
Estimation of the median effective dose and the 95% effective dose of alfentanil required to inhibit the bronchoscopy reaction during painless bronchoscopy with i-gel supraglottic airway device: an Up-and-Down Sequential Allocation Trial
Introduction
Bronchoscopy plays a vital role in the diagnosis and treatment of lung disease (1). The British Thoracic Society guideline for diagnostic flexible bronchoscopy (FB) recommends that sedation be provided to all patients undergoing bronchoscopy unless there are specific contraindications to sedation (2). “Painless bronchoscopy” can reduce perioperative stress, reduce the cough reaction, and avoid intraoperative awareness, which may improve the satisfaction of patients and operators (3). Although propofol alone is safe and effective for bronchoscopy, it is more effective in combination with analgesic drugs (4). The most commonly used analgesic agents for general anesthesia are opioids (5). Alfentanil is known to be a µ-opioid receptor agonist, causing analgesia, sedation, and suppression of the cough reflex. It also has the most rapid analgesic onset and time to peak effect, as well as the shortest distribution and elimination half-life (6). The effects of alfentanil have favorable pharmacological profiles for bronchoscopy.
Alfentanil combined with propofol produces synergistic sedation in patients with FB. A recent study demonstrated that the combination of alfentanil and propofol achieved fast onset and quick recovery, and may therefore be ideal for FB sedation, providing good physician satisfaction and patient tolerability (7). Conversely, other research has suggested that induction with alfentanil immediately before propofol target-controlled infusion (TCI) sedation for bronchoscopy is unsafe, particularly for hypoxemia (8). Furthermore, the dose of 5 µg/kg alfentanil could only achieve sedation of the patient, and was insufficient for the anesthesia intubation and endoscopic operation. Another study reported that 10 µg/kg alfentanil with 2.5 mg/kg propofol is an optimum dose for inserting an i-gel (9). Further studies are warranted to determine the effective dose of alfentanil required during fiberoptic bronchoscopy under laryngeal mask general anesthesia.
This trial determined the ED50 and the ED95 of an intravenous (IV) bolus of alfentanil that, when combined with propofol, could effectively blunt the bronchoscopy reaction to painless bronchoscopy. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-412/rc).
Methods
Ethics statement and study design
This study was an Up-and-Down Sequential Allocation Trial approved by the Hospital Ethics Committee of Taizhou Hospital of Zhejiang Province (No. K20200780). The study was registered at The Chinese Clinical Trial Registry (trial registration number: ChiCTR 2000035855, date of registration: August 18, 2020). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients who underwent a fiberoptic bronchoscopy at the Taizhou Hospital of Zhejiang Province, from January 1, 2021 to July 30, 2021, were included in this study. All patients signed informed consent forms prior to the trial. The following inclusion criteria were applied: (I) patients underwent fiberoptic bronchoscopy under general anesthesia; (II) patients were designated with American Society of Anesthesiologists (ASA) physical status I–II; and (III) patients were aged 18–70 years. The following exclusion criteria were applied: (I) patients with contraindications to bronchoscopy; (II) patients with opioid allergies; (III) patients with severe airway stenosis and acute infections; (IV) patients with arrhythmia, presenting with (i) sinus bradycardia with ventricular rate <45 beats/min and a negative atropine test; (ii) atrioventricular block or ventricular block of grade II or above; or (iii) other arrhythmias with a high risk of sinus arrest, such as sick sinus syndrome (SSS); (V) patients with severe cardiac insufficiency classified as New York Heart Association (NYHA) class III or above; (VI) patients with liver dysfunction characterized by abnormal biochemical indexes of liver function; (VII) women in labor, childbirth, or lactation period; (VIII) patients whose preoperative state of consciousness was difficult to determine independently or accurately; (IX) patients with a history of psychotropic drug use; and (X) patients who met any other conditions considered by the researchers as unsuitable for inclusion in this study.
