
Serum protein level as a predictor of therapeutic response and adverse effects associated with afatinib use
Introduction
Afatinib can be used as either first or second line treatment in patients with EGFR mutated lung cancer (1-8). Randomized controlled trials demonstrated a significant longer progression free survival than platinum-based chemotherapy as well as the first generation EGFR-TKIs (2,9,10). Afatinib also demonstrated a longer overall survival for 12 months than platinum-based chemotherapy (11). Afatinib was also used in second line setting for EGFR mutant non-small cell lung cancer (NSCLC) after failure of first-generation EGFR-TKI.
Serum protein binding capacity of afatinib is 95%. This includes both non-covalent and covalent binding to serum protein (12). In a Japanese study, significant covariates in the population pharmacokinetics model included aspartate aminotransferase and creatinine clearance, and age and body mass index. Higher trough plasma concentrations of afatinib were shown in patients with adverse effects of grade 3 or higher (13). Factors associated with increased plasma afatinib levels include body weight, ECOG performance status, renal impairment, lactate dehydrogenase level, alkaline phosphatase level and total protein level (14). Low body weight (<45 kg), female gender and older age (≥60 years) were identified as major independent risk factors of severe diarrhea in lung cancer patients taking afatinib (15). In a community-based cohort, there were no survival differences between patients taking afatinib or other EGFR-TKI when patients were stratified by age, baseline albumin level and the types of EGFR mutations. This study, however, only included 80 patients on first-line treatment with EGFR-TKI and only 3% of patients were on afatinib (16). In a prognostic tool developed for advanced stage EGFR mutant NSCLC based on pre-treatment clinic-pathological factors, NSCLC patients treated with first-line afatinib, total protein level was not shown to be associated with significant progression free survival (PFS) or overall survival (OS) benefits (17).
Common adverse effects of afatinib include diarrhea, rash, mucositis, paronychia, etc. (2,9,10). Patients experiencing adverse effects on afatinib were shown to have higher plasma afatinib levels than patients without adverse effects. The tolerability-guided dose adjustment reduced the frequency and severity of adverse effects from afatinib. This dose adjustment of afatinib had no significant impact on efficacy outcomes (18,19). Other factors associated with adverse effects from afatinib were plasma trough afatinib level, older age, female gender (15), ECOG performance status, body weight, body surface area (20), nutritional status and baseline hemoglobin level (14).
Not all factors reported to be associated with adverse effects from Afatinib, and plasma drug level could be easily explained. Serum lactate dehydrogenase level was shown likely related to tumor burden; alkaline phosphatase level could be related to bone and liver metastases; association with renal impairment was also doubtful as afatinib is not renal excreted; while baseline hemoglobin level could be affected by non-cancer related conditions (14).
As Afatinib is heavily protein-bound, serum protein and albumin levels may affect its efficacy. In this study, the associations between serum protein and albumin levels and the clinical response to afatinib, as well as the occurrence of adverse effects, were explored to identify possible risk factors for development of adverse effects of using afatinib in advanced stage NSCLC patients. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1649/rc).
Methods
Subjects
We conducted a retrospective cohort study to investigate the association between baseline serum protein and albumin levels and the development of afatinib-related adverse effects. Between 1st January 2014 and 31st December 2018, all patients with metastatic NSCLC harboring EGFR mutations treated with afatinib in Queen Mary Hospital, Hong Kong, were included. Demographic data (age, gender, smoking status), clinical data/investigations (driver mutation status, metastatic sites, hepatitis B status), prescription details of afatinib and the adverse effects associated with Afatinib use were collected. For baseline laboratory results, the laboratory tests right before the initiation of afatinib were taken as the baseline for both patients on first and second line afatinib. The co-primary outcomes of interest were the response to afatinib and the development of adverse effects associated with Afatinib use. The response was graded according to the Response Evaluation Criteria In Solid Tumors (RECIST 1.1). Severity of adverse effects was graded according to the CTCAE V5.0 published by the US National Cancer Institute (NCI) of the National Institutes of Health (NIH) (21). Disease under control with Afatinib was defined as achieving stable disease, partial response and complete response. Response to Afatinib was defined as achieving partial response and complete response. The study was approved by the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW) IRB (Approval No. UW 20-539). No patient consent was needed as only anonymous data were analyzed.
