
Multifocal locules including the anterior mediastinum side as a surgical indicator in pleural infection
Introduction
Although the treatment strategy for pleural infection was updated in the most recent guidelines, surgery is indicated for cases resistant to non-surgical treatment (1,2). Andrews classified pleural infection based on the intrathoracic condition as exudative, fibropurulent, or organized; these stages were dependent mainly on the duration of the illness (3). Patients in the exudative and early fibropurulent stage can be treated by non-surgical therapy, including tube drainage, antibiotics, and intrapleural fibrinolytic therapy; surgical management is often required for the advanced fibropurulent and organized stages of empyema (2,4,5). However, these clinical stages cannot be defined exactly because of the difficulty in diagnosing intrathoracic phases based only on clinical findings and the fact that the disease duration depends on the self-reported appearance of symptoms (6).
Light classified pleural parapneumonic effusions and empyema according to the anatomical, bacteriological, and chemical characteristics of the effusion and concluded that surgical management is often required for patients showing an accumulation of pus (classified as empyema) and multiple locules (7,8). However, Light’s criteria were reported to be unreliable in patients with multiple locules because of the variations in chemical and bacteriological characteristics of the locules (9-11). Moreover, non-surgical management was associated with a higher recurrence rate of empyema (12); thus, surgical management is desirable for selected patients with pleural infection.
The treatment strategy for pleural infection and the surgical indications remain topics of debate. Wozniak et al. determined that failure of the first procedure was strongly related to the prognosis of empyema patients and recommended early consultation with a surgeon (13). Thus, identifying additional predictors of surgical conversion for pleural infection, including specific imaging results, may facilitate decision-making regarding early surgery and the formulation of a treatment strategy for improving the prognosis. Our institution has used a guideline-based strategy to treat many patients with pleural infection over a long period; therefore, we retrospectively analyzed these patients to identify the predictors for surgical intervention in pleural infection. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1812/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Toho University Omori Medical Center (No. M20218_19218) and informed consent was taken from all the patients.
Patients
We retrospectively analyzed two regularly updated medical databases of the Division of Chest Surgery, Department of Surgery, and Division of Respiratory Medicine, Department of Internal Medicine, Toho University School of Medicine. We extracted the data of the patients who were treated for pleural infection between January 2008 and December 2020 at Toho University Omari Medical Center from the databases. All participants provided informed consent for using their data for analyses in the form of an opt-out feature on the website of the center. The study protocol was approved by the Ethics Committee of Toho University Omori Medical Center (approval number: M20218_19218).
Evaluation of pleural infection
Acute empyema was defined as a pleural infection with symptoms appearing within 3 months. All cases were diagnosed to acute empyema via blood test, chest X-ray, ultrasound, computed tomography (CT) scan, and thoracentesis, and classified based on the American College of Chest Physicians (ACCP) category and Light’s classification. Patients having images or surgical findings for chronic empyema were excluded from the study dataset. Patients with iatrogenic empyemas, such as empyema occurring after neck and chest surgery, port-site infection, or lung abscess after bronchoscopic biopsy, were also excluded because the infection route and treatment strategy differed from pleural parapneumonic effusions and empyema. Patients with empyema due to Aspergillus infections and tuberculosis were also excluded because their treatment differed from pleural infection caused by bacteria.
Image findings
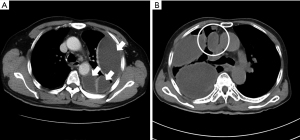
Image findings in CT scans, including multifocal locules, locules on the mediastinal side (Figure 1), pleural microbubbles (14), and the split pleura sign (15), were reviewed by two different respiratory doctors and confirmed by all co-authors. A “locule” was defined by an isolated fluid retention in the thoracic cavity on the scan; these were confirmed by recalibrating multi-planar reconstruction images in the axial, sagittal, and coronal views. If isolated locule on the anterior mediastinum side (LAMS), locules in the space surrounded by the lung, anterior mediastinum, and chest wall were observed, we defined the condition as LAMS.
Treatment strategy and patient follow-up
The treatment strategy was based on Light’s classification (Figure 2). All patients underwent thoracentesis and initial treatment with antibiotic therapy. Generally, ampicillin and β-Lactam antibiotics were chosen; patients with systemic inflammatory response syndrome and sepsis received initial broad-spectrum antibiotic treatment using carbapenem or piperacillin and tazobactam.