Introduction and anesthetic management
Prior to fiber bronchoscopy under general anesthesia, all patients were fasted for no less than 8 hours and did not receive any premedication. Electrocardiography, pulse oximetry [oxygen saturation (SPO2)], and noninvasive blood pressure (NBP) assessments were performed to monitor all patients. In the stable period of 5 minutes after entering the operating room, heart rate (HR) and NBP were measured twice, and the average was taken as the baseline (T0). The lactate ringer’s solution was infused with a 22-gauge IV cannula placed in the arm. Each patient was preoxygenation for 3 minutes. A trained physician administered the TCI sedation (Injectomat TIVA Agilia, Fresenius Kabi, France), monitored the sedation and vital signs, and provided supportive interventions when necessary. Alfentanil (5–25 µg/kg, Yichang, China) was administered 2 minutes before propofol administration. After induction of anesthesia, the i-gel was positioned and the correct position was verified with chest auscultation and capnography. During the induction period, the state entropy (SE) value was maintained between 40 and 60. If the patient remained apneic for more than 30 seconds after i-gel O2 insertion, the lungs were manually ventilated via the i-gel to maintain SPO2 above 95% and end-tidal carbon dioxide tensions between 40 and 50 mmHg, until spontaneous ventilation was achieved. Blood pressure (BP) and HR were immediately recorded after induction of general anesthesia (T1), before the i-gel laryngeal mask was positioned (T2), and immediately after the i-gel laryngeal mask was positioned (T3). An experienced endoscopist performed the procedures using a flexible bronchoscope with an insertion tube outer diameter of 4.8 mm (Olympus Q290) (T4). BP and HR were assessed again at 1 minute after the fiberoptic bronchoscopy entered the airway (T5). A second anesthesiologist, who was blinded to the dose of alfentanil, administered the anesthesia. The bronchoscopy feasibility was assessed using the bronchoscopy score, which was computed by combining the following three variables that were graded from 1–4: movement of the vocal cords, cough occurrence, and limb movement. The final bronchoscopy score varied between 3 and 12, where 3 represented the optimal score and 12 represented the worst score. The primary objective was the bronchoscopy score, which refers to a reaction that ultimately influences the FB procedure and requires an additional administration of alfentanil or propofol. The bronchoscopy reaction was defined as the bronchoscopy score >6. A bronchoscopy score >6 suggested an obvious reaction to the FB, and a bolus dose of alfentanil (10–20 µg) was intravenously administered. A bronchoscopy score ≤6 suggested a negative reaction to the bronchoscopy. The conditions of the FB insertion were only assessed at the first attempt. During the FB procedure, 10–20 µg alfentanil could also be administered intravenously if necessary. Patients with BP below 60 mmHg or HR below 45 beats/min during the procedure were excluded from this study. In patients whose HR decreased to <45 beats/min, 0.5 mg of atropine was administered.
If the mean BP (MBP) was ≤60 mmHg for 30 seconds, 6 mg of ephedrine was administered. Patients with tachycardia or hypertension [defined as HR and systolic BP (SBP)/diastolic BP (DBP) >20% of the baseline values] after FB were treated with esmolol or additional propofol. After the FB procedure, all of the patients were transferred to the post-anesthesia recovery room for close observation until the standards of exiting the Operating Room were satisfied.
Dixon’s up-and-down
The ED50 of alfentanil was calculated using the modified Dixon’s up-and-down method (10). Given our previous experience and pre-experimental data, the initial dose of alfentanil was set at 20 µg/kg. The next patient received an increased dose of alfentanil (5 µg/kg) if the patient had a positive response, or a reduced dose (5 µg/kg) if there was no positive response. After seven inflection points had been obtained, patient recruitment was terminated. The patients were divided into two groups based on gender, namely, a male group and a female group.
Statistical methods
Clinical data was expressed as count (percentage), or as mean ± standard deviation (mean ± SD). Normality for continuous variables was assessed using the Shapiro-Wilk test (P value >0.05). Between-group comparisons were made using Student’s t-test or a corrected t-test for normally distributed continuous variables. Two-sided P values <0.05 were considered statistically significant. ED50 values were compared between groups using the t-tests. The dose-response curve was calculated by probit regression analysis. All of statistical analyze were performed with GraphPad Prism 8.0 and SPSS 26.0 software.
Results
A total of 48 adult patients were recruited into the study from January 1, 2021 to July 30, 2021, including 25 females and 23 males. No patients were excluded from the study due to BP readings below 60 mmHg or HR below 45 beats/min. The demographic data of the patients are shown in Table 1. The median age was 54 years (range, 25–70 years) (Table 1). There was no significant difference in patient characteristics [such as age, ASA, and body mass index (BMI)] between males and females (P>0.05; Table 1). The total dosage of alfentanil administered was 15.1±4.9 µg/kg during the FB procedure. The i-gel was successfully positioned in all patients. No failed positioning was recorded.