Statistical analysis
The demographic and clinical data were described in actual frequency or mean ± standard deviation (SD), or median and interquartile ranges where appropriate. To identify whether serum protein and albumin levels and their ratios were associated with clinical response to afatinib and specific adverse effects from afatinib, univariate logistic regression analyses were performed with the protein, albumin and globulin level and their ratios being continuous variable in the univariate logistic regression analysis. Multivariate analysis was conducted with adjustment for potential confounding factors including age, gender, smoking status, EGFR mutation and performance status receiver operating characteristic (ROC) curve techniques were used to analyze the usefulness of serum protein, albumin levels and their ratios as predictors of response and occurrence of adverse events. The statistical significance was determined at the level of P=0.05. All the statistical analyses were done using the 26th version of SPSS statistical package. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Baseline characteristics
A total of 217 patients with afatinib treatment for metastatic EGFR mutant NSCLC were included in this study. The mean age of these patients was 65.5±12.0 (range, 38–90) years, with 127 (58.5%) females and 90 (41.5%) non-smokers. The EGFR mutations were mostly exon 19 deletion (n=149; 65.9%) and exon 21 L858R (n=45; 20.7%), while 17 (7.9%) and 12 (5.5%) patients had uncommon or complex EGFR mutations. 91 patients were on afatinib as first line treatment and 126 were on afatinib as second line treatment. The median progression free survival was 8.2±1.8 months for patients on first line afatinib, and 5.1±0.7 months for patients on second line afatinib. The baseline demographics and clinical features are shown in Table 1.
Table 1
| Baseline demographics/clinical characteristics | First line afatinib (N=91) | Second line afatinib (N=126) |
|---|---|---|
| Gender, (female), n (%) | 49 (53.8) | 78 (61.9) |
| Age (years), mean ± SD | 62.22±11.66 | 67.78±11.82 |
| Non-smoker, n (%) | 65 (71.4) | 103 (81.7) |
| EGFR mutations, n (%) | ||
| Exon 19 deletion | 59 (64.8) | 84 (66.7) |
| L858R | 14 (15.4) | 31 (24.6) |
| Uncommon EGFR mutations | 10 (11.0) | 7 (5.6) |
| Complex EGFR mutations | 8 (8.8) | 4 (3.2) |
| Liver metastasis, n (%) | 10 (11.0) | 20 (15.8) |
| Brain metastasis, n (%) | 14 (15.4) | 29 (23.0) |
| Malignant pleural effusion, n (%) | 35 (38.5) | 56 (44.4) |
| Prior EGFR-TKI, n (%) | ||
| Gefitinib | – | 85 (67.5) |
| Erlotinib | – | 41 (32.5) |
| ECOG performance status, n (%) | ||
| 0 | 23 (25.3) | 10 (7.9) |
| 1 | 55 (60.4) | 88 (69.8) |
| 2 | 9 (9.9) | 17 (13.5) |
| 3 | 4 (4.4) | 10 (7.9) |
| 4 | 0 (0) | 1 (0.8) |
| Best response to afatinib, n (%) | ||
| Progressive disease | 12 (13.2) | 48 (38.1) |
| Stable disease | 29 (31.9) | 65 (51.6) |
| Partial response | 46 (50.5) | 13 (10.3) |
| Complete remission | 4 (4.4) | 0 (0) |
| Baseline white cell count | 7.63±2.98×109/L | 7.25±2.61×109/L |
| Baseline lymphocyte count | 1.26±0.55×109/L | 1.31±0.75×109/L |
| Baseline hemoglobin | 12.56±1.91 g/dL | 11.97±1.62 g/dL |
| Baseline serum protein level | 73.30±8.09 g/L | 71.88±7.54 g/L |
| Baseline serum albumin level | 38.81±5.51 g/L | 38.06±5.10 g/L |
| Baseline serum globulin level | 35.17±8.86 g/L | 33.87±5.80 g/L |
NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitors; ECOG, Eastern Cooperative Oncology Group.
Regarding the starting dose of afatinib, 7 (3.2%), 1 (0.5%), 77 (35.5%), 40 (59.0%) and 4 (1.8%) were started on afatinib as 20 mg daily, 20/30 mg alternate day, 30 mg daily, 40 mg daily and 50 mg daily respectively. 53 (24.4%) of patients required dose reduction because of adverse effects.