After placing tube drain, irrigation was performed every day using 500 or 1,000 mL of saline depending on the size of the cavity. Thrombolytic therapy was performed using 120,000 units of urokinase on day 1 and 60,000 units on days 2 and 3. Irrigation and thrombolytic therapy were not performed or were discontinued for patients diagnosed with or suspected to have a fistula. Monotherapy using urokinase was selected for thrombolytic therapy because combination therapy using tissue plasminogen activator and DNase was not allowed in Japan (16).
Tube thoracostomy was performed under fluoroscopy or CT in addition to ultrasound; tube thoracostomy with multiple tubes was performed for patients with multifocal locules who were resistant to single-tube thoracostomy. If tube thoracostomy was not possible due to a lack of space or adhesion of the lungs, only thoracentesis and administration of antibiotics were performed initially.
Patients who showed improvement after the initial treatment were discharged with oral antibiotics and outpatient follow-up. A surgical approach was considered after a respiratory-center discussion if the locules persisted and the symptoms, condition, or inflammatory data did not improve or worsened. If the respiratory or general condition worsened rapidly or led to sepsis, early surgical management was considered.
All thoracotomy procedures were performed via two- or three-port approaches. The surgical procedure was selected according to the thoracoscopy findings. Irrigation with complete video-assisted thoracoscopic surgery (VATS) or a two-port approach with wounds of 5–8 and 1 cm was performed for patients with exudative and early-phase fibropurulent stage empyema; patients with late-phase fibropurulent and organized-stage empyema underwent decortication with wounds of 8–15 and 1–5 cm.
Antibiotics were continued until clinical condition, radiological findings, and inflammatory parameters including the white blood cell count and C-reactive protein level, improved. Patients who underwent surgery were followed-up for at least 6 months postoperatively. Follow-up assessments were terminated for patients who showed improvements in the inflammatory parameters and overall condition after the initial treatment and for patients who were re-administered antibiotics on presenting to our hospital for other reasons; the re-administration duration was included in the follow-up period.
Statistical analysis
Differences in categorical variables were analyzed using Fisher’s exact test, and those in continuous variables were analyzed using the Mann-Whitney test. Univariate and Multivariate analyses were performed using logistic regression analysis. Multivariate analysis used the significantly different variables detected by the univariate analysis and variables used for classification in the ACCP category and guidelines. The statistical significance level was set at P<0.05. JMP statistics version 14.2.0 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics
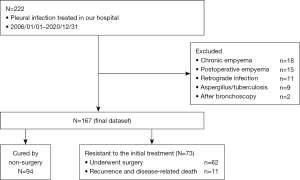
The flowchart for patient selection is shown in Figure 3. A total of 222 consecutive patients with pleural infection were treated between January 2008 and December 2020. Among them, we excluded patients who were diagnosed as showing chronic empyema (n=18), postoperative empyema (n=15), retrograde infection (n=11), pleural infection with Aspergillus or tuberculosis (n=9), and empyema after bronchoscopy (n=2). The remaining 167 patients were analyzed.
The patient characteristics are shown in Table 1. The median age was 66 years; 139 patients (83.2%) were men, 117 patients (70.1%) were smokers, and 49 patients (29.3%) had received treatment for diabetes mellitus. The median interval from the appearance of symptoms to hospitalization was 7 days (range, 0–74 days).
Table 1
| Variables (n=167) | N (%) or median (range) |
|---|---|
| Age, years | 66 [26–89] |
| Male | 139 (83.2) |
| Body mass index, kg/m2 | 22.6 (12.2–38.4) |
| ECOG-PS 3–4 | 19 (11.3) |
| Smoker | 117 (70.1) |
| Pack-year smoking | 17 [0–145] |
| Heavy drinker | 40 (24.0) |
| From appearance of symptoms to hospitalization (days) | 7 [0–74] |
| PaO2/FiO2 | 341 [63–592] |
| Comorbidity/anamnesis | |
| Diabetes mellitus | 49 (29.3) |
| History of malignancy | 22 (13.2) |
| Stroke | 17 (10.2) |
| Chronic kidney disease (creatinine >1.5 mg/dL) | 15 (9.0) |
| Blood test | |
| C-reactive protein (mg/L) | 199 [10–401] |
| White blood cell (/µL) | 14,400 (1,100–75,300) |
| Albumin (g/dL) | 2.5 (1.6–3.9) |
| Side | |
| Right | 84 (50.3) |
| Left | 83 (49.7) |
| Detection of causative bacteria in pleural effusion | 99 (59.3) |
| Streptococcus anginous group | 43 (25.7) |
| Anaerobic bacteria | 38 (22.8) |
| Staphylococcus aureus | 10 (6.0) |
| Mixed aerobic and anaerobic bacteria | 12 (7.2) |
| First dose antibiotics | |
| β-lactamase | 80 (47.9) |
| Carbapenem | 58 (34.7) |
| Piperacillin/Tazobactum | 21 (12.6) |
| Others | 8 (4.8) |
| Procedure | |
| Tube thoracostomy | 157 (94.0) |
| Irrigation | 119 (71.3) |
| Thrombolytics | 100 (59.9) |
| Surgery | 62 (37.1) |
ECOG-PS, Eastern Cooperative Oncology Group-performance status.
