
Study design for a multicenter, randomized controlled trial evaluating the diagnostic value of ultrathin bronchoscope compared to thin bronchoscope without fluoroscopy for peripheral pulmonary lesions
Introduction
Lung cancer is a leading cause of cancer deaths in the world (1). With the popularization of low-dose computed tomography (CT) screening, the discovery rate of lung nodules has increased and lung cancer mortality has been reduced, but it has a higher false positive rate (2). Therefore, it is significant to clarify the nature of peripheral pulmonary lesion (PPL) prior to surgery. It is important as well to obtain specimens of primary lesions for patients with advanced lung cancer to guide diagnosis and treatment in the era of precision treatment.
There are two minimally invasive diagnostic techniques, transthoracic needle aspiration (TTNA) with the guidance of CT or B mode ultrasound and transbronchial lung biopsy (TBLB), commonly used for the diagnosis of PPL at present. TTNA is associated with severe complications including pneumothorax and hemorrhage, especially higher pneumothorax rate with 3.1–41.7%, due to violating the pleural space. Moreover, some lesions are difficult to reach due to the position and TTNA has the potential risk of tumor pleural metastasis (3,4). TBLB is performed through the natural cavity for the diagnosis of PPL while examining the lumen. Complications such as pneumothorax and bleeding are relatively low and has advantages over other methods. However, traditional TBLB is performed with blind biopsy based on image positioning and has a low diagnostic yield. The diagnostic yield varies greatly depending on lesion size with the guidance of fluoroscopy (5,6). In particular, it is difficult to find and locate the lesion with a diameter less than 2 cm, and both the operator and patient receive extra radiation that can be evitable (7). The improvements of bronchoscopy with adjunct techniques, such as the use of endobronchial ultrasound and guide sheath (EBUS-GS) and virtual bronchoscopic navigation (VBN) in TBLB, have increased the diagnostic yield of bronchoscopy. TBLB with the guidance of endobronchial ultrasound and guide sheath (EBUS-GS-TBLB) began to be applied to the clinical scenario in 2004.
EBUS combined with GS allows to clearly observe the lesions around the small airway and insert biopsy forceps or brushes repeatedly, reducing the occurrence of bleeding. Many studies have shown that it is more convenient and safer of TBLB with the guidance of GS compared with traditional TBLB, especially in the improvement of diagnostic yield for solitary pulmonary nodules less than 3 cm (8-10). TBLB can be performed with the guidance of EBUS without fluoroscopy, reducing X-ray radiation, which has high clinical application value (11).
Detecting the lesion rapidly and accurately in complicated tracheobronchial tree is the key to improve the diagnosis and treatment of PPLs. VBN is a technology for diagnosing PPL that can transfer thin-slice CT data to virtual bronchoscopic images, creating a path to the target lesion automatically when the lesion is depicted in this system, which provide powerful help for lung biopsy. Previous studies have shown that VBN combined with EBUS-GS can improve the diagnostic yield of PPL and shorten the examination time, which has become the standard method for the diagnosis of PPL (12,13).
With the development of bronchoscopy technology, ultrathin bronchoscope (UTB) with a 3.0-mm outer diameter and a 1.7-mm working channel has appeared, which can be used combined with a diameter of 1.4-mm ultrasound probe. UTB can reach the more distal bronchus compared with the current thin bronchoscope (TB) (outer diameter 4.0/4.2 mm, working channel 2.0 mm). PPL invisible under conventional bronchoscopy may become a lumen lesion that can be seen directly under UTB. Thus, UTB can improve the diagnostic yield of TBLB by reaching the more distal bronchus accurately where combined with VBN. Studies have shown that the diagnostic yield is close to 70% in PPL less than 3 cm using UTB combined with VBN-EBUS and fluoroscopy, which is significantly higher than that of TB combined with VBN-EBUS-GS, no matter what sampling method is used of TB, especially in external 1/3 lesions (14-16). There is still a high diagnostic yield of PPLs by EBUS-GS without fluoroscopy (11,17). Our previous research found that there was no significant difference in the diagnostic yield of PPL using UTB with a 3.0-mm outer diameter combined with VBN and EBUS with or without X-ray fluoroscopy (18). Therefore, UTB can be used without fluoroscopy, avoiding or reducing X-ray radiation exposure and saving cost of GS. However, there is no report comparing the diagnostic yield of UTB to TB without fluoroscopy guidance. This study aims to clarify the diagnostic value of UTB by comparing with TB combined with different sampling methods without fluoroscopy. We present the following article in accordance with the SPIRIT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-20/rc).
