
Surgical outcomes of oro-intestinal continuity reconstruction after total esophagectomy in patients with cervicothoracic malignancy: a thoracic surgeon’s perspective
Introduction
In patients with cervicothoracic malignancy and esophageal invasion, oro-intestinal continuity reconstruction is required to achieve complete resection (1). The procedure involves cervicomediastinal exenteration, tracheal relocation with tracheostomy, and immediate reconstruction of the mediastinal defect and continuity of the digestive tract (1,2). In case of cervicothoracic malignancy with minimal esophageal invasion, oro-intestinal continuity reconstruction can be done using a local flap (2). However, when total esophagectomy is needed for complete resection, oro-intestinal continuity reconstruction becomes challenging as it involves the continuity of a great length of the digestive tract, as well as mediastinal tracheostomy and filling of the cervicomediastinal defect (1,2). Previous studies have reported high rates of morbidity and mortality following this surgery due to potential complications such as mediastinal sepsis, rupture of major vessels, and necrosis of anastomosis (1-3). However, few studies have analyzed the feasibility and safety of this surgery, and the number of patients enrolled in these studies was relatively small.
In this study, we sought to investigate the perioperative and long-term surgical outcomes of oro-intestinal continuity reconstruction after total esophagectomy in patients with cervicothoracic malignancy. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1768/rc).
Methods
Patients
From July 2011 to September 2018, 14 patients underwent oro-intestinal reconstruction surgery at the Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea. Electronic medical records of patients were retrospectively reviewed to determine the characteristics of the patients, surgical profiles, postoperative profiles, postoperative complications, oncologic outcomes, and recovery of dietary function. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by institutional board of the Asan Medical Center (No. 2022-0144), and the requirement for informed consent was waived because of the retrospective nature of the study design.
Preoperative work-up
As a preoperative evaluation, neck, chest, and abdominopelvic computed tomography (CT); whole-body positron emission tomography (PET); gastroduodenoscopy; bronchofluoroscopy; laryngoscopy; oropharyngoscopy; colonoscopy; superior mesenteric artery (SMA)/inferior mesenteric artery (IMA) angiography; pulmonary function test; and echocardiography were performed. Whether the surgery would be beneficial in terms of survival and palliative benefits and the resectability of the tumor was evaluated by a multidisciplinary team that included medical oncologists, radiologists, a radiation oncologist, a gastrointestinal (GI) physician, a head and neck surgeon, and a thoracic surgeon. The surgical indication of patients with cervicothoracic malignancy and esophageal invasion was not definite, but patients of advanced age (>75 years), or in poor physical condition (Eastern Cooperative Oncology Group performance ≥2), with decreased pulmonary function test (FEV1% <60% of predicted), were generally contraindicated. When surgical treatment was decided as the path forward, head and neck, stomach, colorectal, and plastic surgeons were consulted to participate in the oro-intestinal continuity reconstruction. Prior to the surgery, a feeding jejunostomy was employed in some patients with esophageal obstruction to optimize their nutritional status for perioperative care and recovery.
Operative technique
The oro-intestinal continuity reconstruction with total esophagectomy consisted of three phases: (I) exposure and tumor resection phase, (II) conduit formation phase, and (III) reconstruction phase. A gastric conduit, a colon conduit, or a jejunal free-flap was selected depending on the extent of resection and reconstruction required. A transhiatal or transthoracic approach was selected for esophagectomy, and conventional or mediastinal tracheostomy was performed to keep the air way intact.
(I) Exposure and tumor resection
A low collar incision was done with the patient in the supine position. Complete neck exploration was performed to assess the tumor involvement in the neck and mediastinum. The surgical field was exposed from the hyoid bone superiorly to the sternal notch inferiorly. Then, the underlying trachea and esophagus were exposed. The prevertebral fascia was checked for any tumor invasion. For better exposure of the mediastinum, the manubrium was split, and both clavicular heads were removed along with the medial clavicles and first and second rib cartilages.
