
Initial experience with and surgical outcomes of da Vinci single-port system in general thoracic surgery
Introduction
Minimally invasive surgery in the field of general thoracic surgery has evolved with a reduction in the number of ports. Recently, single-port thoracoscopic surgery has gained attention; however, it is not widespread due to its limitations, such as poor ergonomics. Early robotic surgery introduced the use of multiple arms; however, robotic single-site approaches using the da Vinci Si system (Intuitive Surgical Inc., Sunnyvale, CA, USA) have also been attempted through enlarged 3–4-cm single incisions. We previously reported our experience with robotic single-site thoracic surgery in the excision of mediastinal masses (1,2). Even though the number of incisions and ports can be reduced using the single-site platform of the da Vinci system, the approach has limitations, such as non-articulating instruments with a lack of free wrist movements and long distance (8 cm) between the incision and the target lesion. Consequently, it is difficult to perform complex procedures using the single-site approach.
In April 2014, the first flexible robotic single-port system (SPS) (da Vinci SPTM Surgical System, Model SP999, Intuitive Surgical Inc.) was used in genitourinary surgery in a clinical study in France (3). The next generation system Model SP1098 was then approved for general thoracic surgery in August 2020 in South Korea. The SPS includes three flexible instruments in contrast to the two non-flexible arms in the previous robotic single-site platform as well as a stereoscopic binocular wristed camera, all contained within a cannula of 2.8-cm diameter. However, there are some limitations to the use of the da Vinci SPS in thoracic surgery through the intercostal spaces (ICS) due to the size of the 2.8-cm single-port cannula. Additionally, SPS requires a distance of 10 cm between the tip of the cannula and the target anatomy to triangulate the instruments and enable complete articulation of both the elbow and wrist instrument joints. To overcome these limitations, we previously performed animal and cadaver experiments to investigate the appropriate approach for SPS in thoracic surgery (4). Based on these experiments, two independent thoracic surgeons in South Korea began performing robotic SPS surgeries for simple general thoracic surgeries. The aim of this study was to report the initial experiences and surgical outcomes of robotic SPS in general thoracic surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1739/rc) (5).
Methods
Patients
This retrospective study included data from two institutions. Two thoracic surgeons, surgeon A (SYP) and surgeon B (HKK) began performing robotic SPS surgery independently at their respective institutions since August 2020 following approval from the Ministry of Food and Drug Safety in Korea. Data from each institution were merged and analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the institutional review board of each institution (IRB No. 2021-0957-001 for Severance Hospital and 2019GR0400 for Korea University Guro Hospital) which waived the informed consent from the patients as a retrospective study. This study included 17 patients who underwent SPS surgery. Preoperative work-up included chest computed tomography, evaluations for myasthenia gravis, and pulmonary function tests. The demographic, intraoperative, and postoperative data were collected.
Approaches and operations
This was the initial experience with robotic SPS thoracic surgery; therefore, the patients were carefully selected. Simple mediastinal mass excision was the main indication for surgery. Patients with thymic tumors invading the great vessels or heart, based on radiological findings, and patients who previously underwent cardiovascular or thoracic surgery were excluded. The surgeons evaluated various approaches, such as subxiphoid, subcostal, and intercostal approaches, based on the location, size, and relative anatomy of the lesion to identify the access with the best clinical outcome.
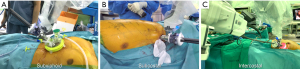
The approaches for the SPS were based on a previous cadaver study (4) and are illustrated in Figure 1. For the subxiphoid approach, a vertical 3–4-cm skin incision below the xiphoid process over the subxiphoid area was placed in the supine position without partial resection of the xiphoid process (Figure 1A). Following the incision through the linea alba, blunt dissection of the preperitoneal and retrosternal spaces was performed using a finger. A third-party access system for single-port surgery, such as GelPoint Mini system (Applied Medical Corporation, Rancho Santa Margarita, CA, USA) or Lapsingle VR (Sejong Medical, Paju, South Korea), was inserted through the incision, and CO2 was insufflated at a pressure of 6–10 mmHg. For the subcostal approach, a 5-mm assistant port was created in the posterior axillary line in the 8th ICS to insufflate the chest cavity with 6–10 mmHg of CO2 and displace the diaphragm inferiorly (Figure 1B). Subsequently, a 3–4-cm skin incision was made right below the subcostal margin in the mid-clavicular line, and a tunnel was dissected superiorly using long Metzenbaum scissors and fingers. The connection of the tunnel between the skin incision and the thoracic cavity was confirmed using an endoscope through the 5-mm port. The wound retractor of the port access system was placed through the skin incision but not “rolled” down to the skin level to create a “sleeve” for the SPS cannula and instruments. To enable triangulation of the SPS instruments with sufficient working distance to the tissue, the SPS cannula was initially fixed within the cap of the single-port access system floating above the patient. If the target lesion was located far from the subcostal incision (more than 10 cm, such as an apical lesion), the cannula was inserted directly into the subcostal incision. For the intercostal approach, an incision in the 6th or 7th ICS was placed in the sub-mammary line (Figure 1C). The cap of the single-port access system and SPS cannula were fixed as in the subcostal approach.
