708501), the Fundamental Research Funds for the Central Public Welfare Research Institutes (No.YZ-202026), and the Jiangsu College graduate research and innovation projects (No. SJCX21-0753).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-261/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-261/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020;324:488-504. [Crossref] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. [Crossref] [PubMed]
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30-41. [Crossref] [PubMed]
- Francis GS. Neurohormonal control of heart failure. Cleve Clin J Med 2011;78:S75-9. [Crossref] [PubMed]
- Hu SS, Gao RL, Liu LS, et al. Summary of the 2018 Report on Cardiovascular Diseases in China. Chinese Circulation Journal 2019;34:209-20.
- Wu Y, Li P, Wu H. Rehospitalization Rate of Chronic Heart Failure. Advances in Cardiovascular Diseases 2021;42:691-4.
- Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res 2016;119:159-76. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016;68:1476-88. [Crossref] [PubMed]
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368-78. [Crossref] [PubMed]
- Senni M, Adamo M, Metra M, et al. Treatment of functional mitral regurgitation in chronic heart failure: can we get a 'proof of concept' from the MITRA-FR and COAPT trials?. Eur J Heart Fail 2019;21:852-61. [Crossref] [PubMed]
- Ahn YM, Cho KW, Kang DG, et al. Oryeongsan (Wulingsan), a traditional Chinese herbal medicine, induces natriuresis and diuresis along with an inhibition of the renin-angiotensin-aldosterone system in rats. J Ethnopharmacol 2012;141:780-5. [Crossref] [PubMed]
- Jin WR, Zhang FE, Diao BZ, et al. Clinical Outcomes of Wulingsan Subtraction Decoction Treatment of Postoperative Brain Edema and Fever as a Complication of Glioma Neurosurgery. Evid Based Complement Alternat Med 2016;2016:5078689. [Crossref] [PubMed]
- He H, Wang J. Research progress of Wulingsan in the treatment of chronic heart failure. World Journal of Integrated Traditional and Western Medicine 2017;12:589-92.
- Xue XR, Xue JJ, Cui X, et al. Wulingsan (Oryeongsan/Goreisan) ameliorate lipid metabolism of obesity rats via regulation of the plasma metabolic profiling. Biotechnol Appl Biochem 2019;66:654-63. [Crossref] [PubMed]
- Ahmed S, Uchida R, Hussain M, et al. Evaluation of the safety and adverse effects of goreisan/wulingsan, a traditional Japanese-chinese herbal formulation (kampo), in a rat model: a toxicological evaluation. Trop Med Health 2014;42:127-32. [Crossref] [PubMed]
- He L, Rong X, Jiang JM, et al. Amelioration of anti-cancer agent adriamycin-induced nephrotic syndrome in rats by Wulingsan (Gorei-San), a blended traditional Chinese herbal medicine. Food Chem Toxicol 2008;46:1452-60. [Crossref] [PubMed]
- Wu H, Fan Z. Treatment of 60 cases of chronic heart failure with Zhenwu Decoction and Wuling powder combined with Western Medicine. Zhejiang Journal of Traditional Chinese Medicine 2013;48:171.
- Zhang D. Zhenwu decoction combined with Wuling powder in the treatment of chronic heart failure and its effect on serum BNP and left ventricular ejection fraction. Shaanxi Journal of Traditional Chinese Medicine 2014;35:1472-3.
- Wang C, Zhou Y. Bazhen decoction combined with Wuling powder in the treatment of 35 cases of chronic congestive heart failure. Henan Traditional Chinese Medicine 2010;30:989.
- Wang Q. Effect of Wuling powder on serum TGF in patients with chronic heart failure-β1. Chinese Medical Guidelines 2011;9:165-6.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Gu R, Gao Y, Zhang C, et al. Effect of Tai Chi on Cognitive Function among Older Adults with Cognitive Impairment: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2021;2021:6679153. [Crossref] [PubMed]
- Chen H, Zhuo Q, Yuan W, et al. Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database Syst Rev 2008;CD006090. [Crossref] [PubMed]
- Ning R, Zhi L. Summary of 70 cases of chronic heart failure treated with modified Wuling powder. Journal of Zhejiang University of Traditional Chinese Medicine 2012;36:143-4.