Resection of the trachea, larynx, pharynx, and nodal tissue was performed by an experienced head and neck surgeon (Figure 1). Resection of the trachea was performed preserving a 1.5 cm margin of disease-free tissue. The strategy for the management of the tracheal stump was determined based on the residual length of the trachea. If the residual tracheal stump was several centimeters above the sternal notch, a conventional cervical tracheostomy was performed. However, when the trachea was resected just behind the sternum or when there was a recurrence at the primary tracheostomy site, an anterior mediastinal tracheostomy was created during the reconstruction phase. The tracheal stump margin was sent for frozen section analysis to assess for the absence of residual cancer. Meticulous dissection was performed from the mediastinum to the neck superiorly. After reaching the level of the larynx, the suprahyoid muscles, hyoid bone, and laryngeal muscles were transected. The superior laryngeal nerve and vessels were also resected. Continuing the dissection posteriorly, the pharynx and epiglottis were resected. The superior resection margin was also sent for frozen biopsy. Cervical node dissection was also performed together.
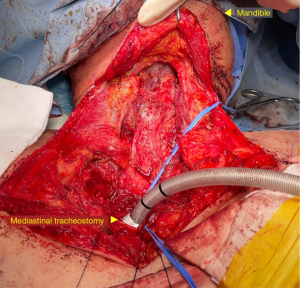
To completely remove the entire esophagus, a transhiatal or transthoracic approach was followed by the thoracic surgeon. The transhiatal approach was performed with the patient in the supine position. During transhiatal approach esophagectomy, blunt mediastinal dissection of the esophagus and resection of tumor with pharyngo-laryngectomy were consequently performed. In the transthoracic approach, the patient was repositioned in a left-sided lateral position for a conventional posterolateral thoracotomy or a left-sided semiprone position for a robot assisted, minimally invasive esophagectomy with four ports. During the surgery, the lymph nodes including both recurrent laryngeal, subcarinal, hilar, azygous vein, upper, middle, lower para-esophageal, upper and lower paratracheal, and inferior pulmonary ligament lymph nodes, were resected along with the esophagus and tumor.
(II) Conduit formation
Conduit formation via a median laparotomy was performed with the patient in a supine position by an experienced stomach, colorectal, or plastic surgeon based on the organ selected for conduit construction. When using the stomach as the conduit, it was essential to prepare sufficient omentum together for wrapping the anastomosis site securely. During the abdomen phase, a feeding jejunostomy was routinely performed on patients who needed appropriate nutritional support required to endure the long hospital stay and the highly probable postoperative complications.
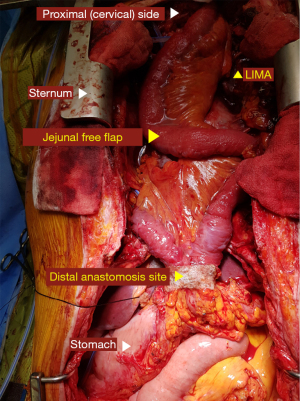
When using the jejunal free-flap approach, flaps were harvested by the stomach surgeon. The principal vessel for the appropriate arcades was meticulously dissected. The bowel was divided using an intestinal stapler at both ends, and the jejunum was prepared for perfusion prior to the ligation of the pedicle vessels. The flap was transferred to the neck region, and a subsequent microsurgical vascular anastomosis was performed by the plastic surgeon. The feeding vessels for the jejunal free-flap were determined based on the distance from the flap and the degree of vascular flow (e.g., carotid artery/internal jugular vein, right internal mammary artery/right internal mammary vein).
(III) Reconstruction
After the formation of the conduit abdominal phase, oro-intestinal continuity reconstruction was performed by a single thoracic surgeon. Although the posterior mediastinal route was routinely used as the reconstruction route, the substernal route was occasionally adopted for a colon conduit. The proximal end of the conduit was hand-sewn and anastomosed to the tongue base (Figure 2). A size discrepancy between the conduit and the tongue base was inevitable. Therefore, suturing of the adjacent tissue was needed for size adjustment. The hand-sewn anastomosis was accomplished using a double layer of continuous or interrupted sutures. In brief, the outer row of the anastomosis was carried out across the longitudinal muscle fibers of the esophagus with interrupted horizontal mattress sutures of fine silk (4–0). The mucosa of the esophagus and stomach were then approximated with absorbable monofilament sutures (4–0 Maxon, polyglyconate; Davis and Geck, Danbury, CT) (Figure 3).