The causative bacteria were detected by culture or Gram staining in 99 patients (59.3%). Streptococcus anginosus was the most frequently detected bacterium (25.7%, 43 patients). Anaerobic bacteria were detected in 38 (22.8%) patients, and a mixed infection of aerobic and anaerobic bacteria was detected in 12 patients (7.2%). β-lactam antibiotics were selected for most of the initial antibiotic treatments (80 patients, 47.9%).
Tube thoracostomy was performed in 157 (94.0%) patients; tube insertion in the remaining 10 (6.0%) patients was difficult because of lung adhesions and a lack of adequate space. Irrigation and thrombolytic therapy with urokinase were performed in 119 (71.3%) and 100 (59.9%) patients, respectively. Surgical intervention was performed in 62 patients (37.1%): 39 patients underwent irrigation, and 23 patients underwent decortication.
Classifications
The data for the variables related to ACCP (17) and Light’s classification (8) are shown in Table S1. LAMS was observed in the pleural space anatomy, a single locule, and multiple locules in 38 (22.8%), 100 (59.9%), and 63 (37.7%) patients, respectively. Pleural microbubbles were observed in 38 patients (22.8%), and the split pleura sign was observed in 107 patients (64.1%).
Analysis of surgical indications
To identify the indicators for resistance to non-surgical therapy, patients were divided into those cured by non-surgical therapy (n=94) and those resistant to non-surgical therapy (n=73); the latter included patients who underwent surgical intervention after the initial therapy (n=62) and those that showed recurrence or disease-related death after non-surgical therapy (n=11). The characteristics are shown in Table S2.
The findings of the logistic analyses are shown in Table 2. The existence of multiple locules was identified as the only significant variable. The existence of multiple locules without LAMS was a significant variable compared to free-flowing effusion [odds ratio (OR) =3.34, 95% confidence interval (CI): 1.05–10.60, P=0.0409]. The presence of LAMS was a significant indicator compared to a free-flowing effusion (OR =11.47, 95% CI: 3.78–34.78, P<0.0001), single locule (OR =7.70, 95% CI: 3.04–19.48, P<0.0001), and multifocal locules without LAMS (OR =3.43, 95% CI: 1.58–7.97, P=0.0041). Multivariate analysis detected the existence of multiple locules and the presence of LAMS was a significant indicator for resistance to non-surgical therapy.