Methods
Study population
Individuals with PPLs on chest imaging need to undergo TBLB and those who meet the following inclusion and exclusion criteria are considered the target population of this study (Table 1). The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol had been approved by Ethics Committee of Shanghai Chest Hospital (approval No. KS2027) as well as other participating centers, and was registered under ClinicalTrials.gov (NCT04571476). If the patient is willing to participate in the study, information will be provided and the informed consent will be asked by the local investigator. The eligible participants will have time until the scheduled procedures to reconsider their consent. The patients must be aware of and give consent to the fact that monitors will be granted direct access to the study patients source medical records without violating subject confidentiality. All subjects should provide written informed consent prior to participating the study.
Table 1
| Inclusion criteria: |
| Subjects meeting all of the following criteria will be enrolled: |
| (I) Patients older than 18 years old |
| (II) Chest imaging shows the presence of PPLs (defined as those lesions that are surrounded by pulmonary parenchyma and located beyond the segmental bronchus) that need to be confirmed by pathology. The length diameter of the lesion is no less than 8 mm and no more than 5 cm |
| (III) Patients without contraindications of bronchoscopy |
| (IV) Patients have good medical adherence and signed informed consent |
| Exclusion criteria: |
| Subjects meeting any of the following criteria will be excluded: |
| (I) PPL is pure ground-glass opacity |
| (II) Absence of bronchus leading to or adjacent to the lesion on thin-slice chest CT |
| (III) *Visible lumen lesions in segment and above segment bronchus during bronchoscopy (evidence of endobronchial lesion, extrinsic compression, submucosal tumor, narrowing, inflammation, or bleeding) |
| (IV) Diffuse pulmonary lesions |
| (V) Target PPL has received chemotherapy, target therapy, radiotherapy or immunotherapy, etc. |
| (VI) The investigators believe that patient has other conditions that are not suitable for the study |
*, this criterion implements after enrollment and randomization. PPLs, peripheral pulmonary lesions; CT, computed tomography.
Study design
This study is a prospective, randomized, controlled, non-inferior, multicenter study. Patients are recruited at five academic hospitals in Chinese mainland, and details are presented in the Table S1. All procedures will be performed by experienced bronchoscopists with the guidance of VBN and EBUS, but without fluoroscopy. Patients with PPLs eligible for the study will be randomly divided into three groups (1:1:1), UTB-VBN-EBUS group, TB-VBN-EBUS-GS group, and TB-VBN-EBUS-non-GS group based on stratified factors with dynamic randomization (Figure 1). Stratified factors include lesion size (≤3 or >3 cm), lesion location from the hilum [three elliptical regions on CT scans: central third, intermediate third, or peripheral third of the lung field (19), and bronchus sign (leading to or adjacent to the lesion)]. The investigators generate the allocation sequence, enroll participants, and assign participants to interventions. The pathologist and data analysts are blinded to the assignment.
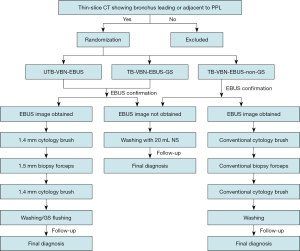
Instruments and procedures
The UTB (BF-MP290F; Olympus, Tokyo, Japan), TB (BF-P260F or BF-P290; Olympus), radial-type probe EBUS (UM-S20-17S; Olympus), GS (SG-200C; Olympus), biopsy forceps and cytology brushes used in this study are shown in Figure 2.