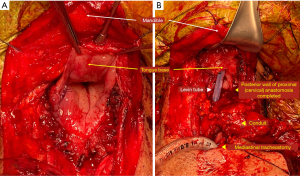
If it was impossible to perform a conventional tracheostomy, an anterior mediastinal tracheostomy was performed. Considering the possibility of an innominate artery rupture, the tracheostomy was located at the right side of the reconstruction conduit. Moreover, the tracheal stump was wrapped with omentum from the conduit to prevent erosion of the innominate artery. The mediastinal defect was covered with the cutaneous flap created by the pectoral fascia or pectoralis major flap with its entire muscle and skin island.
Postoperative care
Patients were admitted to the intensive care unit for ventilator care and hemodynamic monitoring. The nasogastric tube was routinely inserted in the operating room. On postoperative day 4, esophagography was performed to evaluate the patency of the bowel conduit and identify any leaks or strictures at the site of anastomosis. A clear liquid diet was started if the esophagography was normal. The chest tube was removed when patients could tolerate a soft diet, and there was no evidence of chylothorax, air leakage, empyema, and excessive tube drainage (>3 mL/kg/day).
Statistical analysis
Categorical variables were presented as frequencies and percentages of the relevant denominators, and continuous variables were presented as medians. Due to the limited number of patients and descriptive nature of our study, analysis for identifying factors associated with events could not be performed.
Results
The baseline characteristics of the patients are summarized in Table 1. There were 10 male and 4 female patients. The median age of the patients was 61 (range, 42–72) years. The median follow-up was 18.6 (range, 0–52.9) months. Among the 14 patients, 10 (71.4%) had undergone surgery for the primary cancer and 2 of them were diagnosed with double primary cancers. Four patients (28.6%) underwent surgeries for salvage operation and one for secondary primary esophageal cancer. In total, six patients (42.8%) had esophageal cancer, five (35.7%) had head and neck squamous cell carcinomas (HNSCC), and the remaining three (21.4%) had HNSCC with synchronous or metachronous secondary primary esophageal cancer (double primary cancers). The cancer stages of most of the patients were above stage III according to the American Joint Committee on Cancer (AJCC) 8th edition TNM classification. Details of clinical characteristics, surgical procedures, and pathological findings are described in Table 2.
Table 1
| Variable | Value |
|---|---|
| Age (year) | 61 (range, 42–72) |
| Sex (male) | 10 (71.4) |
| BMI, kg/m2 | 21.3 (range, 14.0–29.0) |
| Smoking history | 10 (71.4) |
| Charlson comorbidity index | |
| ≤3 | 3 (21.4) |
| 4–6 | 4 (28.6) |
| ≥7 | 7 (50.0) |
| ECOG Performance status | |
| 0 | 2 (14.3) |
| 1 | 10 (71.4) |
| 2 | 2 (14.3) |
| Pulmonary function | |
| FEV1 (%) of predicted | 72.5 (range, 45–120) |
| Primary cancer | 10 (71.4) |
| Esophageal cancer | 5 (35.7) |
| Head and neck cancer | 3 (21.4) |
| Double primary cancer | 2 (14.3) |
| Salvage operation | 4 (28.6) |
| Esophageal cancer | 1 (7.1) |
| Head and neck cancer | 2 (14.3) |
| Double primary cancer | 1 (7.1) |
Values are numbers (%), or median (range; minimum value – maximum value), unless otherwise noted. BMI, body mass index; ECOG, eastern cooperative oncology group; FEV1, forced expiratory volume during the first second.