Among patients on second line afatinib, 85 (67.5%) patients received gefitinib and 41 (32.5%) received erlotinib in first line setting. Among patients treated with gefitinib and erlotinib as first line, 1 (0.8%) had primary progressive disease, 58 (46.0%) had stable disease, 66 (52.4%) had partial response and 1 (0.8%) had complete response.
Overall clinical responses to afatinib
Among the 91 patients treated with afatinib in first line setting, 29 (31.9%) of them achieved stable disease, 46 (50.5%) with partial response, 4 (4.4%) with complete response and 12 (13.2%) had progressive disease. Among the 126 patients treated with afatinib in the second line setting, 65 (51.6%) of them achieved stable disease, 13 (10.3%) achieved partial response, 48 (38.1%) had primary progressive disease.
Afatinib-induced adverse effects
Among the 217 patients treated with afatinib, cutaneous reactions occurred in 172 (79.3%) patients [123 (56.7%) grade 1, 38 (17.6%) grade 2, 10 (4.6%) grade 3 and 1 (0.5%) grade 4]. Gastrointestinal adverse effects were seen in 158 (72.8%) [107 (49.5%) grade 1, 31 (14.4%) grade 2, 17 (7.9%) grade 3 and 3 (1.4%) grade 4]. Thirty-eight (17.4%) had hepatotoxicity with 36 (16.5%) having grade 1 hepatotoxicity and 2 (0.9%) having grade 3 hepatotoxicity. Nine (4.13%) had pneumonitis. The incidence and grading of these adverse reactions are summarized in Table 2. No mortality was attributable to the use of Afatinib in this cohort.
Table 2
| Adverse effects | First line (N=91) | Second line (N=126) |
|---|---|---|
| Cutaneous adverse effects, n (%) | ||
| Nil | 17 (18.7) | 27 (21.6) |
| Grade 1 | 52 (57.1) | 71 (56.8) |
| Grade 2 | 16 (17.6) | 22 (17.6) |
| Grade 3 | 6 (6.6) | 4 (3.2) |
| Grade 4 | 0 (0) | 1 (0.8) |
| Gastrointestinal adverse effects, n (%) | ||
| Nil | 26 (28.6) | 32 (25.6) |
| Grade 1 | 44 (48.4) | 63 (50.4) |
| Grade 2 | 12 (13.2) | 19 (15.2) |
| Grade 3 | 8 (8.8) | 9 (7.2) |
| Grade 4 | 1 (1.1) | 2 (1.6 ) |
| Hepatotoxicity, n (%) | ||
| Nil | 63 (81.8) | 84 (77.8) |
| Grade 1 | 14 (18.2) | 22 (20.4) |
| Grade 2 | 0 (0) | 0 (0) |
| Grade 3 | 0 (0) | 2 (1.9) |
| Grade 4 | 0 (0) | 0 (0) |
Clinical response to afatinib
All patients
Serum protein, albumin, and albumin to globulin ratio, were found to be associated with clinical response to afatinib. A higher baseline protein level was associated with disease control with afatinib, with an odds ratio (OR) of 1.051 [95% confidence interval (CI): 1.011–1.094, P=0.013]. With multivariate analysis adjusted for age, gender, smoking status, EGFR mutation, tumor size, lymph node metastasis and presence of distant metastasis, the association was significant with an OR of 1.045 (95% CI: 1.002–1.090, P=0.038). With ROC analysis, the area under the curve (AUC) for serum protein level and disease control with afatinib was 0.605 (95% CI: 0.515–0.695) (Figure S1A).
A higher serum albumin level was also associated with disease control with afatinib with an OR of 1.119 (95% CI: 1.052–1.190, P<0.001). With multivariate analysis adjusted for age, gender, smoking status, EGFR mutation, performance status, tumor size, lymph node metastasis and presence of distant metastasis, the association was significant with an OR of 1.101 (95% CI: 1.016–1.160, P=0.015). The AUC for serum albumin level and disease control with afatinib was 0.703 (95% CI: 0.623–0.783) (Figure S1B).