All procedures will be performed by experienced bronchoscopist under local anesthesia with or without moderate sedation, or general anesthesia. Chest CT (slice width 0.5–1 mm, interval 0.5–1 mm) data will be obtained from all patients prior to bronchoscopy. Individual CT data sets are transferred to a workstation on which VBN software (DirectPath; Olympus) created virtual bronchoscopic images automatically. The consecutive images can be moved back and forth and rotated, just like a bronchoscope in a monitor next to the video-bronchoscopic screen in the endoscopy suite. When assistant physician controlled the virtual bronchoscopic images during bronchoscopy, the bronchoscopist inserts the bronchoscope as instructed. The specific procedure of each group is as follows.
UTB-VBN-EBUS group
UTB with a 3.0-mm outer diameter and a 1.7-mm working channel will be used in this group. Specimens will be obtained using 1.5-mm biopsy forceps and 1.4-mm cytology brush with the guidance of VBN and EBUS. EBUS is performed using an endoscope ultrasound system, which is equipped with a 20-MHz mechanical radial-type probe with an external diameter of 1.4 mm. The EBUS probe is inserted into the UTB working channel and advanced to the PPL to obtain an EBUS image. The operator adjusts the probe continuously based on EBUS image during examination until it shows “within” or “adjacent to” features, which indicates the probe reaching the lesion. Once a typical EBUS image is seen, the probe is withdrawn from the UTB working channel. Specimens are obtained through the UTB working channel using biopsy forceps and cytology brush, respectively, then washing the biopsy site with saline and collecting fluid for cytology and/or microbiology after biopsy and brushing. The acquisition of specimens follows the sequence of cytology brush, biopsy forceps (at least 5 but no more than 10 specimens visible to the naked eye are recommended), cytology brush, and washing (for patients with suspected infectious disease, washing can be given priority). If the EBUS image cannot be obtained, as a supplementary procedure, the bronchoscopist would determine that the area around the bronchial target is washed with 20 mL of saline. Instruments used are shown in Figure 3A.

TB-VBN-EBUS-GS group
TB with a 4.0/4.2-mm outer diameter and a 2.0-mm working channel will be used in this group. Specimens will be obtained using 1.5-mm biopsy forceps and 1.4-mm cytology brush with the guidance of VBN-EBUS and a 1.95-mm outer diameter GS. The usage of VBN is the same as UTB group. EBUS probe is inserted into the GS beforehand, and the GS-covered probe is introduced via the working channel of the bronchoscope and advanced to the PPL to obtain an EBUS image. The probe and GS are confirmed to reach the lesion by EBUS images that had “within” or “adjacent to” EBUS features. EBUS probe is withdrawn from GS when the probe reaching the lesion and the GS is left in place. Biopsy forceps and cytology brush are introduced through the GS to obtain specimens. GS is flushed with saline to collect liquid specimens for cytology and/or microbiology after biopsy and brushing. The acquisition of samples is the same as UTB group. If the EBUS image cannot be obtained, then withdraw the GS and the area around the bronchial target is washed as determined by the bronchoscopist with 20 mL of saline as a supplementary procedure. Instruments used are shown in Figure 3B.
TB-VBN-EBUS-non-GS group
TB with a 4.0/4.2-mm outer diameter and a 2.0-mm working channel will be used in this group. Specimens will be obtained using conventional biopsy forceps and cytology brush with the guidance of VBN and EBUS, but without GS. The procedure is performed the same as UTB-VBN-EBUS group. Instruments used are shown in Figure 3C.
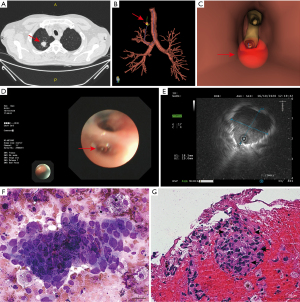
A representative case of UTB-VBN-EBUS method in diagnosing PPL is depicted in Figure 4.