Table 2
| Patient No. | Sex | Age | Diagnosis | Malignancy History | Stage | pTNM | Operative procedure | Resection margin (R0, R1, R2) | Recurrence free survival (months) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 49 | Esophageal SqCC corrosive stricture | IIA | pT3N0M0 | TPL, ThE-RA, OG, C-T, cholecystectomy | R1 | – | Dead, 26 mo | |
| 2 | F | 54 | Subglottic SqCC & 2nd primary esophageal SqCC | IVb/Ia | pT3N3bM/pT1aN0M0 | TPL, TtE, OG, C-T | R0 | 42.6 | Alive, 42.6 mo follow-up | |
| 3 | M | 68 | Subglottic SqCC | Tongue SqCC s/p hemiglossectomy, RTx. | IVa | pT4aN0M0 | TPL, TT, ThE, OG, C-T | R1 | – | Dead, 13.7 mo |
| 4 | M | 72 | Hypopharyngeal SqCC invading esophagus | Glottic SqCC s/p RTx. | IVa | pT4aN2bM0 | TPL, HT, ThE, OG, C-T | R0 | 1.5 | Dead, 1.6 mo |
| 5 | M | 63 | Recurred supraglottic SqCC at hypopharynx & upper esophagus | Supraglottic SqCC s/p supraglottic laryngectomy, MRND, s/p CCRT | IVa | pT4aN0M0 | TPL, HT, ThE, OG, C-T | R0 | 15.6 | Dead, 23.4 mo |
| 6 | M | 63 | Recurred glottic SqCC with neck metastasis | Glottic SqCC s/p RTx. | IVb | pT4aN3bM0 | TPL, TT, ThE, OG, ALT FF, AMT redo OJG d/t conduit failure | R0 | 17.9 | Dead, 24.5 mo |
| 7 | M | 65 | Esophageal SqCC | IIIc | pT4N1M0/pT1aN0M | TPL, ThE-RA, OG, AMT | R0 | 52.9 | Alive, 52.9 mo follow-up | |
| 8 | M | 56 | Thyroid cancer, anaplastic | IVb | pT4bN1bM0 | TPL, TT, ThE, OG, C-T TdL, redo PMMC flap for anastomosis leakage | R0 | 0.2 | Dead, 2.3 mo | |
| 9 | M | 64 | Esophageal SqCC | IIIa | pT1bN2M0 | TPL, TtE-RA OG, C-T | R0 | 6.8 | Dead, 11 mo | |
| 10 | F | 54 | Esophageal SqCC | IIIc | pT4bN3M1 | TPL, TT, TtE-RA OG, C-T, TdL, lung wedge resection | R0 | 1.5 | Dead, 29 mo | |
| 11 | F | 42 | Esophageal SqCC | NSCLC, ADC, LLL s/p CCRT | IIIc | pT4bN0M0 | TPL, HT, TtE, OG, AMT, PMMC flap, TdL | R1 | – | In-hospital death, POD#74 |
| 12 | M | 68 | Subglottic SqCC & 2nd primary esophageal SqCC |
IVa/Ib | pT4aN2M/pT1bN0M0 | TPL, HT, ThE, OCG, C-T | R0 | 9.0 | Dead, 31.4 mo | |
| 13 | M | 61 | Recurred esophageal SqCC | Esophageal SqCC s/p CCRT | IIIc | pT4aN1M0 | TPL, TtE, OCG, AMT, TdL | R1 | – | Dead, 3 mo |
| 14 | M | 59 | Recurred hypopharyngeal SqCC & 2nd primary esophageal SqCC | Hypopharyngeal SqCC s/p RTx. | III/Ia | pT3N0M0/pT1bN0M0 | TPL, ThE, OJG, C-T redo OJG d/t conduit failure | R0 | 0.8 | In-hospital death, POD#25 |
SqCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; ADC, adenocarcinoma; LLL, left lower lobe; s/p, status post; CCRT, concurrent chemoradiation; d/t, due/to; RTx., radiation therapy; MRND, modified radical neck dissection; TPL, total pharyngo-laryngectomy; TT, total thyroidectomy; HT, hemithyroidectomy; ThE, transhiatal esophagectomy; TtE, transthoracic esophagectomy; RA, robot-assisted; OG, oro-gastrostomy; OCG, oro-colo-gastrostomy; OJG, oro-jejuno-gastrostomy; C-T, conventional tracheostomy; AMT, anterior mediastinal tracheostomy; TdL, thoracic duct ligation; PMMC, pectoralis major myocutaneous; ALT FF, anterolateral thigh free flap; R0, resection margin clear; R1, microscopic resection margin positive; R2, gross residual disease; POD, postoperative day.
