Establishing a TNM-like risk classification for metachronous second pulmonary adenocarcinoma in patients with previously resected pulmonary adenocarcinoma
Introduction
Non-small cell lung cancer (NSCLC) is one of the most tedious malignancies. Adenocarcinoma (ADC) represents approximately 40% of cases of NSCLC (1). In past decades, with great advances in screening techniques and treatment modalities involving surgery, cytotoxic drugs, radiotherapy, targeted therapy, and immunotherapy, the number of survivors from lung cancer is greatly increased (2). Because the reported risk to develop a metachronous second lung cancer varied from 1% to 7% per survivor per year, the number of second lung cancer is expected to rapidly increase (3-5). For patients with second lung cancer, the physical condition is commonly limited, which makes the clinical decision more cautious and complex. Particularly, when the pathological type of metachronous second lung cancer is the same as the first one, it is hard to determine its origin (primary or metastatic lung cancer). Although assessment on several clinical parameters, including the location of the primary tumor and metastatic node, tumor diameter, histology, and cancer-free survival, have long been used to distinguish metachronous primary lung cancer (MPLC) from metastasis (6-8). However, these suggestions remain controversial owing to contradictory results reported by series of studies (9-11). This makes it difficult to obtain an accurate stage on a current staging system, and restrict the development of effective prognostication and appropriate treatment decision. Therefore, establishing a TNM-like risk stratification system in the premise of suspending the dispute of tumor clonality for metachronous second pulmonary adenocarcinoma (msPAD) patients with previously resected PAD is still merit.
In this study, we used the population-based Surveillance, Epidemiology, and End Results (SEER) registry to include msPAD patients with previously resected PAD. This study aims to establish a TNM-like risk stratification system on the premise of laying aside the dispute of tumor clonality for these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1982/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Guangzhou First People's Hospital (K-2021-186-01). A statement that the participants gave informed consent before taking part is not required because this study is performed on an established retrospective database. The population was selected from the SEER 18 Custom Database using SEER*Stat 8.3.5 software (http://seer.cancer.gov/seerstat/).
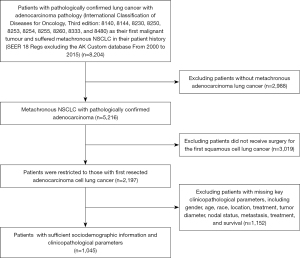
Patients from the SEER 18 Regs excluding AK Custom database (2000 to 2015) with additional treatment fields who had pathologically confirmed lung cancer with adenocarcinoma (International Classification of Disease for Oncology, Third edition: 8140, 8144, 8230, 8250, 8253, 8254, 8255, 8260, 8333, and 8480) as their first malignant tumor and suffered metachronous NSCLC in their patient history were screened. In this cohort, we identified patients according to the following criteria: (I) received surgical resection (lobectomy, sublobectomy, or pneumonectomy) for the primary; (II) the pathology for metachronous NSCLC was ADC pathology (International Classification of Disease for Oncology, Third edition: 8140, 8144, 8230, 8250, 8253, 8254, 8255, 8260, 8333, and 8480). msPAD was defined as the second PAD which occurred after diagnosis of the first PAD, therefore patients with interval survival ≤1 month were excluded in this study. According to the 2015 World Health Organization Classification of Lung Tumors, patients with grade IV (undifferentiated) were excluded (12).
Information on the sociodemographic and clinicopathological features of patients of the primary PAD and msPAD were collected. For the primary tumors, the stage was manually performed according to the 8th TNM staging system (13). Because the tumor characteristic (primary or metastatic cancer) of msPAD is ambiguous, the pathological parameters of msPAD were recorded in the premise of laying aside the dispute of tumor clonality, including tumor diameter, node metastasis (negative, intrapulmonary metastasis, mediastinal metastasis), and extrapulmonary metastasis (no, yes). To verify the efficacy of the risk stratification system, we also extracted the stage record of the msPAD from the SEER database as well. Two recorded variables, “site-specific surgery codes” and “surgery of primary site codes” were adopted to identify the surgical procedure.