Outcomes
The primary endpoint is the diagnostic yield. The secondary endpoints include total examination time, duration of finding lesions, the proportion of lesions visible by radial EBUS, factors affecting the diagnostic yield, difference in the bronchus level reached with the bronchoscope, difference in diagnostic yield, and complication rate (Table 2). If mediastinal lymph node staging and PPL sampling are performed at the same time, lymph node sampling is not counted either in the diagnostic yield or the examination time.
Table 2
| (I) Total examination time |
| Defined from the time that the bronchoscope is inserted beyond the glottis, until the bronchoscope has been removed from the glottis after examination |
| (II) Duration of finding lesions |
| Defined from insertion of ultrasound probe to withdrawal of ultrasound probe when see the location of lesions under radial EBUS |
| (III) The proportion of lesions visible by radial EBUS |
| Defined as the proportion of lesions obtained ultrasound images in all lesions |
| (IV) Factors affecting the diagnostic yield of UTB and TB for PPLs |
| Lesion nature, lesion size, lesion location, the relationship of the ultrasound probe relative to the lesion, sampling method etc. |
| (V) Difference in the bronchus level reached with the bronchoscope |
| Level: main bronchi are level 0, lobar bronchi are level 1, segmental bronchi are level 2, subsegmental bronchi are level 3, and so on, such as: LB3a is level 3 |
| (VI) Difference in diagnostic yield |
| Difference in diagnostic yield between TB with GS combined with small sampling tools and without GS combined with conventional sampling tools |
| (VII) Complication rate |
| The complications referring to serious adverse events related to the procedure during or within 1 month after the operation |
EBUS, endobronchial ultrasound; UTB, ultrathin bronchoscope; TB, thin bronchoscope; PPLs, peripheral pulmonary lesions; GS, guide sheath.
Final diagnosis and follow-up
All patients will be followed-up for at least 6 months post-procedure. The final diagnosis is based on histopathology, cytopathology, microbiological evidences of specimens obtained by bronchoscopy procedure and the follow-up results. If there is definite malignant histological or cytological pathologic evidence or the characteristic pathological or microbiological evidence of benign disease of bronchoscopic obtained specimens that is confirmed by the follow-up, they are considered to be diagnosed by bronchoscopy. Otherwise, if the specimens obtained by bronchoscopy don’t have specific benign or malignant pathological evidence or specimens are unqualified (specimens with normal lung tissue, bronchial mucosa etc.), they will not be regarded as diagnosed by bronchoscopy. For these non-diagnostic lesions, the final diagnosis will be made through repeated biopsy of bronchoscopy or additional procedures, including CT-guided TTNA, surgical biopsy, or clinical and imaging follow-up for at least 6 months.
Sample size
This study is designed to compare the diagnostic yields of UTB and TB for the diagnosis of PPLs. We hypothesize that the diagnostic yield of UTB method is not inferior to that of TB method. Based on the expected diagnostic yield of 75% using both the UTB and TB methods, sample size of UTB versus TB as 1:2, demonstration of noninferiority with difference within 10% (δ =−0.1) and a statistical power of 80% at a one-sided significance level of 0.05 would require 175 patients in UTB group and 350 patients in TB group. We arranged to enroll a total of 578 patients with 193 and 385 patients in UTB group and TB group, respectively, to account for 10% dropouts.
Statistics
The means and percentages are presented as appropriate. With the noninferiority analyses of the primary endpoint, if noninferiority is demonstrated, then its superiority will be analyzed. Categorical variables are analyzed using the Pearson χ2 test or Fisher’s exact test. Use the t-test or U test to analyze continuous variables. Statistical analyses are performed using SPSS 25.0. P<0.05 is taken to indicate statistical significance.
Safety
The names and grading of adverse events and serious adverse events are evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (20).
Data collection and management
Data collection will be performed in the participating centers. Electronic patient record forms will be provided web based. Data collection and analysis will be monitored according to good clinical practice. Clinical monitoring will be organized in case report form files undergoing a quality check.