Operative profiles are listed in Table 3. The median operation time was 620 min (405–914 min). According to the patients’ profiles, there were 11 cases of oro-gastrostomy (78.6%), 2 of oro-colo-gastrostomy (14.3%), and 1 of oro-jejuno-gastrostomy (7.1%). Redo-oro-jejuno-gastrostomy in two patients and anastomosis repair using a pectoralis major musculocutaneous (PMMC) flap in one patient were performed in a total of three reoperations (21.4%). Transhiatal and transthoracic esophagectomy was performed in 9 (64.3%) and 5 (35.7%) patients, respectively. Complete resection was achieved in 10 (71.4%) patients, whereas it was not achieved in 4 (28.6 %). Most of the microscopic resection margin-positive (R1 resection) sites were tracheal margins.
Table 3
| Variable | Value |
|---|---|
| Operation time, minutes | 620 [405–914] |
| ICU stay, days | 2 [0–17] |
| Ventilation time, days | 1 [0–17] |
| Hospital stay, days | 30 [17–85] |
| Conduit selection | |
| Oro-gastrostomy | 11 (78.6) |
| Oro-jejuno-gastrostomy | 1 (7.1) |
| Oro-colo-gastrostomy | 2 (14.3) |
| Conduit failure | 3 (21.4) |
| Redo-oro-jejuno-gastrostomy | 2 (14.3) |
| Repair via PMMC flap | 1 (7.1) |
| Method of esophagectomy | |
| Transhiatal | 9 (64.3) |
| Transthoracic | 5 (35.7) |
| Method of tracheostomy | |
| Conventional | 10 (71.4) |
| Mediastinal | 4 (28.6) |
| Robot-assisted procedure | 4 (28.6) |
| Resection margin | |
| R0 | 10 (71.4) |
| R1 | 4 (28.6) |
Values are numbers (%), or median (range: minimum value – maximum value), unless otherwise noted. R1 resection site: 1 patient of trachea; 1 patient of trachea, thyroid cartilage, cricoid cartilage, thyroid; 1 patient of trachea, spine, sternocleidomastoid muscle; 1 patient of distal esophagus. ICU, intensive care unit; PMMC, pectoralis major myocutaneous; R0, resection margin negative; R1, resection margin positive.
The postoperative clinical outcomes of patients are described in Table 4. The postoperative mortality within 30 days, 90 days, and 1 year was 7.1%, 28.6%, and 42.8%, respectively. Overall mortality occurred in 12 patients (86%), including two in-hospital deaths (14%). Except for two cases of in-hospital deaths, 10 patients died, 6 of whom died due to disease progression, 1 due to bleeding, and 3 without definite causes. In-hospital deaths (n=2, 14.0%) were due to postoperative pneumonia (n=1) and massive bleeding that originated from a trachea-innominate vein fistula (n=1).
Table 4
| Variable | Value |
|---|---|
| Early mortality | |
| Within 30 days | 1 (7.1) |
| Within 90 days | 4 (28.6) |
| Within 1 year | 6 (42.8) |
| Recurrence free survival (months) | 7.9 (range, 0.2–52.9) |
| Postoperative major complication | |
| Overall complications | 7 (50.0) |
| Surgical complications | |
| Anastomotic leakage or stricture | 3 (21.4) |
| Conduit necrosis | 2 (14.3) |
| Bleeding | 1 (7.1) |
| Reoperation | 3 (21.4) |
| Chylothorax | 1 (7.1) |
| Wound problem | 2 (14.3) |
| Pulmonary complications | |
| Pneumonia | 1 (7.1) |
| Prolonged ventilation (>24 hours) | 6 (42.8) |
Values are numbers (%), or median (range: minimum value – maximum value), unless otherwise noted.