- Huang X, Huang J, Niu Y, et al. Effects of Dangewuling powder on cardiac function and left ventricular ejection fraction in patients with chronic heart failure. Yunnan Journal of Traditional Chinese Medicine 2013;34:10-12+89.
- Shen F. Treatment of chronic heart failure with Linggui Zhugan decoction combined with Wupi Decoction and Wuling powder. Medical Information 2013;230.
- Zhou B. Observation on the efficacy of integrated traditional Chinese and Western medicine in the treatment of 38 cases of Yang deficiency chronic heart failure. National Medical Forum 2014;29:55.
- Yang D. Zhenwu decoction combined with Wuling powder in the treatment of 35 cases of Yang deficiency water generalized heart failure. Modern Distance Education of Chinese Traditional Medicine 2014;12:35-6.
- Qing P. Clinical observation of integrated traditional Chinese and Western medicine in the treatment of heart Yang depression in chronic heart failure. Journal of Practical Traditional Chinese Medicine 2015;31:36-7.
- Cao J. Observation on the efficacy of integrated traditional Chinese and Western medicine in the treatment of chronic heart failure. Journal of Practical Traditional Chinese Medicine 2016;32:148-9.
- Liu M, Zhao S. Treatment of 60 cases of chronic heart failure with Wuling powder. Western Traditional Chinese Medicine 2017;30:2.
- Yi X, Liu M. Clinical observation of 46 cases of chronic heart failure treated with Zhenwu Decoction and Wuling powder. Yunnan Journal of Traditional Chinese Medicine 2017;38:2.
- Su X, He Z, Luo S, et al. Clinical observation of 30 cases of cor pulmonale heart failure treated with Wuling powder and Taohong Decoction. Hunan Journal of Traditional Chinese Medicine 2017;33:3.
- Hong J, Hou L, Chen H. Observation on the efficacy of Wuling powder reasonable medium pill in the treatment of 41 cases of chronic left ventricular insufficiency. Chinese Practical Medicine 2018;13:3.
- Chen Y. Analysis of integrated traditional Chinese and Western medicine in the treatment of acute attack of chronic heart failure. Health Required Reading 2019.
- Peng X, Wu S, Deng L, et al. Clinical observation on 90 cases of Yang deficiency and water flooding syndrome of chronic heart failure treated with Wuling powder combined with furosemide. Hunan Journal of Traditional Chinese Medicine 2019;35:4.
- Tang Q. Clinical observation of Buzhong Yiqi Decoction Combined with Wuling powder combined with sakubatrovalsartan in the treatment of chronic heart failure. Modern Distance Education of Chinese Traditional Medicine 2020;18:3.
- Wang X. Clinical effect analysis of modified Wuling powder in the treatment of chronic heart failure. Contemporary Medicine 2020;26:2.
- Hu F. Efficacy of modified Wuling powder combined with western medicine in the treatment of chronic heart failure and its effect on cardiac function, fibrosis index and inflammatory factor level. Shaanxi traditional Chinese medicine 2021;42:5.
- Li J, Wang M. Clinical effect and safety analysis of Wuling powder in the treatment of patients with chronic heart failure. Journal of Clinical Rational Drug Use 2021;14:2.
- Savarese G, Musella F, D'Amore C, et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: a meta-analysis. JACC Heart Fail 2014;2:148-58. [Crossref] [PubMed]
- Guan X, Deng B. Effect of WulingSan on ET and BNP in patients with chronic congestive heart failure. Lishizhen Medicine and Materia Medica Research 2013;24:1906-7.
- Chen J. Clinical Observation of Taohongsiwu Decoction Combined with Wuling Powder in Treatment of Heart Failure Edema. Journal of Hubei University of Chinese Medicine 2014;16:59-61.
(English Language Editor: A. Kassem)