Statistical analysis
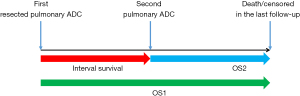
The statistical analysis was performed using the SPSS 22.0 software package (SPSS, inc., Chicago, IL, USA) and R 3.3.2 (http://www.r-project.org). Survival data of patients with the primary tumors were extracted and defined as overall survival 1 (OS1), and the survival data of the msPAD were extracted and defined as the overall survival 2 (OS2). The interval between the diagnosis of the two PADs was recorded as the interval survival (Figure 1). The survival rate was calculated using the Kaplan-Meier method. Univariate and multivariate Cox regressions were constructed to identify independent predictors for interval survival, OS1, and OS2. In this study, the main objective is OS2. According to the criteria for the diagnosis of metachronous second primary lung cancer (MSPLC) proposed by the American College of Chest Physicians (ACCP) in 2013, 24 and 48 months were selected as the cut-off points for interval survival (8). Statistical significance was assumed at a two-sided P<0.05.
Then, we built a nomogram system involving independent pathological parameters through the survival and rms package. A new decision tree group through recursive partitioning analysis (RPA) was established for risk stratification for OS2. To validate the effectiveness of the proposed TNM-like risk stratification system, we calculated the Akaike information criterion (AIC) and the concordance index (c-index) and carried out a time-dependent receiver operating curve (ROC) analysis (14). In this research, the nomogram score is the only predictor, and the PRA and time-dependent ROC curves were performed using R 3.3.2 (http://www.r-project.org) with the rpart package and survival ROC package, all parameters were set as default values.
Results
Patients’ characteristics
A total of 1,045 patients were met the mentioned criteria and included in this study. The median age of the primary and msPAD was 64 (range, 37 to 88) and 69 (range, 39 to 93) years, respectively. The median tumor diameters of the primary and msPAD were 23 (range, 4 to 95) and 17 (range, 2 to 95) mm, respectively. There were 751 (71.9%) msPAD located in the contralateral side to the primary. Third metachronous PAD was observed in 63 patients. Time distribution of the diagnosis of the primary PAD and msPAD was shown in Figure S1. The median survival time for the interval survival, OS1 and OS2 were 42, 112, and 51 months, respectively. The patients’ characteristics were listed in Table 1. Flow chart of patient recruitment is shown in Figure 2.
Table 1
| Variable | Case number (%) |
|---|---|
| Gender | |
| Male | 425 (40.7) |
| Female | 620 (59.3) |
| Race | |
| White | 874 (83.6) |
| Black | 104 (10.0) |
| Others | 67 (6.4) |
| Age (1st) (years) | |
| <70 | 749 (71.7) |
| ≥70 | 296 (28.3) |
| Location (1st) | |
| Left upper | 305 (29.2) |
| Left lower | 132 (12.6) |
| Right upper | 361 (34.5) |
| Right middle | 57 (5.5) |
| Right lower | 156 (96.7) |
| Unknown | 34 (3.3) |
| Tumor diameter (1st) (mm) , mean ± SD | 26.7±14.6 |
| T status (1st) | |
| T1 | 525 (50.2) |
| T2 | 384 (36.7) |
| T3 | 103 (9.9) |
| T4 | 33 (3.2) |
| Nodal status (1st) | |
| N0 | 790 (75.6) |
| N1 | 100 (9.6) |
| N2 | 132 (12.6) |
| N3 | 23 (2.2) |
| Grade (1st) | |