Handling and storage of data and documents
The investigators will maintain adequate records, including signed patients informed consent forms and information on adverse events. The anonymity and confidentiality will be guaranteed and patients’ identification will be coded.
Amendments
Amendments are changes made to the research after a favorable opinion by the accredited research institute has been given. All amendments will be notified to the research institute that gives a favorable opinion. Non-substantial amendments will not be notified to the accredited research institute and the competent authority, but will be recorded and filed by the sponsor.
Quarterly progress report
The investigator will submit a summary of the progress of the trial to the sponsor every quarter. Information will be provided on the date of inclusion of the first subject, numbers of subjects included and numbers of subjects that have completed the trial, serious adverse events, other problems, and amendments.
End of study report
The investigator will notify the sponsor of the end of the study within a period of 8 weeks. The end of the study is defined as the last patient’s last visit.
In case the study is ended prematurely, the investigator will notify the sponsor within 15 days, including the reasons for the premature termination.
Within one year after the end of the study, the investigator will submit a final study report with the results of the study, including any publications/abstracts of the study, to the sponsor.
Study organization
The study will utilize a medical monitoring committee to provide a medical review and adjudication of pre-specified adverse events in support of endpoint data defined by the protocol. The medical monitoring committee is a qualified physician certified by the board of directors and is not affiliated with an investigative center or the research sponsor.
Discussion
At present, minimally invasive surgery including bronchoscopic biopsy is recommended in clinical consensus guideline of pulmonary nodules evaluation to minimize unnecessary thoracotomy (21). However, current thin bronchoscopy still cannot meet the clinical needs for diagnosis of PPLs (22), thinner bronchoscopy and technical improvements are required to increase the diagnostic yield by accessing to smaller, more peripheral lesions under the navigation with a low risk of pneumothorax.
Our preliminary study shows that UTB combined with VBN and EBUS is an efficient method for the diagnosis of PPLs with diagnostic yield of 81.7% and 73.3% with or without fluoroscopy, respectively (P=0.38) (18), significantly higher than TB combined with VBN-EBUS (49–59%) (15,22). In addition, there are no significant difference in the diagnostic yield of PPL via UTB whether X-ray fluoroscopy is used from our preliminary experience.
The purpose of this study is to compare the diagnostic yield of UTB and TB under the guidance of VBN without X-ray fluoroscopy. The presence of CT bronchus sign was reported to be a predictor associated with high bronchoscopic diagnostic yield (8,23,24). Therefore, CT bronchus sign is considered as one of the stratification factors. Positive CT bronchus sign is defined as a bronchus leading to the PPL on thin-slice CT. Negative bronchus sign indicates a bronchus adjacent to or not involved to the PPL. Patients with lesions having bronchus leading to or adjacent to will be enrolled in the study, but no bronchus detection in relation to the lesion will not be enrolled. From others studies (23,24) and our previous experience, the proportion of lung lesions having no bronchus involved was very low, most susceptible malignant lesions have involved bronchus when observing on thin-slice CT, as lung cancers are mainly originated from bronchial epithelium. Also, large lesions are more likely to have involved bronchus. In addition, lesions without involved bronchus are difficult to diagnose (23). Usually peripheral transbronchial needle aspiration (TBNA) under the guidance of fluoroscopy is needed for accessing no bronchus involved lesions. But all the procedures will be performed without fluoroscopy in the current study. We considered it may be unsafe to conduct peripheral TBNA without fluoroscopy, despite it can be associated with increased diagnostic yield (23).
UTB is efficient and promising for the diagnosis of PPL and the diagnostic value of UTB compared with TB without fluoroscopy needs to be clarified. The current study is designed as a prospective, multicenter, randomized controlled three-arm clinical study with a large sample size to further evaluate the diagnostic value and safety of UTB compared with TB under the guidance of VBN and EBUS without X-ray fluoroscopy. Enrollment began in March 2021.