Table 2
| Variables | Reference | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Age >70 years | ≤70 years | 0.71 | 0.48–1.32 | 0.275 | ||||
| Sex: male | Female | 1.24 | 0.54–2.85 | 0.605 | ||||
| Body mass index >25 kg/m2 | ≤25 | 1.77 | 0.77–4.05 | 0.179 | ||||
| ECOG-PS 3–4 | 0–2 | 1.48 | 0.57–3.86 | 0.421 | ||||
| PaO2/FiO2 <300 | ≥300 | 0.85 | 0.45–1.60 | 0.621 | ||||
| Pack-year smoking>60 | ≤60 | 0.56 | 0.21–1.45 | 0.232 | ||||
| Comorbidity/anamnesis | ||||||||
| Diabetes mellitus | No | 1.71 | 0.87–3.34 | 0.118 | ||||
| Chronic kidney disease | No | 1.53 | 0.53–4.43 | 0.434 | ||||
| From appearance of symptoms to hospitalization >7 days | ≤7 days | 1.64 | 0.88–3.03 | 0.117 | 1.71 | 0.84–3.51 | 0.140 | |
| Fistula | No | 1.31 | 0.41–4.25 | 0.649 | ||||
| Septic shock at the hospitalization | No | 1.31 | 0.36–4.70 | 0.680 | ||||
| Serum albumin level <2.5 g/dL | ≥2.5 g/dL | 1.02 | 0.55–1.89 | 0.946 | ||||
| White blood cell >15,000/µL | ≤15,000/µL | 0.79 | 0.42–1.47 | 0.461 | ||||
| CRP >200 mg/L | ≤200 mg/L | 1.42 | 0.77–2.62 | 0.263 | ||||
| Pus | No | 1.11 | 0.56–2.21 | 0.755 | 1.08 | 0.43–2.70 | 0.873 | |
| Glucose <40 mg/dL (pleural effusion) | ≥40 mg/dL | 1.11 | 0.59–2.08 | 0.743 | 1.01 | 0.51–2.37 | 0.800 | |
| pH <7.2 (pleural effusion) | ≥7.2 | 1.53 | 0.79–2.96 | 0.211 | 1.49 | 0.63–3.50 | 0.356 | |
| Bacteria positive (pleural effusion) | Negative | 0.80 | 0.43–1.48 | 0.470 | 0.63 | 0.29–1.40 | 0.259 | |
| Image findings | ||||||||
| Pleural space anatomy >1/2 hemithorax | ≤1/2 hemithorax | 1.34 | 0.71–2.53 | 0.368 | 0.81 | 0.38–1.77 | 0.608 | |
| Locules | ||||||||
| Single locule | Not loculated | 0.52 | 0.44–5.04 | 0.522 | 1.73 | 0.49–6.17 | 0.397 | |
| Multiple locules without LAMS | Not loculated | 3.34 | 1.05–10.60 | 0.041* | 3.67 | 1.11–12.14 | 0.033* | |
| Single locule | 2.24 | 0.84–5.99 | 0.108 | 2.12 | 0.72–6.21 | 0.171 | ||
| Multiple locules + LAMS | Not loculated | 11.47 | 3.78–34.78 | <0.001* | 14.15 | 4.30–46.56 | <0.001* | |
| Single locule | 7.70 | 3.04–19.48 | <0.001* | 8.17 | 3.06–21.82 | <0.001* | ||
| Multiple locules without LAMS | 3.43 | 1.58–7.97 | 0.0041* | 3.86 | 1.52–9.77 | 0.0044* | ||
| Microbubbles | No | 1.06 | 0.51–2.19 | 0.885 | ||||
| Split Pleural Sign | No | 1.57 | 0.82–3.01 | 0.171 | ||||
*, significant difference (P<0.05). OR, odds ratios; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group-performance status; LAMS, locule on the anterior mediastinum side; CRP, C-reactive protein.
Treatment courses and prognosis
Treatment courses and prognoses were analyzed by comparing the findings across three groups: patients who underwent surgery within 7 days of admission (n=33), patients who underwent surgery more than 7 days after the admission (n=29), and patients who were treated by non-surgical therapy (n=105) using median days from admission to surgery, as shown in Table S3. Among the prognoses, recurrence of the pleural infection and disease related death were observed only in the non-surgical group. Mortality was not observed among the patients who underwent surgery within 7 days of admission.
The comparisons between patients who underwent surgery within 7 days of admission and patients who underwent surgery more than 7 days after admission are shown in Table 3. Among the patients who underwent surgery within 7 days of admission, the duration of hospitalization was significantly shorter (P=0.0071), and there were significantly more patients whose C-reactive protein level improved within 30 days (P<0.0001).
Table 3
| Outcome | Surgery ≤7 days (n=33) | Surgery >7 days (n=29) | P value |
|---|---|---|---|
| Duration between admission and surgery (average, days) | 2.9 | 18.6 | |
| Hospitalization (average, days) | 26.9 | 42.0 | 0.0071* |
| Using antibiotics (average, days) | 31.0 | 38.5 | 0.0619 |
| Duration of CRP improvement <30 days, n (%) | 23 (69.7) | 6 (20.7) | <0.0001* |
| Recurrence of empyema, n (%) | 0 (0.0) | 0 (0.0) | 0.2480 |
Surgery ≤7 days: patients underwent surgery within 7 days of admission; surgery >7 days: patients underwent surgery over 7 days of admission; duration of CRP improvement: CRP level improved within 10 mg/L during the observation period. *, significant difference (P<0.05). CRP, C-reactive protein.