A higher albumin to globulin ratio was associated with disease control with afatinib with an OR of 4.015 (95% CI: 1.133–14.223, P=0.031). With multivariate analysis adjusted for age, gender, smoking status, EGFR mutation, performance status, tumor size, lymph node metastasis and presence of distant metastasis, the association was not significant with an OR of 3.933 (95% CI: 0.913–16.950, P=0.066). The AUC for serum albumin to globulin ratio and disease control with afatinib was 0.619 (95% CI: 0.533–0.705) (Figure S1C).
A higher baseline protein level was associated with response to afatinib, with an OR of 1.077 (95% CI: 1.028–1.128, P=0.002). At multivariate analysis adjusted for age, gender, smoking status, EGFR mutation, performance status, tumor size, lymph node metastasis and presence of distant metastasis the association was still significant with 1.077 (95% CI: 1.022–1.136, P=0.006). The AUC for serum protein level and response to afatinib was 0.664 (95% CI: 0.580–0.748) (Figure S1D).
Higher serum albumin level was also associated with response to afatinib with an OR of 1.073 (95% CI: 1.007–1.143, P=0.03). However, the result was statistical insignificant after adjustment for age, gender, smoking status, EGFR mutation, tumor size, lymph node metastasis and presence of distant metastasis in multivariate analysis, with an OR of 1.055 (95% CI: 0.986–1.128, P=0.121). The AUC for serum albumin level and response to afatinib was 0.622 (95% CI: 0.537–0.706) (Table 3, Figure S1E).
Table 3
| All patients | Univariate analysis | Multivariate analysis | AUC of ROC (95% CI) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Disease control with afatinib (SD, PR, CR), baseline protein level | 1.051 (1.011–1.094) | 0.013* | 1.045 (1.002–1.090) | 0.038* | 0.605 (0.515–0.695) | |
| Disease control with afatinib (SD, PR, CR), baseline albumin level | 1.119 (1.052–1.190) | <0.001* | 1.101 (1.016–1.160) | 0.015* | 0.703 (0.623–0.783) | |
| Disease control with afatinib (SD, PR, CR), baseline albumin to globulin ratio | 4.015 (1.133–14.223) | 0.031* | 3.933 (0.913–16.950) | 0.066 | 0.619 (0.533–0.705) | |
| Response to afatinib (PR, CR), baseline protein level | 1.077 (1.028–1.128) | 0.002* | 1.077 (1.022–1.136) | 0.006* | 0.664 (0.580–0.748) | |
| Response to afatinib (PR, CR), baseline albumin level | 1.073 (1.007–1.143) | 0.030* | 1.055 (0.986–1.128) | 0.121 | 0.622 (0.537–0.706) | |
| Patients on first line afatinib | ||||||
| Disease control with afatinib (SD, PR, CR), baseline albumin level | 1.131 (1.018–1.257) | 0.013* | 1.190 (1.029–1.397) | 0.020* | 0.816 (0.723–0.910) | |
| Patients on second line afatinib | ||||||
| Disease control with afatinib (SD, PR, CR), baseline albumin level | 1.131 (1.029–1.206) | 0.008* | 1.104 (1.010–1.206) | 0.029* | 0.667 (0.566–0.769) | |
| Response to afatinib (PR, CR), baseline protein level | 1.111 (1.010–1.221) | 0.030* | 1.111 (1.003–1.231) | 0.043* | 0.660 (0.482–0.838) | |
*, factors that are statistically significant in multivariate analysis adjusted for age, gender, smoking status, EGFR mutation, tumor size, lymph node metastasis and presence of distant metastasis. OR, odds ratio; CI, confidence interval; AUC, area under the curve; ROC, receiver operator characteristic; SD, stable disease; PR, partial response; CR, complete response.
Patients on first line afatinib
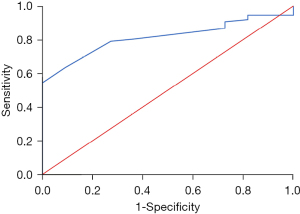
For patients on first line afatinib, those with higher baseline albumin levels were more likely to have disease control with afatinib with an OR of 1.131 (95% CI: 1.018–1.257, P=0.022). The result was significant after adjustment for age, gender, smoking status, EGFR mutation, tumor size, lymph node metastasis status and presence of distant metastasis with an OR of 1.199 (95% CI: 1.029–1.397, P=0.02). The AUC for serum albumin level and disease control with afatinib was 0.816 (95% CI: 0.723–0.910) (Figure 1).