| I | 160 (15.3) |
| II | 483 (46.2) |
| III | 359 (34.4) |
| Unknown | 43 (4.1) |
| Distant metastasis (1st) | |
| M0 | 892 (85.4) |
| M1 | 153 (14.6) |
| Stage (1st) | |
| I | 562 (53.8) |
| II | 196 (18.8) |
| III | 134 (12.8) |
| IV | 153 (14.6) |
| Surgery (1st) | |
| Sublobectomy | 165 (15.8) |
| Lobectomy | 860 (82.3) |
| Pneumonectomy | 20 (1.9) |
| Chemotherapy (1st) | |
| Yes | 227 (21.7) |
| No/unknown | 818 (78.3) |
| Radiotherapy (1st) | |
| Yes | 92 (8.8) |
| No/unknown | 953 (91.2) |
| Interval survival (months) | |
| <24 | 317 (30.3) |
| 24–47 | 284 (27.2) |
| ≥48 | 444 (42.5) |
| Age (2nd) (years) | |
| <70 | 565 (54.1) |
| ≥70 | 480 (45.9) |
| Location (2nd) | |
| Left upper | 278 (26.6) |
| Left lower | 210 (20.1) |
| Right upper | 257 (24.6) |
| Right middle | 74 (7.1) |
| Right lower | 189 (18.1) |
| Unknown | 37 (3.5) |
| Tumor diameter (2nd) (mm) , mean ± SD | 20.3±12.9 |
| Node metastasis (2nd) | |
| Negative | 852 (81.5) |
| Intrapulmonary metastasis | 72 (6.9) |
| Mediastinal metastasis | 121 (11.6) |
| Extrapulmonary metastasis (2nd) | |
| No | 991 (94.8) |
| Yes | 54 (5.2) |
| Grade (2nd) | |
| I | 269 (25.7) |
| II | 461 (44.1) |
| III | 315 (30.1) |
| Stage (2nd) | |
| I | 444 (42.5) |
| II | 82 (7.8) |
| III | 84 (8.0) |
| IV | 376 (36.0) |
| Unknown | 59 (5.6) |
| Surgery (2nd) | |
| No surgery | 325 (31.1) |
| Sublobectomy | 442 (42.3) |
| Lobectomy | 278 (26.6) |
| Chemotherapy (2nd) | |
| Yes | 248 (23.7) |
| No/unknown | 797 (76.3) |
| Radiotherapy (2nd) | |
| Yes | 251 (24.0) |
| No/unknown | 794 (76.0) |
| Followed ADC | |
| No | 982 (94.0) |
| Yes | 63 (6.0) |
ADC, adenocarcinoma.
Predictors for interval survival, OS1, and OS2
After univariate and multivariate analysis, several independent prognostic factors were identified (Table 2). For interval survival, these parameters included gender, age (1st), side of second ADC, chemotherapy (1st), surgery (1st), tumor diameter (2nd), and node metastasis (2nd). For OS1, these parameters included gender, age (1st), surgery (1st), T status (1st), tumor diameter (2nd), node metastasis (2nd), grade (2nd), extrapulmonary metastasis, and interval survival. For OS2, these parameters included gender, race, age (1st), tumor diameter (2nd), node metastasis (2nd), grade (2nd), and extrapulmonary metastasis.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | Ptrend | Adjusted HR | 95% CI | P | Ptrend | ||
| Interval survival | |||||||||
| Gender | 0.827 | 0.731–0.936 | 0.003 | 0.807 | 0.710–0.918 | 0.001 | |||
| Age (1st) | 1.293 | 1.129–1.480 | <0.001 | 1.304 | 1.132–1.502 | <0.001 | |||
| Side of second ADC (ipsilateral/contralateral) | 1.321 | 1.154–1.513 | <0.001 | 1.365 | 1.185–1.573 | <0.001 | |||
| Grade difference (same/different) | 0.880 | 0.777–0.997 | 0.045 | 0.889 | 0.784–1.007 | 0.064 | |||
| Chemotherapy (1st) | 0.852 | 0.735–0.987 | 0.033 | 0.858 | 0.736–1.000 | 0.050 | |||
| Surgery (1st) | |||||||||
| Sublobectomy | 1 | <0.001 | 1 | 0.002 | |||||
| Lobectomy | 0.700 | 0.592–0.827 | <0.001 | 0.727 | 0.611–0.866 | <0.001 | |||
| Pneumonectomy | 0.676 | 0.906–1.441 | 0.676 | 0.765 | 0.467–1.253 | 0.287 | |||
| Tumor diameter (2nd) | 0.983 | 0.978–0.988 | <0.001 | 0.984 | 0.979–0.990 | <0.001 | |||
| Node metastasis (2nd) | |||||||||
| Negative | 1 | <0.001 | 1 | 0.024 | |||||
