
Patterns of recurrence after intracranial stereotactic radiosurgery for brain-only metastases from non-small cell lung cancer and the impact of upfront thoracic therapy with synchronous presentation
Introduction
Lung cancer is the leading cause of cancer mortality in the United States with >130,000 estimated deaths, comprising >20% of all cancer deaths (1). Early symptoms are often non-specific and over half of patients with non-small cell lung cancer (NSCLC) present with metastatic disease. Lung cancer is associated with the greatest absolute risk of brain metastases in the United States (2-7). Central nervous system-directed therapies, including whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), and/or surgery are standard for brain metastases. For patients with limited brain metastases, surgery and/or SRS have been shown to improve local control and overall survival (OS) compared to WBRT alone (8,9). Numerous studies have shown that patients with solitary or multiple brain metastases from NSCLC may experience 5-year survival rates ranging from 7–29% with aggressive local treatment, consisting of surgery and/or definitive dose radiation treatment, to all evident brain and thoracic disease (10,11).
The role of aggressive thoracic therapy in patients with synchronous oligometastatic NSCLC was analyzed in a systematic review and pooled analysis of 7 studies (one abstract only), 4 of which included brain-only metastases, of which 2 were specific to solitary brain metastases (12). Aggressive thoracic therapy was associated with a significantly improved OS among patients with 1 [n=108 patients; hazard ratio (HR) =0.49; 95% confidence interval (CI): 0.31–0.75] or 2–4 brain metastases (n=33 patients; HR =0.44; 95% CI: 0.26–0.73).
In this current study, we sought to retrospectively characterize the patterns of recurrence and survival outcomes among patients with NSCLC treated with single-fraction SRS for solitary or multiple brain-only metastases using modern staging techniques, in the pre-immunotherapy era. We hypothesized that treatment of the primary site with surgery or radiation in addition to treatment of the brain metastases would result in more favorable outcomes as compared to no thoracic treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1640/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Rochester’s Research Studies Review Board (Study #00000862), and individual consent for this retrospective analysis was waived.
We identified all patients with NSCLC treated with SRS for brain metastases between October 2001 and October 2015 (n=366) from a prospectively maintained database at the University of Rochester. Patient records were retrospectively reviewed and those with evidence of extracranial metastases at the time of SRS, or incomplete staging records, were excluded from these analyses. For the remaining patients (n=137), follow-up imaging and clinic records were reviewed to determine the timing and location of recurrences. Patients with available follow-up imaging (n=124) were analyzed for patterns of recurrence; all patients were analyzed for OS.
All patients were treated with linear accelerator-based SRS using the BrainLab Novalis ExacTrac system. In each patient, the SRS dose was prescribed to isocenter with the 80% isodose line covering 99% of the target with the planning target volume (PTV) equal to gross tumor volume. Upfront thoracic therapy (UTT) was defined as either thoracic surgery and/or radiotherapy prior to any locoregional progression. Patients typically underwent brain magnetic resonance imaging (MRI) and body computerized tomography (CT) or positron emission tomography (PET)-CT to detect disease progression at 3–4 months intervals post-treatment. Progression was defined as: (I) an increase in the measured size of a treated brain lesion, not subsequently found to be necrosis on salvage craniotomy or follow-up MRI with perfusion or spectroscopy; (II) recurrence of initial thoracic disease; and/or (III) interval development of new intra- or extra-cranial metastases.
Statistical analysis
Baseline patient characteristics were compared using the Wilcoxon rank-sum or Chi-squared tests. The cumulative intracranial tumor volume (CITV) was calculated from the Novalis BrainLab treatment planning system. OS was calculated from the time of brain metastasis diagnosis to death from any cause or censored at last patient contact for survivors. Patterns of first and cumulative recurrences were determined through retrospective review of patient imaging. Analyzed recurrence patterns included: local brain recurrence, local lung recurrence, regional nodal recurrence and distant sites of brain, lung, non-regional lymph nodes, bone, liver, adrenal gland and other distant sites. Time to progression (TTP) was calculated from the time of brain metastasis diagnosis until progression or last follow-up. OS and TTP were estimated using the Kaplan-Meier method and compared using the log-rank test. Overall TTP, extracranial TTP, and intracranial TTP were analyzed separately. Organ-specific TTP (adrenal gland, bone, distant lung, liver, non-regional lymph nodes, and others) were also analyzed. The frequency and type of salvage therapy after intracranial progression are described. Both univariable and multivariable Cox proportional hazards models were used to assess patient factors associated with TTP and OS accounting for age, performance status, sex, histology, thoracic stage (i.e., T and N stage ignoring M1 for brain metastases), timing (synchronous vs. metachronous presentation) of brain metastases, number of brain metastases and treatment for brain and primary lung cancers. Hazard ratios (HR) are presented. All P values are two-sided. Statistical analysis was performed using STATA version 14 (13).