Patients on second line afatinib
For patients on second line afatinib, those with higher baseline albumin levels were more likely to have disease control with afatinib with an OR of 1.131 (95% CI: 1.029–1.206, P=0.008). The result was still significant after adjustment for age, gender, smoking status, EGFR mutation, tumor size, lymph node metastasis, presence of distant metastasis, first line EGFR-TKI used and response to first line EGFR-TKI with an OR of 1.108 (95% CI: 1.016–1.209, P=0.021). The AUC for serum albumin level and disease control with afatinib was 0.667 (95% CI: 0.566–0.769) (Figure S2A).
Higher baseline protein level was shown to be associated with response to afatinib, with an OR of 1.111 (95% CI: 1.010–1.221, P=0.03). The results remained significant after adjustment for age, gender, smoking status, EGFR mutation, tumor size, lymph node metastasis, presence of distant metastasis, first line EGFR-TKI used and response to first line EGFR-TKI with an OR of 1.111 (95% CI: 1.003–1.231, P=0.043) (Figure S2B).
Adverse effects of afatinib
Patients on first line afatinib
Among patients on first line afatinib and with ECOG PS at 1 or above, patients with lower serum protein level at baseline had higher risks of developing grade 2 or above gastrointestinal adverse effects, with an OR of 1.082 (95% CI: 1.009–1.161, P=0.027). With multivariate analysis adjusted for age, gender and performance status, the result was significant with OR of 1.083 (95% CI: 1.007–1.165, P=0.031). There was a trend to suggest lower serum albumin to globulin level at baseline was associated with increased risks of all cutaneous adverse effects among patients with ECOG PS at 1 or above, with an OR of 1.163 (95% CI: 0.996–1.359, P=0.057). The AUC for serum protein level and grade 2 or above gastrointestinal adverse effects was 0.742 (95% CI: 0.613–0.817) (Table 4, Figure S3).
Table 4
| Univariate analysis | Multivariate analysis | AUC of ROC (95% CI) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Patients on first line afatinib, PS ≥1 | ||||||
| Grade 2 or above gastrointestinal adverse effects, baseline protein level | 1.082 (1.009–1.161) | 0.027* | 1.083 (1.007–1.165) | 0.031* | 0.742 (0.613–0.817) | |
| All cutaneous adverse effects, baseline albumin to globulin ratio | 1.163 (0.996–1.359) | 0.057 | 0.703 (0.623–0.783) | |||
| Patients on second line afatinib, PS >1 | ||||||
| Grade 3 or above cutaneous adverse effects, baseline protein level | 1.146 (1.016–1.292) | 0.027* | 1.149 (1.016–1.290) | 0.027* | 0.791 (0.594–0.988) | |
| Patients on second line afatinib, PS >1, no prior exposure to systemic chemotherapy | ||||||
| Grade 3 or above gastrointestinal adverse effects, baseline albumin to globulin ratio | 1.058 (1.004–1.114) | 0.034* | 1.077 (1.005–1.154) | 0.035* | 0.932 (0.857–0.988) | |
*, factors that are statistically significant in multivariate analysis adjusted for age, gender and performance status. PS, performance status; OR, odds ratio; CI, confidence interval; AUC, area under the curve; ROC, receiver operator characteristic.
Patients on second line afatinib
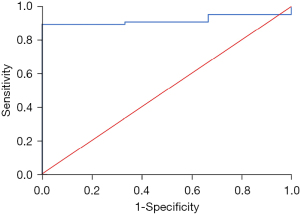
Among patients on second line afatinib and with ECOG PS at or above 1, patients with lower serum protein level at baseline had higher risks of developing grade 3 or above cutaneous adverse effects, with an OR of 1.146 (95% CI: 1.016–1.292, P=0.027). With multivariate analysis adjusted for age, gender and performance status, the result was significant with OR of 1.149 (95% CI: 1.016–1.298, P=0.027). Lower serum albumin to globulin ratio at baseline was associated with increased risks of grade 3 or above gastrointestinal adverse effects among patients with ECOG PS at 1 or above and no prior systemic chemotherapy, with an OR of 1.058 (95% CI: 1.004–1.114, P=0.034). With multivariate analysis adjusted for age, gender and performance status, the result was significant with OR of 1.077 (95% CI: 1.005–1.154, P=0.035). The AUC for serum protein level and grade 3 or above cutaneous adverse effects was 0.791 (95% CI: 0.594–0.988) (Figure S4). The AUC for serum albumin to globulin ratio and grade 3 or above gastrointestinal adverse effects was 0.932 (95% CI: 0.857–0.988) (Figure 2).