Definitions of variables
Pain was assessed using a numeric pain intensity scale; the score was recorded by nurses every 8 h until discharge (6). The highest score was documented. The robot console time started with the initiation of dissection and ended with the completion of the dissection with the robotic system. The total operation time included the duration between the first skin incision for port placement and skin closure with the placement of a chest tube. Complications were defined according to the Clavien-Dindo classification (7).
Statistical analysis
The surgical outcomes of robotic SPS thoracic surgery were compared with those of robotic single-site surgery. The two groups were compared using the Fisher’s exact test for discrete variables and the Mann-Whitney U test for continuous variables because of the small number of patients in the SPS group. Variables with P values <0.05 were considered statistically significant. All statistical analyses were performed using R v2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Operative outcomes of robotic SPS surgery
Since August 2020, 17 patients underwent robotic SPS surgery and 48 patients underwent robotic single-site surgery at the two institutions. The basic characteristics of the 17 patients who underwent robotic SPS surgery are summarized in Table 1. The patients included 6 males, and the overall median age was 52 years (range, 28–83 years). Thymoma (n=8) and benign cystic lesions (n=6) were the commonest pathologies. The median size of the masses to be excised was 3.0 cm (range, 1.2–9.7 cm). The approach for the SPS cannula included subxiphoid, subcostal, and intercostal approaches in 11, 4, 2 patients, respectively. The docking time was not measured in all patients; however, it was usually less than 10 min with the SPS. The median operation time and peak pain score were 120 minutes (range, 58–250 minutes) and 3 (range, 2–4). The median duration of in situ chest tube and hospital stay was 1 day (range, 1–2 days) and 3 days (range, 2–7 days), respectively. All operations were completed successfully without conversion to conventional multiport robotic surgery or thoracoscopic surgery.
Table 1
| No. | Age, years | Sex | Position | Approach | Pathologic diagnosis | Mass size (cm) |
|---|---|---|---|---|---|---|
| 1 | 28 | Female | Supine position | Subxiphoid | Non-Hodgkin’s lymphoma | 3.3 |
| 2 | 52 | Female | Supine position | Subxiphoid | Thymoma AB | 6.0 |
| 3 | 53 | Female | Right lateral position | Subcostal | Bronchogenic cyst | 3.0 |
| 4 | 47 | Male | Supine position | Subxiphoid | Thymic atypical carcinoid | 2.7 |
| 5 | 65 | Female | Supine position | Subxiphoid | Pericardial cyst | 9.7 |
| 6 | 73 | Female | Supine position | Subcostal | Thymoma B3 | 1.7 |
| 7 | 35 | Male | Left lateral position | Subcostal | Extrapulmonary sequestration | 4.4 |
| 8 | 51 | Male | Supine position | Subxiphoid | Thymoma B1 | 3.0 |
| 9 | 60 | Male | Supine position | Intercostal | Thymoma AB | 1.8 |
| 10 | 42 | Female | Supine position | Intercostal | Thymic cyst | 2.0 |
| 11 | 45 | Female | Supine position | Subxiphoid | Thymic cyst | 4.5 |
| 12 | 83 | Female | Supine position | Subxiphoid | Thymoma A | 4.9 |
| 13 | 44 | Female | Supine position | Subxiphoid | Thymoma B2 | 1.8 |
| 14 | 56 | Male | Supine position | Subxiphoid | Thymoma B2 + B3 | 3.0 |
| 15 | 62 | Male | Lateral decubitus position | Subcostal | Schwannoma | 3.6 |
| 16 | 72 | Female | Supine position | Subxiphoid | Thymic carcinoma | 1.6 |
| 17 | 39 | Female | Supine position | Subxiphoid | Thymic cyst | 1.2 |
Comparison between robotic SPS surgery and robotic single-site surgery
Comparisons between the SPS surgeries and single-site surgeries are summarized in Table 2. Regarding the approaches, the subxiphoid (54.2%) and intercostal (43.8%) approaches were commonly used in single-site surgeries, whereas the subxiphoid approach (64.7%) the predominant one with SPS surgery. Due to the limitations of cannula size in the SPS, we predominantly used the subxiphoid approach in SPS surgeries. No surgical mortalities were observed in either group and all patients underwent complete resection. SPS showed shorter duration of in situ chest tube (1 vs. 2 days, P=0.004) even though the size of mass was smaller in SPS group (3.0 vs. 4.0 cm, P=0.049) than robotic single-site surgeries. The other operative outcomes were similar in both groups. Three operative complications reported with single-site surgeries included pleural effusion, dysrhythmia, and aggravation of myasthenia gravis. All complications were managed conservatively (Clavien-Dindo classification grade II).