Results
Patient and treatment characteristics
Table 1 summarizes the patient and treatment characteristics. Median age at diagnosis of brain metastases was 63 (range, 41–88) years. Eight-eight (64.2%) had presented with synchronous occurrence of primary NSCLC and brain metastases. The median CITV was 4.71 (range, 0.03–268.1) cc. One-hundred and three (75.2%) patients had symptomatic brain metastasis at presentation. Thirty-eight (27.8%) initially underwent craniotomy and resection of brain metastases. The median diagnosis-specific graded prognostic assessment (DS-GPA) (14) was 2.5 (range, 1.0–4.0). Staging was performed with MRI brain in 96.3% and PET-CT in 68.1% with all others undergoing CT head and/or body CT imaging plus bone scan to complete staging. Eighty-five (62.0%) received upfront systemic therapy with first line chemotherapy (for at least 1 cycle) or a molecular targeted therapy for a known epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) rearrangement prior to disease progression. Of patients with adenocarcinoma histology (n=92), 38 patients were assessed with molecular testing and 8 (21%) had a targetable mutation. Of these 8 patients, 4 received upfront tyrosine kinase inhibitor (TKI) targeted therapy, 2 received upfront chemotherapy and ultimately TKI therapy for salvage, and 2 were assessed for mutation status at the time of disease progression (as targeted drugs became standard of care) but were unable to receive systemic therapy due to declining performance status.
Table 1
| Characteristic | Number (%) |
|---|---|
| Age at diagnosis of brain metastases, years | |
| <60 | 52 (38.0) |
| ≥60 | 85 (62.0) |
| Karnofsky performance status | |
| <70 | 6 (4.4) |
| 70–80 | 74 (54.0) |
| >80 | 57 (41.6) |
| Sex | |
| Male | 75 (54.7) |
| Female | 62 (45.3) |
| Histology | |
| Adenocarcinoma | 92 (67.2) |
| Squamous cell carcinoma | 13 (9.5) |
| Other* | 32 (23.4) |
| Thoracic stage (ignoring brain metastases)† | |
| I | 24 (17.5) |
| II | 8 (5.8) |
| III | 105 (76.6) |
| Timing if brain metastases and primary NSCLC | |
| Synchronous | 88 (64.2) |
| Metachronous | 49 (35.8) |
| Number of brain metastases | |
| 1 | 85 (62.0) |
| 2–3 | 33 (24.1) |
| ≥4 | 19 (13.9) |
| Upfront brain-metastases directed therapy | |
| SRS alone | 48 (35.0) |
| WBRT + SRS | 51 (37.2) |
| Surgery + SRS | 23 (16.8) |
| Surgery + WBRT + SRS | 15 (11.0) |
| Upfront thoracic therapy‡ | |
| Surgery ± radiotherapy | 9 (10.2)§ |
| Chemoradiotherapy | 18 (20.5) |
| SBRT | 15 (17.0) |
| Conventionally fractionated radiotherapy | 14 (15.9) |
| None | 32 (36.4) |
*, including NSCLC not otherwise specified and large cell carcinoma. †, using 8th edition of AJCC staging manual. Initial T stage was unknown, 1, 2, 3, 4 in 1.4%, 26.1%, 30.4%, 22.5% and 18.8% respectively. Initial N stage was 0, 1, 2, and 3 in 22.5%, 11.6%, 47.8% and 18.1% respectively. Staging was clinical in all but the 9 patients who underwent definitive resection; ‡, this applies only for the 88 patients who developed brain metastases synchronously with their primary NSCLC. Those with metachronous treatment previously had their primary tumor addressed, and controlled, with local therapy; §, thoracic resection included lobectomy and lymph node dissection (n=3) and sublobar resection (n=6); with lymph node dissection or sampling. NSCLC, non-small cell lung cancer; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy; AJCC, American Joint Committee on Cancer.
The median cumulative dose and number of fractions for patients receiving WBRT was 30 (range, 24–50.4) Gy and 10 (range, 8–28) fractions. All but 1 patient received ≤15 fractions and only 2 received <30 Gy; all others received 30 Gy in 10 fractions or 35–37.5 Gy in 14–15 fractions. All SRS was delivered in 1 fraction. The median SRS isocenter dose and PTV size were 16.5 (range, 9–25) Gy and 4.4 (0.06–193.8) cc, respectively.