| Intrapulmonary metastasis | 0.987 | 0.776–1.256 | 0.917 | 1.094 | 0.850–1.407 | 0.485 | |||
| Mediastinal metastasis | 0.629 | 0.519–0.763 | <0.001 | 0.767 | 0.624–0.943 | 0.012 | |||
| Extrapulmonary metastasis | 0.619 | 0.469–0.817 | 0.001 | 0.814 | 0.604–1.099 | 0.179 | |||
| Overall survival 1 | |||||||||
| Gender | 0.741 | 0.624–0.881 | 0.001 | 0.792 | 0.664–0.945 | 0.009 | |||
| Age (1st) | 1.595 | 1.323–1.924 | <0.001 | 1.509 | 1.246–1.827 | <0.001 | |||
| Side of second ADC (ipsilateral/contralateral) | 1.278 | 1.051–1.554 | 0.014 | 1.208 | 0.839–1.258 | 0.793 | |||
| Surgery (1st) | |||||||||
| Sublobectomy | 1 | 0.013 | 1 | 0.039 | |||||
| Lobectomy | 0.708 | 0.561–0.894 | 0.004 | 0.743 | 0.584–0.934 | 0.016 | |||
| Pneumonectomy | 0.866 | 0.473–1.585 | 0.641 | 0.611 | 0.330–1.132 | 0.117 | |||
| T status (1st) | |||||||||
| T1 | 1 | 0.019 | 1 | 0.001 | |||||
| T2 | 1.020 | 0.847–1.230 | 0.832 | 1.110 | 0.917–1.344 | 0.285 | |||
| T3 | 0.928 | 0.690–1.249 | 0.623 | 0.911 | 0.673–1.232 | 0.543 | |||
| T4 | 1.994 | 1.278–3.114 | 0.002 | 2.542 | 1.606–4.024 | <0.001 | |||
| Tumor diameter (2nd) | 1.010 | 1.004–1.016 | <0.001 | 1.013 | 1.007–1.020 | <0.001 | |||
| Node metastasis (2nd) | |||||||||
| Negative | 1 | <0.001 | 1 | <0.001 | |||||
| Intrapulmonary metastasis | 1.739 | 1.276–2.370 | <0.001 | 1.642 | 1.196–2.254 | 0.002 | |||
| Mediastinal metastasis | 1.529 | 1.210–1.932 | <0.001 | 2.013 | 1.544–2.623 | <0.001 | |||
| Grade (2nd) | |||||||||
| I | 1 | 0.005 | 1 | 0.009 | |||||
| II | 1.262 | 1.003–1.587 | 0.047 | 1.332 | 1.056–1.681 | 0.015 | |||
| III | 1.488 | 1.170–1.892 | 0.001 | 1.465 | 1.143–1.877 | 0.003 | |||
| Extrapulmonary metastasis | 1.552 | 1.132–2.128 | 0.006 | 1.458 | 1.041–2.044 | 0.028 | |||
| Interval survival, months | |||||||||
| <24 | 1 | <0.001 | 1 | <0.001 | |||||
| 24–47 | 0.536 | 0.429–0.670 | <0.001 | 0.483 | 0.385–0.607 | <0.001 | |||
| ≥48 | 0.254 | 0.206–0.312 | <0.001 | 0.183 | 0.146–0.231 | <0.001 | |||
| Overall survival 2 | |||||||||
| Gender | 0.834 | 0.702–0.991 | 0.039 | 0.791 | 0.664–0.942 | 0.009 | |||
| Race | |||||||||
| White | 1 | 0.049 | 1 | 0.034 | |||||
| Black | 0.950 | 0.711–1.269 | 0.727 | 0.887 | 0.660–1.191 | 0.425 | |||
| Others | 0.585 | 0.381–0.898 | 0.014 | 0.573 | 0.371–0.884 | 0.012 | |||
| Age (1st) | 1.369 | 1.137–1.648 | 0.001 | 1.446 | 1.197–1.747 | <0.001 | |||
| Age (2nd) | 1.335 | 1.123–1.587 | 0.001 | 1.120 | 0.873–1.437 | 0.372 | |||
| Tumor diameter (2nd) | 1.028 | 1.023–1.033 | <0.001 | 1.022 | 1.016–1.028 | <0.001 | |||
| Node metastasis (2nd) | |||||||||
| Negative | 1 | <0.001 | 1 | <0.001 | |||||
| Intrapulmonary metastasis | 1.985 | 1.457–2.704 | <0.001 | 1.673 | 1.219–2.296 | 0.001 | |||
| Mediastinal metastasis | 3.066 | 2.418–3.886 | <0.001 | 2.489 | 1.937–3.199 | <0.001 | |||
| Grade (2nd) | |||||||||
| I | 1 | <0.001 | 1 | 0.001 | |||||
| II | 1.313 | 1.044–1.651 | 0.020 | 1.260 | 1.000–1.588 | 0.050 | |||
| III | 1.825 | 1.435–2.321 | <0.001 | 1.566 | 1.227–1.999 | <0.001 | |||
| Extrapulmonary metastasis | 2.944 | 2.144–4.044 | <0.001 | 2.342 | 1.677–3.271 | <0.001 | |||
| Followed ADC | 0.644 | 0.456–0.908 | 0.012 | 0.723 | 0.510–1.024 | 0.068 | |||
HR, hazard ratio; 95% CI, 95% confidence interval; ADC, adenocarcinoma.