Table 2
| Variables | Single-port surgery (n=17) | Single-site surgery (n=48) | P value |
|---|---|---|---|
| Age (years), median [range] | 52 [28–83] | 50 [19–73] | 0.501 |
| Males, n (%) | 6 (35.3) | 29 (60.4) | 0.094 |
| Pathology, n (%) | 0.624 | ||
| Thymoma | 8 (47.0) | 16 (33.3) | |
| Thymic carcinoma | 1 (5.9) | 2 (4.2) | |
| Benign cystic lesions* | 6 (35.3) | 18 (37.5) | |
| Others | 2 (11.8) | 12 (25.0) | |
| Size (cm), median [range] | 3.0 [1.2–9.7] | 4.0 [0.9–22.1] | 0.049 |
| Approaches, n (%) | 0.003 | ||
| Subxiphoid | 11 (64.7) | 26 (54.2) | |
| Subcostal | 4 (23.5) | 1 (2.1) | |
| Intercostal | 2 (11.8) | 21 (43.8) | |
| Operation time (minutes), median [range] | 120 [58–250] | 146 [27–262] | 0.085 |
| Duration of in situ chest tube (days), median [range] | 1 [1–2] | 2 [1–5] | 0.004 |
| Hospital stay duration (days), median [range] | 3 [2–7] | 4 [2–11] | 0.147 |
| Peak pain score, median [range] | 3 [2–4] | 3 [1–8] | 0.719 |
| Complication, n (%) | 0 | 3 (6.3) | 0.561 |
*, benign cystic lesions include thymic, pericardial, and bronchogenic cysts.
Discussion
This is the first report on the surgical outcomes of robotic SPS in general thoracic surgery. Even though we enrolled simple cases for the initial experience, the SPS demonstrated acceptable surgical outcomes. In comparison with the duration of in situ chest tube stay in robotic single-site surgeries, that in SPS surgeries was shorter. The better outcomes than single-site surgery results might be related to the meticulous dissections with articulating instruments of SPS.
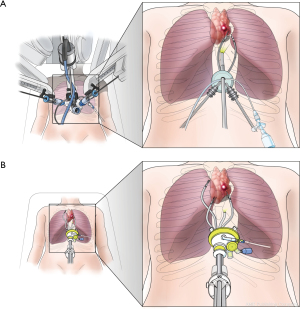
The advantages of the SPS compared with the robotic single-site system are the following: (I) flexible instruments that allow for complex movements and meticulous dissection; (II) reduced collision and interference of instruments due to parallel entrance of the SPS instruments through a single port; and (III) freedom to use additional instruments (three instruments simultaneously) whereas only two non-articulated instruments are available in the robotic single-site platform (Figure 2). Therefore, the SPS offers better ergonomics than thoracoscopic single-port surgery and better maneuverability than robotic single-site surgery. Even though we only performed simple surgeries in this initial series, we believe that more complex procedures that cannot be performed using the robotic single-site platform can be performed with the SPS following additional experience. The minimum distance required between the target lesion and the port increased from 8 cm in the single-site system to 10 cm with the SPS; however, this limitation can be overcome by ‘floating the cannula’ as described in the methods section.