Discussion
This study demonstrated the potential role of plasma protein and albumin levels, as well as albumin to globulin ratio, in the prediction of clinical response to afatinib in advanced stage EGFR mutated lung cancer patients, and the association with such clinical response to the occurrence of afatinib-induced cutaneous and gastrointestinal adverse effects. Our finding is consistent with the findings from previous clinical trials (14), which suggested the roles of performance status and nutritional status in predicting the occurrence of adverse effects. From ROC analysis, serum albumin levels showed relatively high sensitivity and specificity in predicting clinical responses to afatinib. While serum albumin to globulin ratio showed relatively high sensitivity and specificity in predicting grade 3 or above gastrointestinal adverse effects among patients on second line Afatinib, who had ECOG PS at 1 or above and no prior exposure to systemic chemotherapy. Serum protein levels were also likely to have reasonable sensitivity and specificity in predicting grade 2 or above gastrointestinal adverse effects among patients on first line afatinib and with ECOG PS at 1 or above; as well as predicting grade 3 or above gastrointestinal adverse effects among patients on second line Afatinib and with ECOG PS at 1 or above.
As afatinib is heavily protein-bound in the circulation, a low serum protein level may increase the plasma afatinib level, which can lead to an increase in afatinib toxicity. Together with impaired performance status, plasma afatinib level was shown to be correlated to risks of developing adverse effects from afatinib (20). At the same time, having increased serum free afatinib due to low serum protein and albumin levels may also shorten the presence of afatinib in circulation. This reduction in serum afatinib concentrations may lead to suboptimal clinical response to afatinib. Serum albumin to globulin ratio may reflect reduced availability of albumin for binding drugs such as afatinib. Our finding is consistent with results from clinical trials as well as pharmacokinetic studies of afatinib. This has important clinical implication for the use of afatinib in EGFR mutated lung cancer patients. Among patients with Stage IV NSCLC, it is not uncommon to have impaired performance status due to the overall cancer disease, which may only be improved with appropriate anti-cancer treatment. A relatively non-invasive blood tests on serum proteins can help to guide selection of afatinib as treatment in advanced stage lung cancer. It is also not uncommon to find low serum protein levels, especially low albumin levels, among these patients, usually attributable to increased catabolism from underlying active malignancy. The association between the performance status and the occurrence of adverse effects could be related to both hypoproteinemia as well as weight loss. While EGFR-TKI is usually prescribed with fixed dosage, there are proposed ways of dose reduction for patients with low body weight or body mass index, and this may improve tolerance to treatment (19,22-25). As dose reduction was a commonly acceptable option to manage adverse effects, pre-emptive dose reduction may be a reasonable option for patients with plasma hypoproteinemia or suboptimal performance status.
Another possible way to manage this problem is to have nutritional supplementation for patients with low serum protein levels before starting afatinib treatment. Having nutritional supplementation would not only help to increase the serum protein level, it can also help to improve the general well-being and the performance state of patients. The importance of nutritional support for patients with advanced stage malignancy cannot be over-emphasized. Personalized anti-cancer treatment with flexible dose adjustment and holistic patient care with emphasis on nutritional status and general well-being of patients will not only have psycho-social benefits but may also give physical benefits, with reduced occurrence of adverse effects.
There are some limitations of this study. Among patients on second line afatinib treatment, there was more heterogeneity of clinical conditions than those on first line treatment. Yet, this should have minimal impact on the results that were based on serum protein level measurement. afatinib could still be considered as a salvage treatment option for heavily pre-treated patients that progressed after EGFR-TKI and chemotherapy. For these patients, they were likely to have poorer performance status and lower serum protein level. Thus, this association between serum protein and albumin levels, and the occurrence of adverse events with use of afatinib, may help clinicians in selection of management options in patients with advanced stage EGFR mutated lung cancer.
Conclusions
Serum protein and albumin levels, and serum albumin to globulin ratio, are useful clinical markers for prediction of clinical response to afatinib and the occurrence of adverse effects from afatinib, in patients with advanced stage EGFR mutated lung cancer.