Nomogram and RPA stratification for OS2
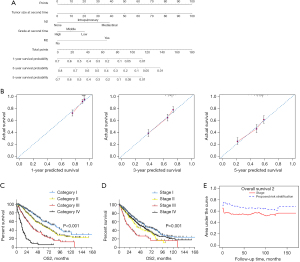
A nomogram that incorporated aforementioned independently pathological factors was established for OS2 (Figure 3A). The calibration plots presented well agreement between the nomogram prediction and actual observation for 1-, 3-, and 5-year survival rate (Figure 3B). Then, we perform RPA for the dichotomous OS according to the nomogram score, partitioned the patient population into three risk strata defined as the followings: low risk (nomogram score <35), moderate risk (nomogram score ≥35 & <76), and high risk (nomogram score >76) (Figure S2A). The RPA stratification system present well-operating characteristics for stratification of OS2 (P<0.001) (Figure S2B).
Proposed a TNM-like risk stratification for OS2
A TNM-like risk stratification system for OS2 was established on tumor diameter (2nd), node metastasis (2nd), grade (2nd), and extrapulmonary metastasis (2nd), based on the nomogram and PRA analysis (Table 3). The median survival after msPAD for category I, II, III, and IV was 88, 58, 32, and 12 months, respectively (P<0.001) (Figure 3C). However, according to the extracted stage information, survival curves were overlapped as the long-term survival of cases with stage IV was similar to that of cases with stage II (P=0.308) but better than that of cases with stage III (P<0.001) (Figure 3D). The AIC value for the proposed risk classification was smaller than that for the applied staging system (5,890.612 vs. 6,015.516). The c-index value was larger for the proposed version than for the applied staging system (0.656 vs. 0.572, P<0.001). Meanwhile, according to the time-dependent ROC curve, the predict accuracy of the proposed risk stratification system is better than the TNM stage system at 160 months of follow-up (Figure 3E).
Table 3
| Tumor diameter (2nd) | Node metastasis (2nd) | Grade (2nd) | Extrapulmonary metastasis (2nd) | ||
|---|---|---|---|---|---|
| Negative | Intrapulmonary | Mediastinal | |||
| ≤30 mm | Category I | Category III | Category III | I | No |
| Category II | Category III | Category III | II-III | No | |
| >30 & ≤70 mm | Category III | Category IV | Category IV | Any | No |
| >70 mm | Category IV | Category IV | Category IV | Any | No |
| ≤30 mm | Category III | Category IV | Category IV | I | Yes |
| Category III | Category IV | Category IV | I | Yes | |
| >30 mm | Category IV | Category IV | Category IV | Any | Yes |
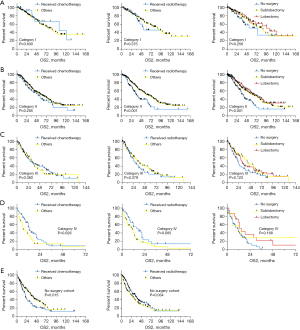
Then, the entire cohort is stratified into three group according to the diagnosis year (2000–2005, 2006–2010, and 2011–2015), the sample size for each group is 117, 394, and 534, respectively. As shown in Figure S3, the calibration plots presented well agreement between the nomogram prediction which is established on the entire cohort and actual observation for 1-, 3-, and 5-year survival in all three subgroups. The proposed risk stratification system presented a higher prediction accuracy on prognosis than the TNM stage system in all three groups according to the time-dependent ROC curve (Figure S3). Furthermore, the proposed risk stratification system could distinguish the OS2 well in all stages (I, P<0.001; II, P=0.004; III, P<0.001; IV, P<0.001) (Figure 4).