The natural approach of choice for thoracic surgeons would be the intercostal route using the SPS system, which is also what most of us are familiar with. A major concern with the SPS is introducing the canula, with a large outer diameter of 2.8 cm, between the ribs. Therefore, we used various approaches with the SPS. The subxiphoid approach is traditionally an extremely useful approach in thymectomy and anterior mediastinal mass excision; furthermore, single-site thymectomy can also be performed using this approach. The subxiphoid approach was technically feasible with SPS. Maintaining CO2 insufflation was difficult using the third-party access system. However, this problem was solved by using the SP Access Port system (Intuitive Surgical Inc.), which has been approved for clinical use and was used by the authors in the initial cases. The additional space required to articulate and move the instruments with SPS in comparison with the single-site system is limited due to the sternum (Video 1). However, this problem can be overcome by adjusting the robotic arms and introducing the third instrument below the SPS camera instrument. We previously demonstrated in cadaveric experiments that the subcostal approach can enable more complex procedures in comparison with other approaches (4). However, to enable safe entry into the chest cavity, we believe that a small 5-mm thoracoscopic port may be needed to insufflate CO2, displace the diaphragm inferiorly, and visualize the entrance into the thoracic cavity. In one of the pre-clinical cadaver experiments, we had entered the abdominal cavity; therefore, we believe that the use of an additional thoracoscopic port is essential during early learning. In the current series with the additional thoracoscopic port, the abdominal cavity was not entered in any of the patients. After placing the wound retractor and cannula in the subcostal incision, the movements of the SPS robotic arm had no limitations and complete surgery was performed through this incision (Video 2). The subcostal approach can reduce postoperative neuralgia when compared with conventional multiport robotic surgery, which uses the intercostal approach. With the use of appropriate positions, we believe that all types of surgeries can be performed using the subcostal approach. As mentioned earlier with regards to the intercostal approach, there are concerns about whether a large cannula can result in rib fractures or higher postoperative pain. Stein & Falk reported that the intercostal approach to the chest is feasible but observed motion-related pressure strain during clutching of the SPS instrument arm and increased potential for the trauma that is dependent on the intercostal distance and trajectory of the instrument arm (8). In two patients, we used the intercostal approach with the SPS; the peak numeric pain scale scores were 4 and 3 in these patients, respectively, without postoperative intercostal neuralgia or rib fracture. However, the intercostal distance is small, especially in female Asian patients; a substantial amount of friction between the ribs and instruments could lead to unintended motion, patient trauma, and damage to the instruments. Therefore, further investigations are required regarding the intercostal approach. Currently, the subxiphoid and subcostal approaches might provide better clinical value to the patient. However, redesigning the access port and SPS cannula to merge the instruments into a single line might reduce the compression of intercostal nerves and make the intercostal approach more feasible.
This study has a few limitations. First, the indications for surgery were heterogeneous. Second, we used a third-party port designed for single-port thoracoscopic or laparoscopic surgery instead of the SP Access Port for the SPS because the latter has not been approved in South Korea yet. Maintaining CO2 insufflation and floating the cannula was extremely difficult with the third-party wound retractors, especially in the subxiphoid approach. We found that CO2 insufflation was well-maintained with the SP Access Port in cadaver experiments with the subxiphoid approach; therefore, after the Intuitive port is approved, the difficulties with this approach could be resolved and our experiences with and impressions of various approaches with the SPS could change. Third, other approaches, such as transcervical or transhiatal approaches, were not attempted. Lastly, we only enrolled simple cases of mediastinal masses for the initial experience. The feasibility and safety of complex cases must be verified in future trials. Another aspect of the SPS in general thoracic surgery is the absence of staplers and energy devices. Simple excision of masses was feasible with grasping instruments, electrocautery, and small clips. Currently, the surgical indications for the SPS could include simple mediastinal mass excision, thymectomy, and enucleation of submucosal tumors. To perform pulmonary resections, an additional port for a laparoscopic stapler might be needed, even though Gonzalez-Rivas and Ismail reported the feasibility of lobectomy using SPS in cadaver (9). Nevertheless, we will continue to evaluate thoracic surgeries using the SPS and consider pulmonary resections in the near future.
In conclusion, the operative outcomes of the SPS were acceptable. To apply this system to more complex thoracic surgeries, advances in instrumentation, as well as more clinical experience, are required to overcome the learning curve of this new approach.
Acknowledgments
The authors thank MID (Medical Illustration & Design), a part of the Medical Research Support Services of Yonsei University College of Medicine, for the artistic support related to this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1739/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1739/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1739/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1739/coif). HS and SYH are the employees of Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park SY, Kim HK, Jang DS, et al. Initial Experiences With Robotic Single-Site Thoracic Surgery for Mediastinal Masses. Ann Thorac Surg 2019;107:242-7. [Crossref] [PubMed]
- Park SY, Han KN, Hong JI, et al. Subxiphoid approach for robotic single-site-assisted thymectomy. Eur J Cardiothorac Surg 2020;58:i34-8. [Crossref] [PubMed]
- Kaouk JH, Haber GP, Autorino R, et al. A novel robotic system for single-port urologic surgery: first clinical investigation. Eur Urol 2014;66:1033-43. [Crossref] [PubMed]
- Park SY, Stein H, Heo SY. Preclinical, cadaveric study of the application of da Vinci single port system in thoracic surgery. J Thorac Dis 2019;11:5586-91. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Rodriguez CS. Pain measurement in the elderly: a review. Pain Manag Nurs 2001;2:38-46. [Crossref] [PubMed]
- Dindo D. The Clavien–Dindo Classification of Surgical Complications. In: Cuesta MA, Bonjer HJ. editors. Treatment of Postoperative Complications After Digestive Surgery. Springer, 2014:13-7.
- Stein H, Falk V. Feasibility of bilateral internal thoracic artery harvesting using the da Vinci SP system. Surg Today 2021;51:303-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]