For patients with metachronous brain metastases, the median interval from diagnosis and initial treatment of NSCLC to brain metastases was 14.2 (range, 1–68) months.
Among those not undergoing UTT, 31 received systemic therapy alone. For patients undergoing UTT, the range of time from initial diagnosis to completion of treatment of thoracic disease (prior to any evidence of progression) was broad (range, 1–12 months); though for most, it was on the order of months (2nd–3rd interquartile range: 1.9–3.9 months). Non-surgical patients undergoing UTT were treated to a median biologically effective dose (BED) of 75.0 (range, 39–141.0) Gy (using α/β=10). Three patients received a BED of <60 Gy.
Among patients with synchronous brain metastases (n=88), those receiving UTT (n=56) were less likely to receive upfront systemic therapy (67.9% vs. 90.6%; P=0.017), but more likely to have lower thoracic stage disease (28.6% vs. 9.4% thoracic Stage I–II) and lower CITV (median 1.71 vs. 8.41 cc, P=0.030) as compared to patients not receiving UTT.
Patterns of recurrence
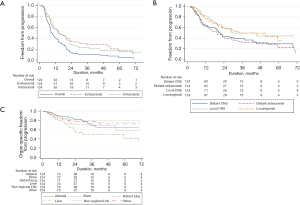
The median follow-up for patients analyzed for patterns of recurrence was 16.0 (range, 1.9–145.6) months. The median overall TTP, intracranial TTP, and extracranial TTP were 9.3, 14.5, and 14.4 months, respectively. Patterns of first and cumulative recurrences are given in Table S1. Intracranial recurrences were symptomatic in 36.8% (n=46), asymptomatic in 59.2% (n=74) and unknown in 4% (n=4). Kaplan-Meier curves of TTP for various organs/sites are shown in Figure 1. At time of death or last follow-up, 60 (48.4%) and 70 (56.5%) patients developed recurrence in distant brain and extracranial sites, respectively, while 31 (25.0%) had developed no discernible distant recurrences.
Patient and treatment factors associated with recurrence
Univariable analyses of TTP revealed male sex (HR 1.642, P=0.012), greater CITV (HR 1.004 per cc, P=0.020), and disease-specific graded prognostic index (HR 0.668, P=0.022) as adverse risk factors; male sex and CITV remained significant with multivariable analysis (Table S2). For intracranial TTP, greater CITV (HR 1.004 per cc, P=0.039) and worse DS-GPA (HR 0.612, P=0.018) were adverse factors with univariable analyses; these factors were not significant with multivariable analysis whereas adenocarcinoma histology (HR 0.584, P=0.038) was (Table S3). For extracranial TTP, only synchronous occurrence of brain metastases (HR 1.912, P=0.014) was a significant factor with univariable analyses whereas no factors were significant with multivariable analysis (Table S4). UTT was the only significant predictor of extracranial TTP (HR 0.447, P=0.003 on univariate analysis and HR 0.366, P=0.001 on multivariable analysis) among patients with synchronous brain metastases (Table S5). Within the subgroup of thoracic stage III patients (n=69), those treated with UTT experienced a median extracranial TTP 19.3 vs. 9 months among stage III patients treated with vs. without UTT.
For patients who received upfront WBRT with SRS, the median brain TTP (19.1 vs. 13.3 months, P=0.34) and median distant brain TTP (23.1 vs. 17.4 months, P=0.11) were numerically (non-significantly) longer; and the rate of distant brain relapse was non- significantly less (39.6% vs. 58.0%, P=0.10).
Analysis of progression among patients with synchronous brain metastases
Among patients with synchronous brain metastases, UTT was associated with improved median extracranial TTP (16.0 vs. 8.6 months, P=0.002), but similar median overall TTP (8.5 vs. 8.4 months, P=0.059) (see Figure S1). Twenty-two (43.1%) patients receiving UTT experienced central nervous system (CNS) progression by 9 months. Extracranial TTP was longer after systemic therapy used in conjunction with UTT vs. systemic therapy alone (19.3 vs. 8.6 months, P=0.001). Extracranial TTP after UTT alone was numerically longer, but not significantly, vs. systemic therapy alone (14.2 vs. 8.6 months, P=0.20). Receipt of upfront systemic therapy was not associated with an improvement in overall or extracranial TTP in either synchronous (8.6 vs. 7.1 months, P=0.12 and 12.8 vs. 14.2 months, P=0.81) or metachronous (7.5 vs. 14.2 months, P=0.073 and 22.9 vs. 59.3 months, P=0.25) patients.