Discussion
Our study aimed to clarify the predictors for surgical indications in patients with pleural infection and identified that multifocal locules, including LAMS, are significant predictors of resistance to non-surgical therapy in these patients. The American and European guidelines do not specify definite indicators for surgical treatment. The British Thoracic Society guideline proposed a therapeutic algorithm for pleural infection and recommended surgical therapy for patients resistant to antibiotics and chest tube replacement (1). The guideline of the American Association for Thoracic Surgery recommends chest tube replacement followed by surgical management for the patients showing pus, positive results in culture or Gram staining, and a pleural fluid pH<7.2, based on the classifications of Light and the ACCP (4,17). Conversely, Himelman et al. reported that Light’s classification was unreliable in patients with locules because the bacteriological and chemical findings of locules in patients with multiple locules are not consistent (9). Consistent with this result, Everts et al. found that 44% of culture findings from catheters or chest tubes were inaccurate; this suggested that the direct aspiration of the potentially infected locules is important (10). Maskell et al. reported differences in the chemical and bacteriological findings for locules in patients with multiloculated pleural infections (11). These reports suggest that negative findings in bacteriological and chemical examinations on thoracentesis are not a definitive factor for excluding surgical procedures from the potential treatment options. Therefore, to detect the existence of a locule with uncontrolled infection (18), imaging results may be valid predictors of resistance to the medical treatment. Many previous studies concluded that multifocal locules on ultrasonography and CT scans predicted surgical management (19). In our study, LAMS, which has not been discussed previously, was a strong indicator for surgical management, because chest tube drainage is quite difficult and thrombolytic therapy may not be effective on the anterior mediastinal side. There is no report about the anatomical positional relationship of the locules and resistance of the non-surgical therapy, the results of this study may be useful for establishing criteria for surgical indication. As ultrasonography has difficulty observing the thoracic cavity on the anterior mediastinum side, the presence of LAMS on the CT scan should be confirmed before a decision is made for the treatment of a pleural infection.
We clarified that early surgery within 7 days of admission could decrease the duration of hospitalization and improved C-reactive protein level faster, which suggests that early consultation with a thoracic surgeon and surgery in patients with these imaging results may improve the prognosis; this is contrary to the conventional guidelines of surgical treatment for the pleural infections resistant to initial non-surgical treatment. The prognosis of a pleural infection remains poor, with a mortality rate of 15–20% (1,2) and long-term hospitalization (20). Possible causes include uncontrolled infection and recurrence of respiratory infections. Tube drainage does not always remove the fluid completely, especially in patients with multiple locules. These drainage-defective cavities may cause uncontrolled infection and recurrence of the pleural infection. Moreover, the lungs are often not fully dilated at discharge in patients with tube drainage, which might induce pneumonia at the site of the poor lung dilation and respiratory failure due to decreased lung function. Wozniak et al. reported that the first choice for management of empyema was strongly related to the prognosis (13). A USA database analysis of patients with pleural infection who had received chest tube drainage showed that they had higher rates of mortality, re-admission, and re-intervention compared with patients who had received surgical treatment (12). These conclusions suggest that earlier surgical management for selected patients with pleural infections can reduce the duration of hospitalization and even improve the prognosis of pleural infection (21,22), because adequate surgical intervention can unify the thoracic cavity and promote sufficient lung dilation (2). In this study, none of the patients who underwent surgery showed recurrence of empyema, and the rate of disease-related death among these patients was lower than that among patients who received non-surgical treatment. The selection of the appropriate patients with pleural infections will require a determination of the indicators for surgical conversion or the need for surgery.
Nayak et al. demonstrated that the inpatient mortality rate for VATS decreased over time (7% until 2011 to 4.3% after 2011) (21), which suggests that the VATS procedure is less invasive and more stable. VATS for pleural infections was reported to reduce the operative time, postoperative pain, duration of chest tube drainage and hospitalization, and mortality rate (22,23). The stereotype that surgical treatment is highly invasive should be dispelled; it has been suggested that surgery benefits patients with pleural infections (24). Since various factors such as causative bacteria, duration of illness, and comorbidities may contribute to the progression of intrathoracic infections such as a pleural infection (25,26), the treatment strategy cannot be determined easily. We propose that surgical indicators such as LAMS are simple and will help determine early consultation with surgeons. Early surgery for patients with these imaging results may improve the prognosis of pleural infections.