Then we estimated the association between treatment decision and OS2 in patients with different risk categories (Figure 5). Chemotherapy would improve prognosis in patients in IV category (P=0.028) and those without surgery (P=0.015). Radiotherapy would improve prognosis in patients without surgery (P=0.034). While surgery could benefit prognosis in patients with II (P<0.001) and III (P=0.049) category. In addition, the effectiveness of sublobectomy is comparable to lobectomy in all categories.
Discussion
In this study, we observed longer interval survival in the younger female patients. This might be correlated to the fact that the risk for lung cancer development is relatively low in this cohort (15). More aggressive resection in the first time is associated with less residual pulmonary tissue, which reduces the rate to develop metachronous lung cancer and thus is associated with shorter interval survival. Besides, shorter interval survival was observed in contralateral msPAD. This might be partially explained by the process of unction compensation. Because the contralateral pulmonary function is accounted for a larger proportion after the first resection, metachronous lung cancer is more likely to be located in the contralateral side. This speculation is in line with the observation that there are most metachronous (80.2%) lung cancers in the contralateral lobe after first resection (16).
The interval survival has long been regarded as an important indicator for the tumor clonality of metachronous multiple lung cancer. In the first edition of diagnostic criteria proposed by Martini et al., time interval >2 years is a necessary condition for the diagnosis of metachronous multiple primary lung cancer (mMPLC) (17). This edition was further modified by the ACCP in 2003. According to their suggestions, interval survival >4 years is a necessary condition for mMPLC, and the interval survival <2 years is a necessary condition for metastatic lung cancer (7). This suggestion is still used in the following editions (6,8). However, in this study, there is no significant association between interval survival between OS2, even in the univariate analysis (P=0.105). A similar result is also reported by Hamaji et al. (9). It has been widely accepted that the characteristic of tumor clonality would greatly impact long-term survival. It is plausible that, because interval survival is not a predictor for OS2, it should not be an essential factor to distinguish tumor clonality. The criterion for mMPLC, especially in the issue of interval survival, might be biased and merit further modification.
According to the extracted stage information, overlaps among OS2 are commonly observed. Because the methodology to distinguish tumor clonality is still biased, some patients with truly primary PAD might be overestimated, and some patients with truly metastatic PAD might be underestimated. To establish a TNM-like stratification system in the context of suspending dispute for the tumor clonality, including pathologic parameters were designed in a compromise way. For example, we applied tumor diameter to describe primary tumor status. Node status was reclassified into three groups, including negative, intrapulmonary metastasis, and mediastinal metastasis. Definition of distant metastasis in the current TNM stage was replaced into expulmonary metastasis. We found that the proposed risk stratification system well stratify the prognosis.
In addition, the proposed risk system yields a smaller AIC, but higher c-index than the TNM staging system. Besides, the AUC value from the proposed risk stratification is usually higher than that from the staging system with 160 months of follow-up. The risk system seems to be reliable for prognostication.
In this study, characteristics of the msPAD were included in the analysis of interval survival. In our opinion, when the msPAD is found and treated in early stage, the interval survival is short; when the msPAD is found and treated in advanced stage, the interval survival is long. In addition, it is plausible that, the characteristics of first PAD should impact OS2 as well. Therefore, in the survival analysis of OS2, characteristics of the first primary lung cancer were involved. We found that, although the T status (1st) present significant association with OS2 in the univariate analysis (P=0.045), however, it missed significance after adjusting other confounders in the multivariate analysis. Thus, the tentative risk stratification system was established on the characteristics of first primary lung cancer. For this phenomenon, there is two potential explanations. The first is that, the characteristics of the msPAD play an more important impact on OS2 than PAD. The second is that, the tumor clonality of the msPAD is still unclear, therefore its association with the first primary lung cancer is still unknown, which would greatly limit the impact of first primary lung cancer on OS2.