This study compensates the limitations of previous research (14,15,25). Firstly, the study is conducting not only at one center of expertise, but also generalized to several other institutions. Then, UTB used here was the commercial end product, not just prototype, so the study is in essence strictly clinical research of this product. Thirdly, we set the groups of TB-VBN-EBUS with small sampling tools and larger ones to determine whether the size of sampling tools is a factor effecting diagnostic yield. Therefore, the results will provide evidence for the diagnostic value of UTB in PPLs and a wealth of information about the uses of this novel bronchoscope.
Acknowledgments
Thanks for the support of Olympus (Beijing) sales and service Co., Ltd.
Funding: The study is supported by Olympus (Beijing) sales and service Co., Ltd. in financial and material.
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-20/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-20/coif). All authors report funding from Olympus (Beijing) sales and service Co., Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol had been approved by Ethics Committee of Shanghai Chest Hospital (approval No. KS2027) as well as other participating centers, and was registered under ClinicalTrials.gov (NCT04571476). If the patient is willing to participate in the study, information will be provided and the informed consent will be asked by the local investigator.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [Crossref] [PubMed]
- Schreiner AM, Jones JG, Swistel AJ, et al. Transthoracic fine needle aspiration resulting in implantation metastasis in the superficial tissues of the breast. Cytopathology 2013;24:58-60. [Crossref] [PubMed]
- Stringfield JT, Markowitz DJ, Bentz RR, et al. The effect of tumor size and location on diagnosis by fiberoptic bronchoscopy. Chest 1977;72:474-6. [Crossref] [PubMed]
- Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest 1995;108:131-7. [Crossref] [PubMed]
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S-28S. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. [Crossref] [PubMed]
- Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788-93. [Crossref] [PubMed]
- Asano F, Ishida T, Shinagawa N, et al. Virtual bronchoscopic navigation without X-ray fluoroscopy to diagnose peripheral pulmonary lesions: a randomized trial. BMC Pulm Med 2017;17:184. [Crossref] [PubMed]
- Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011;66:1072-7. [Crossref] [PubMed]
- Oki M, Saka H, Asano F, et al. Use of an Ultrathin vs Thin Bronchoscope for Peripheral Pulmonary Lesions: A Randomized Trial. Chest 2019;156:954-64. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Ultrathin Bronchoscopy with Multimodal Devices for Peripheral Pulmonary Lesions. A Randomized Trial. Am J Respir Crit Care Med 2015;192:468-76. [Crossref] [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327-33. [Crossref] [PubMed]
- Tachihara M, Tamura D, Kiriu T, et al. Bronchoscopy Using Virtual Navigation and Endobronchial Ultrasonography with a Guide Sheath (EBUS-GS) with or without Fluoroscopy for Peripheral Pulmonary Lesions. Kobe J Med Sci 2018;63:E99-104. [PubMed]
- Zheng X, Xie F, Li Y, et al. Ultrathin bronchoscope combined with virtual bronchoscopic navigation and endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions with or without fluoroscopy: A randomized trial. Thorac Cancer 2021;12:1864-72. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) 2010. Version 4.0. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Tanner NT, Yarmus L, Chen A, et al. Standard Bronchoscopy With Fluoroscopy vs Thin Bronchoscopy and Radial Endobronchial Ultrasound for Biopsy of Pulmonary Lesions: A Multicenter, Prospective, Randomized Trial. Chest 2018;154:1035-43. [Crossref] [PubMed]
- Tokoro Y, Yasuo M, Kobayashi T, et al. Computed tomography-guided bronchoscopy in the diagnosis of small peripheral pulmonary lesions: A retrospective study of 240 examinations in a single academic center. Respir Investig 2016;54:347-54. [Crossref] [PubMed]
- Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging 2015;15:21. [Crossref] [PubMed]
- Sumi T, Ikeda T, Sawai T, et al. Comparison of ultrathin bronchoscopy with conventional bronchoscopy for the diagnosis of peripheral lung lesions without virtual bronchial navigation. Respir Investig 2020;58:376-80. [Crossref] [PubMed]