Acknowledgments
Funding: This work was partly supported by Boehringer Ingelheim.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1649/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1649/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1649/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1649/coif). MSMI serves as an unpaid editorial board member of Journal of Thoracic Disease. DCLL received research support from Boehringer Ingelheim in 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW) IRB (Approval No. UW 20-539). No patient consent was needed as only anonymous data were analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3342-50. [Crossref] [PubMed]
- Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013;31:3335-41. [Crossref] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Non-Small Cell Lung Cancer. Version 6. 2020.
- Harvey RD, Adams VR, Beardslee T, et al. Afatinib for the treatment of EGFR mutation-positive NSCLC: A review of clinical findings. J Oncol Pharm Pract 2020;26:1461-74. [Crossref] [PubMed]
- Tamura K, Nukiwa T, Gemma A, et al. Real-world treatment of over 1600 Japanese patients with EGFR mutation-positive non-small cell lung cancer with daily afatinib. Int J Clin Oncol 2019;24:917-26. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- International BI. Giotrif® Full Prescribing Information2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/201292s000lbl.pdf
- Nakao K, Kobuchi S, Marutani S, et al. Population pharmacokinetics of afatinib and exposure-safety relationships in Japanese patients with EGFR mutation-positive non-small cell lung cancer. Sci Rep 2019;9:18202. [Crossref] [PubMed]
- Wind S, Schnell D, Ebner T, et al. Clinical Pharmacokinetics and Pharmacodynamics of Afatinib. Clin Pharmacokinet 2017;56:235-50. [Crossref] [PubMed]
- Hopkins AM, Nguyen AM, Karapetis CS, et al. Risk Factors for Severe Diarrhea with an Afatinib Treatment of Non-Small Cell Lung Cancer: A Pooled Analysis of Clinical Trials. Cancers (Basel) 2018;10:384. [Crossref] [PubMed]
- Ding PN, Roberts TL, Chua W, et al. Clinical outcomes in patients with advanced epidermal growth factor receptor-mutated non-small-cell lung cancer in South Western Sydney Local Health District. Intern Med J 2017;47:1405-11. [Crossref] [PubMed]
- Hopkins AM, Shahnam A, Zhang S, et al. Prognostic model of survival outcomes in non-small cell lung cancer patients initiated on afatinib: pooled analysis of clinical trial data. Cancer Biol Med 2019;16:341-9. [Crossref] [PubMed]
- Arrieta O, De la Torre-Vallejo M, López-Macías D, et al. Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients With Non-Small Cell Lung Cancer. Oncologist 2015;20:967-74. [Crossref] [PubMed]
- Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 2016;27:2103-10. [Crossref] [PubMed]
- Wada Y, Koyama S, Kuraishi H, et al. Clinical analysis of patients treated with afatinib for advanced non-small cell lung cancer: A Nagano Lung Cancer Research Group observational study. Respir Investig 2016;54:462-7. [Crossref] [PubMed]
- Available online: https://www3.ha.org.hk/data/HAStatistics/DownloadReport/2
- Kwok WC, Ho JCM, Tam TCC, et al. Efficacy of gefitinib at reduced dose in EGFR mutant non-small cell lung carcinoma. Anticancer Drugs 2019;30:1048-51. [Crossref] [PubMed]
- Sim SH, Keam B, Kim DW, et al. The gefitinib dose reduction on survival outcomes in epidermal growth factor receptor mutant non-small cell lung cancer. J Cancer Res Clin Oncol 2014;140:2135-42. [Crossref] [PubMed]
- Schuler M, Tan EH, O'Byrne K, et al. First-line afatinib vs gefitinib for patients with EGFR mutation-positive NSCLC (LUX-Lung 7): impact of afatinib dose adjustment and analysis of mode of initial progression for patients who continued treatment beyond progression. J Cancer Res Clin Oncol 2019;145:1569-79. [Crossref] [PubMed]
- Tu HY, Wu YL. Effect of Dose Adjustments on the Safety and Efficacy of Afatinib in Chinese Patients with EGFR-Mutated Non-Small Cell Lung Cancer Who Participated in the LUX-Lung Clinical Trial Program. Onco Targets Ther 2020;13:12539-47. [Crossref] [PubMed]