It has been widely accepted that surgery is an effective treatment for operable metachronous lung cancer (9,16). Similarly, in this study, surgery is associated with longer survival in patients with category II (P<0.001) and III (P=0.049) category. For patients with category I risk, surgery is associated with longer survival than those without (median OS2, 90 vs. 75 months), although the difference is not statistically significant (P=0.132). Therefore, we recommended performing surgery for patients with categories I, II, and III. Sublobectomy is a preferred plan on the premise of ensuring sufficient margin distance.
The findings of the present study should be considered in the context of certain weaknesses. First, because of the nature of SEER data, some well-known prognostic factors such as ground-glass opacity (GGO) ratio, cigarette smoking, and tumor markers were not included. Second, because the source problem of tumor clonality is not solved by our study, the proposed system could only be considered as a risk stratification rather than a staging system, although it is proved with well capacity in predicting and stratifying prognosis. Because the risk stratification is established in the premise of suspending dispute of tumor clonality, it is not suitable for msPAD when the tumor clonality is identified, such as pathologically confirmed ADC in situ and radiographically observed pure GGO (18). moreover, because the biological behavior of lung squamous cell carcinoma (SCC) is significantly different from PAD, especially in recurrence/metastatic pattern and multiple nodule model, our results could not be applied for metachronous second SCC patients after previously resected pulmonary SCC (19,20). Finally, although we carried out 1000 bootstrap resamples for interval validation, further external validation with other populations is still needed.
In conclusion, the TNM-like risk stratification appears to be suitable for prognostic prediction and risk stratification for msPAD patients with former PAD resection. This model validates and refines the known classification rules based on the easily collected variables, and highlights potential implications for clinical management and study design.
Acknowledgments
Funding: This work was supported by a grant from the Guangzhou Health Science and Technology Project (No. 20211A010001).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1982/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1982/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1982/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Guangzhou First People’s Hospital (K-2021-186-01). A statement that the participants gave informed consent before taking part is not required because this study is performed on an established retrospective database.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fois SS, Paliogiannis P, Zinellu A, et al. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int J Mol Sci 2021;22:612. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Ripley RT, McMillan RR, Sima CS, et al. Second primary lung cancers: smokers versus nonsmokers after resection of stage I lung adenocarcinoma. Ann Thorac Surg 2014;98:968-74. [Crossref] [PubMed]
- Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145:75-81; discussion 81-2. [Crossref] [PubMed]
- Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017;112:156-64. [Crossref] [PubMed]
- Shen KR, Meyers BF, Larner JM, et al. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:290S-305S.
- Detterbeck FC, Jones DR, Kernstine KH, et al. Lung cancer. Special treatment issues. Chest 2003;123:244S-58S. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Hamaji M, Allen MS, Cassivi SD, et al. Surgical treatment of metachronous second primary lung cancer after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;145:683-90; discussion 690-1. [Crossref] [PubMed]
- Detterbeck FC, Marom EM, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11:666-80.
- Loukeri AA, Kampolis CF, Ntokou A, et al. Metachronous and synchronous primary lung cancers: diagnostic aspects, surgical treatment, and prognosis. Clin Lung Cancer 2015;16:15-23. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol 2017;17:53. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Yang X, Zhan C, Li M, et al. Lobectomy Versus Sublobectomy in Metachronous Second Primary Lung Cancer: A Propensity Score Study. Ann Thorac Surg 2018;106:880-7. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [Crossref] [PubMed]
- Gao JW, Rizzo S, Ma LH, et al. Pulmonary ground-glass opacity: computed tomography features, histopathology and molecular pathology. Transl Lung Cancer Res 2017;6:68-75. [Crossref] [PubMed]
- Gomes S, Cavadas B, Ferreira JC, et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci Rep 2019;9:12838. [Crossref] [PubMed]
- Chen JW, Dhahbi J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci Rep 2021;11:13323. [Crossref] [PubMed]