Table 1
| Characteristic | Overall (n=48) | Male (n=23) | Female (n=25) | P |
|---|---|---|---|---|
| Age, years, mean ± SD | 54.2±10.0 | 55.5±6.9 | 53.0±12.2 | 0.380 |
| Weight, kg, mean ± SD | 60.8±8.1 | 63.4±6.0 | 58.5±9.1 | 0.031 |
| Height, cm, mean ± SD | 161.9±7.5 | 167.4±5.1 | 156.8±5.5 | 0.000 |
| BMI, kg/m2, mean ± SD | 23.3±3.1 | 22.8±2.5 | 23.8±3.6 | 0.290 |
| ASA physical status, n (%) | 0.057 | |||
| ASA I | 23 (47.9) | 5 (21.7) | 18 (78.3) | |
| ASA II | 15 (31.3) | 12 (48.0) | 13 (52.0) | |
| HR (T0), B/M, mean ± SD | 80.2±11.5 | 77.2±9.2 | 83.0±13.0 | 0.076 |
| RR (T0), B/M, mean ± SD | 17.0±1.3 | 17.2±1.3 | 17.0±1.3 | 0.270 |
| MAP (T0), mmHg, mean ± SD | 95.3±10.0 | 94.2±8.1 | 96.3±11.5 | 0.470 |
| HR (T1), B/M, mean ± SD | 75.4±10.7 | 74.6±8.8 | 76.2±12.3 | 0.612 |
| RR (T1), B/M, mean ± SD | 10.7±14.3 | 8.7±6.0 | 12.4±19.0 | 0.382 |
| MAP (T1), mmHg, mean ± SD | 87.7±12.5 | 87.7±11.1 | 87.6±13.9 | 0.961 |
| Bronchoscopy score (T4), median (IQR) | 5.5 (4.0–7.0) | 6.0 (4.0–7.0) | 5.0 (3.0–7.0) | 0.478 |
| Bronchoscopy score (T5), median (IQR) | 3.0 (3.0–4.0) | 4.0 (3.0–5.0) | 3.0 (3.0–4.0) | 0.116 |
| Alfentanil dose, μg/kg, mean ± SD | 15.1±4.9 | 16.7±4.4 | 13.6 ±4.9 | 0.025 |
BMI, body mass index; ASA, American Society of Anesthesiologists; HR, heart rate; RR, respiratory rate; B/M, beats/min; MAP, mean arterial pressure.
Figure 1 shows that each group reached 7 crossover points. The ED50 of alfentanil for blunting the FB reaction in the 23 males and the 25 females was 17.96±3.45 µg/kg (Figure 1A) and 13.68±4.75 µg/kg (Figure 1B), respectively. The ED50 of alfentanil for blunting the FB reaction was comparable between males and females (P=0.078>0.05).
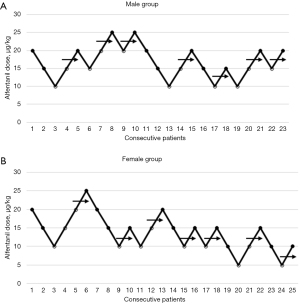
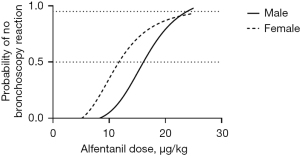
Figure 2 shows the dose-response curve from the probit analysis of the alfentanil dose and the probability of no FB reaction. The ED50 of alfentanil in female bronchoscopies was 12.4 µg/kg (95% CI: 4.5 to 17 µg/kg). In men, the ED50 of alfentanil was 16.4 µg/kg (95% CI: 12.1 to 20.1 µg/kg). According to the probit analysis, the ED95 of alfentanil in females and males was 22.4 µg/kg (95% CI: 17.5 to 67.3 µg/kg) and 23.3 µg/kg (95% CI: 19.8 to 46.2 µg/kg), respectively.
Discussion
Bronchoscopy is a highly stimulating clinical operation, and patients feel a strong sense of discomfort during the diagnosis and treatment process, and a small number of patients may interrupt the examination due to intolerance (11). Painless technology can eliminate the discomfort and fear of patients during the diagnosis and treatment of bronchoscopy, effectively suppressing the patient’s choking reflex, reducing the risk of injury and accidents during diagnosis and treatment, and creating improved diagnosis and treatment conditions for endoscopists (12). However, a standardized anesthesia plan for painless bronchoscopy is still lacking (13,14). The painless protocol for this study involved a laryngeal mask under administration of propofol combined with alfentanil. Alfentanil has been proven to reduce the amount of isobaric propofol required for anesthesia induction in a synergistic manner. None of the patients in this study suffered severe adverse events, such as chest rigidity, difficulty laryngeal mask placement, or cyanosis, after the alfentanil injection. Both BP and HR changed after alfentanil injection, but largely remained within the clinical range. The ED50 of alfentanil required to blunt the FB reaction was 13.68±4.75 and 17.96±3.45 µg/kg in women and men, respectively. In a randomized, double-blinded, controlled trial of using alfentanil in the induction of propofol infusion for inserting an i-gel, the optimum dose for alfentanil was determined to be 10 µg/kg, when coadministered with 2.5 mg/kg propofol (9). This was lower than the dose obtained in our study, and may be due to more intense airway irritation associated with the FB compared to the laryngeal mask.