Postoperative esophagography was performed for every patient. Anastomosis site leakage was observed in three patients (21.4%); one patient underwent reoperation. The patient who underwent oro-gastrostomy with total laryngo-pharyngectomy received primary repair of the anastomosis site and coverage with a PMMC flap. Of two patients who did not undergo reoperation, one adopted conservative management with endoscopic vacuum therapy and the anastomosis site healed spontaneously. The other patient who underwent oro-gastrostomy, total pharyngolaryngectomy, and mediastinal tracheostomy died due to uncontrolled bleeding of the trachea-innominate vein fistula 74 days after the operation.
Conduit necrosis was observed in two patients (14.3%). One patient who underwent oro-gastrostomy with total pharyngolaryngectomy received redo-oro-jejuno-gastrostomy. The other patient who underwent total pharyngolaryngectomy with oro-jejuno-gastrostomy also received redo-oro-jejuno-gastrostomy.
Discussion
In advanced HNSCCs and cervical esophageal cancers (CECs) that are generally considered unresectable (4,5), definitive chemoradiotherapy is currently favored as a treatment method (1,2,6) and shows 3-year overall survival rates of 29% to 66.5% (7). However, up to 60% of locoregional recurrence after definitive chemotherapy has been reported (7-11). From this perspective, total esophagectomy followed by oro-intestinal continuity reconstruction could be considered to achieve complete resection in selected patients with advanced HNSCCs and CECs. Although the surgery might lower the quality of life of the patients, it might be the only treatment option as a salvage therapy for patients who want the life-saving option following the failure of definitive regimens (1,2). However, owing to serious morbidity and mortality, surgical treatment for these patients is limited in the clinical field (1-3) and there are few reports on surgery in these patients. In this study, we evaluated the treatment outcomes of patients, who underwent oro-intestinal reconstruction surgery in our center, from a thoracic surgeon’s perspective.
In this study, postoperative patient mortality within 30 days, 90 days, and 1 year was 7.1%, 28.6%, and 42.8% of, respectively. Operative complications occurred in half of the overall patients. The most common complication was prolonged ventilation over 24 hours. There were three cases of reoperation, two of conduit necrosis, and one of anastomosis leakage. Regarding oncological outcomes, the rate of complete resection was 71.4%, although most of the patients had stage III or IV cancer. Furthermore, 1- and 2-year overall survival was 57.14% and 42.86%, respectively. These outcomes are comparable to those of previous studies (10,11), although those results were obtained from patients with middle and lower esophageal cancer without pharyngolaryngectomy or with less aggressive tumor stage compared to our study.
In terms of treatment modality, the prognoses of patients who underwent surgical resection in this study were relatively inferior to those who received definitive chemoradiation therapy for CECs and advanced-stage HNSCC (12,13). However, the patients in this study had more aggressive cancer, such as tracheal or esophageal invasion, compared with those in previous studies (12,13). In addition, some patients in this study underwent salvage operation due to cancer recurrence. Thus, we believe that reconstruction of oro-intestinal continuity after total esophageal resection is sufficiently beneficial if the tumor seems to be completely resectable and the patient can tolerate the surgery.
Performing an oro-intestinal reconstruction with a total esophagectomy is technically demanding. The HNSCC or CEC is located deep in the cervicothoracic region, close to the neck vessels and adjacent structures. The challenge increases when you consider reconstruction of gastrointestinal continuity itself using a variety of conduits—stomach, colon, and jejunal free flap. Moreover, proper approach routes should be selected and maintained with adequate blood supply to the conduit. Thus, surgical plan should be made thoughtfully to select the most appropriate conduit, surgical approach, and conduit route. Depending on the surgical plan, it may also be necessary to work cooperatively with a surgeon skilled in the specific surgical specialty. Finally, as a conductor, the thoracic surgeon should set the order of the operation, monitor the progress of the operation, appropriately respond to changes from the original plan, and perform postoperative management.