Since the present study was a retrospective and single-center analysis, the number of patients was small and there were biases related to treatment methods and patient groups. Combination therapy using tissue plasminogen activator and DNase could not undergo and only monotherapy using urokinase was performed in our facility, some patients who underwent surgery may treat using combination therapy. Moreover, this study spanned over a long period during which changes in practices and thresholds to refer patients to surgery may have evolved. This analysis did not include the patients with a poor general condition who clearly were not indicated for surgery, and a selection bias may have existed. Therefore, multicenter studies with large sample sizes are required to construct a more definitive strategy.
Conclusions
Multifocal locules, including LAMS, were valid indicators for surgical management in patients with pleural infections. Early surgery for patients with these findings may shorten hospital stay and improve the prognosis of pleural infections.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1812/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1812/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1812/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1812/coif). AI serves as an unpaid editorial board member of Journal of Thoracic Disease from May 2019 to April 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Toho University Omori Medical Center (No. M20218_19218) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Andrews NC. Management of nontuberculous empyema: A statement of the subcommittee on surgery. Am Rev Respir Dis 1962;85:935.
- Light RW. A new classification of parapneumonic effusions and empyema. Chest 1995;108:299-301. [Crossref] [PubMed]
- Weissberg D, Refaely Y. Pleural empyema: 24-year experience. Ann Thorac Surg 1996;62:1026-9. [Crossref] [PubMed]
- Roberts JR. Minimally invasive surgery in the treatment of empyema: intraoperative decision making. Ann Thorac Surg 2003;76:225-30; discussion 229-30. [Crossref] [PubMed]
- Light RW. Parapneumonic effusions and empyema. Clin Chest Med 1985;6:55-62. [Crossref] [PubMed]
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [Crossref] [PubMed]
- Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest 1986;90:852-6. [Crossref] [PubMed]
- Everts RJ, Heneghan JP, Adholla PO, et al. Validity of cultures of fluid collected through drainage catheters versus those obtained by direct aspiration. J Clin Microbiol 2001;39:66-8. [Crossref] [PubMed]
- Maskell NA, Gleeson FV, Darby M, et al. Diagnostically significant variations in pleural fluid pH in loculated parapneumonic effusions. Chest 2004;126:2022-4. [Crossref] [PubMed]
- Semenkovich TR, Olsen MA, Puri V, et al. Current State of Empyema Management. Ann Thorac Surg 2018;105:1589-96. [Crossref] [PubMed]
- Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg 2009;87:1525-30; discussion 1530-1. [Crossref] [PubMed]
- Smolikov A, Smolyakov R, Riesenberg K, et al. Prevalence and clinical significance of pleural microbubbles in computed tomography of thoracic empyema. Clin Radiol 2006;61:513-9. [Crossref] [PubMed]
- Kraus GJ. The split pleura sign. Radiology 2007;243:297-8. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000;118:1158-71. [Crossref] [PubMed]
- Ravaglia C, Gurioli C, Tomassetti S, et al. Is medical thoracoscopy efficient in the management of multiloculated and organized thoracic empyema?. Respiration 2012;84:219-24. [Crossref] [PubMed]
- Chen KY, Liaw YS, Wang HC, et al. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med 2000;19:837-43. [Crossref] [PubMed]
- Cargill TN, Hassan M, Corcoran JP, et al. A systematic review of comorbidities and outcomes of adult patients with pleural infection. Eur Respir J 2019;54:1900541. [Crossref] [PubMed]
- Nayak R, Brogly SB, Lajkosz K, et al. Outcomes of Operative and Nonoperative Treatment of Thoracic Empyema: A Population-Based Study. Ann Thorac Surg 2019;108:1456-63. [Crossref] [PubMed]
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017;3:CD010651. [Crossref] [PubMed]
- Chambers A, Routledge T, Dunning J, et al. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg 2010;11:171-7. [Crossref] [PubMed]
- Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg 2015;48:642-53. [Crossref] [PubMed]
- Sakai T, Sano A, Azuma Y, et al. Streptococcus anginosus group infection as a predictor for the progression of descending necrotizing mediastinitis. Ann Palliat Med 2021;10:4008-16. [Crossref] [PubMed]
- Sakai T, Sano A, Azuma Y, et al. Preoperative undernutrition predicts postoperative complications of acute empyema. Health Sci Rep 2021;4:e232. [Crossref] [PubMed]