Salvage therapy
Of the 76 patients experiencing intracranial progression after SRS, 44 (57.9%) received salvage SRS-alone and 24 (31.6%) received salvage WBRT. Eleven (9.4%) underwent salvage cranial resection due to concern for progression versus refractory symptomatic radionecrosis. Of the 78 patients experiencing extracranial progression, at our institution, 34 (43.6%) received salvage systemic therapy (either chemotherapy, molecular targeted therapy or immunotherapy) following this progression, and seven (30.4%) received palliative radiotherapy to the chest. The remainder of patients were either treated elsewhere or did not undergo additional therapy.
Patient and treatment variables associated with OS
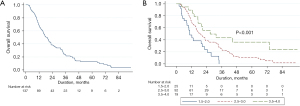
Median OS following diagnosis of brain metastases was 16.6 (range, 2.2–145.6) months and the 2-year survival rate was 38% (Figure 2). There were 111 (79%) deaths with 26 (21%) patients alive at last follow-up. Older age (HR 1.022, P=0.029), symptomatic presentation of brain metastases (HR 1.798, P=0.011), increasing maximal brain metastasis dimension (HR 1.185 per cm, P=0.032), CITV >10 cc (HR 1.543, P=0.028), poorer Karnofsky performance status (KPS) (HR 0.960, P<0.001), and DS-GPA (HR 0.483, P<0.0001) were adverse predictors of OS with univariable analyses; only KPS and adenocarcinoma histology were significant factors with multivariable analysis (Table S6).
Patients with metachronous metastases were less likely to die of extracranial malignancy (27.8% vs. 50.0%, P=0.032).
Analysis of OS among patients with synchronous brain metastases
There were 67 (76%) deaths among the 88 patients presenting with synchronous brain metastases. Adenocarcinoma histology (HR 0.387, P=0.002), greater DS-GPA (HR 0.565, P=0.013), lower thoracic stage (HR 0.609, P=0.004), and UTT (HR 0.515, P=0.029) were associated with improved OS on multivariable analyses (Table 2). Within the subgroup of thoracic stage III patients (n=69), those treated with UTT experienced median OS of 22.7 months and 5-year OS of 15.6%.
Table 2
| Patient/treatment factors | Cox UVA for OS | Cox MVA for OS |
|---|---|---|
| Male | HR 1.075, P=0.772 | HR 1.233, P=0.454 |
| DS-GPA (1.0–4.0) | HR 0.526, P=0.003 | HR 0.565, P=0.013 |
| Adenocarcinoma histology | HR 0.426, P=0.002 | HR 0.387, P=0.002 |
| Thoracic stage (I–II vs. III) | HR 0.746, P=0.055 | HR 0.609, P=0.004 |
| Upfront systemic therapy | HR 0.696, P=0.225 | HR 0.714, P=0.287 |
| Upfront thoracic therapy | HR 0.809, P=0.423 | HR 0.515, P=0.029 |
| CITV (cc) | HR 1.002, P=0.204 | HR 1.000, P=0.827 |
| Upfront whole brain radiotherapy | HR 1.616, P=0.074 | HR 1.086, P=0.762 |
UVA, univariable analysis; OS, overall survival; MVA, multivariable analysis; DS-GPA, diagnosis-specific graded prognostic assessment; CITV, cumulative intracranial tumor volume.
Discussion
To our knowledge, this is the largest study evaluating long-term outcomes in NSCLC patients with solitary or multiple brain-only metastases in the modern staging era. Furthermore, this is the first study of organ-specific patterns of recurrence and the largest series to date examining the association of UTT with OS in this population.