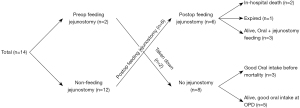
Notably, nutritional support during the perioperative period is critical for patients with oro-intestinal reconstruction with total esophagectomy. Patients with cervicothoracic malignancy are prone to suffer cancer-related dysphagia and subsequent cachexia. Moreover, side effects of neoadjuvant therapy might cause deterioration of the patient’s general condition and worsen malnutrition, which may contribute to an increased rate of postoperative morbidity and mortality (14). To support the nutritional requirements of the patients, we routinely performed preoperative feeding jejunostomy in patients with dysphagia. Due to concerns about potential postoperative malnutrition, patients maintained the previous feeding jejunostomy or underwent feeding jejunostomy at the abdominal phase of the surgery (Figure 4). After confirming stable vital signs, enteral nutrition was offered to patients as soon as possible. Furthermore, nutritional support via feeding tube was performed simultaneously until oral full feeding was possible.
The current study has some limitations. Selection bias is inherent in a retrospective study from a single institution. Analyzing the long-term outcomes was not feasible due to the small number of patients and the relatively short follow-up period. Our results cannot be generalized to other settings as the study was performed at a tertiary, high-volume, and experienced center. A cumulative analysis of these surgical cases and a multicenter study are warranted to further evaluate the actual survival outcomes and prognosis.
In conclusion, oro-intestinal continuity reconstruction after total esophagectomy in patients with HNSCC and CEC revealed acceptable morbidity and mortality. Thus, this reconstruction is feasible and could be considered as one of the treatment options in selected patients with cervicothoracic malignancy. To maximize the oncological outcomes and minimize the surgical risks, careful selection of surgical candidates and multidisciplinary collaboration of experienced specialists are essential.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1768/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1768/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1768/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1768/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by institutional board of the Asan Medical Center (No. 2022-0144), and the requirement for informed consent was waived because of the retrospective nature of the study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conti M, Benhamed L, Mortuaire G, et al. Indications and results of anterior mediastinal tracheostomy for malignancies. Ann Thorac Surg 2010;89:1588-95. [Crossref] [PubMed]
- Sachdeva UM, Lanuti M. Cervical exenteration. Ann Cardiothorac Surg 2018;7:217-26. [Crossref] [PubMed]
- Krespi YP, Wurster CF, Sisson GA. Immediate reconstruction after total laryngopharyngoesophagectomy and mediastinal dissection. Laryngoscope 1985;95:156-61. [Crossref] [PubMed]
- Yousem DM, Gad K, Tufano RP. Resectability issues with head and neck cancer. AJNR Am J Neuroradiol 2006;27:2024-36. [PubMed]
- Grass GD, Cooper SL, Armeson K, et al. Cervical esophageal cancer: a population-based study. Head Neck 2015;37:808-14. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Buckstein M, Liu J. Cervical Esophageal Cancers: Challenges and Opportunities. Curr Oncol Rep 2019;21:46. [Crossref] [PubMed]
- Chang JH, Wu CC, Yuan KS, et al. Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Oncotarget 2017;8:55600-12. [Crossref] [PubMed]
- Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705-15. [Crossref] [PubMed]
- Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg 2007;94:1059-66. [Crossref] [PubMed]
- Schieman C, Wigle DA, Deschamps C, et al. Salvage resections for recurrent or persistent cancer of the proximal esophagus after chemoradiotherapy. Ann Thorac Surg 2013;95:459-63. [Crossref] [PubMed]
- Du XX, Yu R, Wang ZF, et al. Outcomes and prognostic factors for patients with cervical esophageal cancer undergoing definitive radiotherapy or chemoradiotherapy. Bosn J Basic Med Sci 2019;19:186-94. [Crossref] [PubMed]
- Nagasaka M, Zaki M, Issa M, et al. Definitive chemoradiotherapy with carboplatin for squamous cell carcinoma of the head and neck. Laryngoscope 2017;127:2260-4. [Crossref] [PubMed]
- Deans DA, Tan BH, Wigmore SJ, et al. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer 2009;100:63-9. [Crossref] [PubMed]