Animal and human studies have suggested that there are gender differences in opioid-induced analgesia and associated adverse events (15-17). Clinicians need to be aware of the sex differences when administering opioids (18). There is ample literature demonstrating sex differences in morphine analgesia. However, it was unclear whether increased efficacy of certain opioid agonists in women is also observed with alfentanil, a widely used selective opioid agonist. Our current study demonstrated that there was no gender difference in the ED50 of alfentanil required for blunting the FB reaction. The previous study has been shown that alfentanil analgesia is an absence of sex differences (19). A previous study has shown that the pharmacokinetics of remifentanil were influenced by BMI and age, but gender did not affect any pharmacokinetic nor pharmacodynamic parameters (20). Future studies are needed to investigate the analgesic effects of alfentanil at different doses and in the different genders.
In summary, the ED50 of alfentanil required for successful bronchoscopy under i-gel laryngeal mask general anesthesia was 17.96±3.45 µg/kg in males and 13.68±4.75 µg/kg in females. There were no obvious differences between the genders in the effective dose of alfentanil in painless bronchoscopy. The doses of alfentanil found in this study may provide a reference for clinicians.
Acknowledgments
Funding: This work was supported by the Science and Technology Project of Taizhou (No. 21ywb05) and the Medicines Health Research Fund of Zhejiang (No. 2022KY435).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. https://jtd.amegroups.com/article/view/10.21037/jtd-22-412/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-412/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-412/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Hospital Ethics Committee of Taizhou Hospital of Zhejiang Province (No. K20200780). All patients signed informed consent forms prior to the trial.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Andolfi M, Potenza R, Capozzi R, et al. The role of bronchoscopy in the diagnosis of early lung cancer: a review. J Thorac Dis 2016;8:3329-37. [Crossref] [PubMed]
- British Thoracic Society Bronchoscopy Guidelines Committee. a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56:i1-21. [Crossref]
- Gasparini S. It is time for patients to undergo bronchoscopy without discomfort. Eur Respir J 2011;38:507-9. [Crossref] [PubMed]
- Schlatter L, Pflimlin E, Fehrke B, et al. Propofol versus propofol plus hydrocodone for flexible bronchoscopy: a randomised study. Eur Respir J 2011;38:529-37. [Crossref] [PubMed]
- Gupta D. Balanced anesthesia-baseline anesthesia. Ann Neurol 2012;72:629-30. [Crossref] [PubMed]
- Moman RN, Mowery ML, Kelley B. StatPearls Publishing 2022;LLC:2022.
- Lo YL, Lin TY, Fang YF, et al. Feasibility of bispectral index-guided propofol infusion for flexible bronchoscopy sedation: a randomized controlled trial. PLoS One 2011;6:e27769. [Crossref] [PubMed]
- Hsieh CH, Lin TY, Wang TY, et al. The safety and efficacy of alfentanil-based induction in bronchoscopy sedation: A randomized, double-blind, controlled trial. Medicine (Baltimore) 2016;95:e5101. [Crossref] [PubMed]
- Yu AL, Critchley LA, Lee A, et al. Alfentanil dosage when inserting the classic laryngeal mask airway. Anesthesiology 2006;105:684-8. [Crossref] [PubMed]
- Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991;15:47-50. [Crossref] [PubMed]
- Minami D, Takigawa N, Watanabe H, et al. Safety and discomfort during bronchoscopy performed under sedation with fentanyl and midazolam: a prospective study. Jpn J Clin Oncol 2016;46:871-4. [Crossref] [PubMed]
- Wang J, Yang S, Chen J, et al. Painless fiberoptic bronchoscopy in patients with COVID-19: analysis of 33 cases. Nan Fang Yi Ke Da Xue Xue Bao 2021;41:562-6. [PubMed]
- Yarmus LB, Akulian JA, Gilbert C, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc 2013;10:121-6. [Crossref] [PubMed]
- Abouzgheib W, Ben-Jacob TK, Borah A, et al. A Randomized Controlled Trial Comparing a Mapleson Circuit with Nasal Trumpet to Standard Oxygen Supplementation during EBUS Bronchoscopy under Monitored Anesthesia Care. Biomed Hub 2019;4:1-9. [Crossref] [PubMed]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 2004;8:397-411. [Crossref] [PubMed]
- Mogil JS, Wilson SG, Chesler EJ, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A 2003;100:4867-72. [Crossref] [PubMed]
- Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Sci 2019;237:116926. [Crossref] [PubMed]
- Lau T, Hayward J, Vatanpour S, et al. Sex-related differences in opioid administration in the emergency department: a population-based study. Emerg Med J 2021;38:467-73. [Crossref] [PubMed]
- Olofsen E, Romberg R, Bijl H, et al. Alfentanil and placebo analgesia: no sex differences detected in models of experimental pain. Anesthesiology 2005;103:130-9. [Crossref] [PubMed]
- Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 1997;86:10-23. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