Univariable analyses revealed female sex, lower CITV, and higher DS-GPA to be significant predictors of better TTP while younger age, asymptomatic brain metastasis presentation, smaller maximal brain metastasis dimension, smaller CITV, higher KPS, and higher DS-GPA were significantly associated with improved OS. None of these factors, other than the association of higher KPS with OS, were significant on multivariable analyses, possibly reflecting lack of significance of these factors vs. the multivariable analyses being underpowered. These findings on univariate analyses are consistent with other studies in the published literature (10,15-24) with the possible exception of metachronous presentation of brain metastases, which was not a significant factor in our analyses. Metachronous vs. synchronous presentation of oligometastases (not specifically brain metastases) from NSCLC has been shown to be associated with more favorable survival outcomes (25). In studies specific to patients with NSCLC treated with SRS for solitary (n=72 patients) (26) or limited (i.e., 1–4) brain metastases (n=61 patients) (27), metachronous presentation of brain metastases was the only factor significantly favorable for OS. The lack of improved OS associated with metachronous presentation in our series may be due to the high proportion of patients with synchronous brain metastases treated with UTT. A German study of patients with oligometastases (>60% with brain metastases) also showed no difference in OS or progression free survival between metachronous and synchronous presentation when the primary was treated with definitive local therapy (28). Importantly, patients with metachronous brain metastases in our series had decreased distant brain and increased local brain recurrences along with significantly decreased risk of extracranial recurrence or death from extracranial malignant causes supporting aggressive local CNS-directed therapy in this population. We also observed that lower CITV, as well as asymptomatic brain metastasis presentation, but not number of brain metastases was more predictive of OS which is consistent with other studies showing intracranial tumor burden to be prognostic (29).
In this series, the majority of recurrences were in thoracic and distant brain sites (Table S1). Aside from distant lung, other distant extracranial sites were uncommon as sites of first recurrences. It is possible that some late distant lung recurrences were metachronous second primary lung cancers, as these develop in approximately 4–10% of patients with NSCLC (30) and are often not biopsied in the setting of stage IV disease, however the lower risk with metachronous presentation (14.6% vs. 47.4%, P<0.05) would suggest otherwise.
Notably, 54 (43.5%) patients were free of distant extracranial failure and 31 (24.8%) patients were free of distant brain and distant extracranial failure at time of last follow-up or death. As a substantial percentage of patients will not develop progression outside of known areas of disease, local control of existing brain metastases and (for synchronous presentation) initial thoracic disease, becomes paramount. Additionally, as aggressive CNS-directed local therapy with SRS and/or surgery has been shown to provide durable brain local control and improve OS (8,9), the burden of disease progression and mortality may increasingly shift toward known thoracic disease.
There is a growing body of literature supporting the use of definitive thoracic therapy in the setting of oligometastatic NSCLC, typically defined as <3–5 lesions (11). Although brain metastases are considered to be an adverse prognostic factor among patients with oligometastatic NSCLC (25), several studies limited to patients with synchronous brain-only metastases from NSCLC have shown prolonged survival is possible with aggressive local therapy (10,15-22,31-33).
In randomized phase II studies from Gomez and colleagues (from MD Anderson Cancer Center, University of Colorado and London, Ontario) (34,35) and Iyengar and colleagues (from University of Texas Southwestern) (36) patients with extracranial oligometastases were randomized to receive or not receive local consolidative therapy—radiation or resection of all lesions (including primary sites). Both studies were closed early because of a significant progression-free survival benefit with local consolidative therapy. In the study by Gomez et al. (34), local consolidative therapy was associated with significantly improved OS and lower rates of new metastases. Greater than a quarter of patients in the study by Gomez, and more than half of the patients in the study by Iyengar, had treated brain metastases; though not specifically treated with radiosurgery (most receiving upfront WBRT in the study by Iyengar, and none receiving upfront WBRT in study by Gomez, with some receiving pemetrexed chemotherapy-alone). All patients in these two studies had metastatic sites outside of the brain (whereas none of the patients in our study did), and patients with brain metastases were not analyzed separately.
Similar to the results from Gray and colleagues (10), our study shows UTT in patients with brain metastases significantly alters patterns of recurrence and is associated with improved OS (HR 0.515, P=0.029). Among patients with synchronous presentation, receipt of UTT was associated with improved locoregional control (73.3% vs. 28.3%, P<0.05) and extracranial TTP (16.0 vs. 8.6 months, P=0.002), as well as increased brain-only first recurrences (47.8% vs. 20.0%, P=0.012). While other studies have shown the importance of radiation dose with UTT (37,38), the heterogeneity of UTT techniques (surgery, chemoradiation, stereotactic body radiotherapy (SBRT), or conventional radiotherapy alone) in our series made uncovering a dose effect on TTP and OS infeasible. Of note, the vast majority of patients in our study were treated definitively, though 3 received a BED of <60 Gy; however palliative radiotherapy dosing of NSCLC has been associated with favorable control and survival outcomes in select patients (39).
Remarkably, patients in our study with synchronous stage III thoracic disease (n=69) had median extracranial TTP and OS of 19.3 and 22.7 months, respectively. As such, we would discourage withholding aggressive thoracic therapy from thoracic stage III patients who are fit and without other contraindications. Of note, among our patients with synchronous brain metastases, relatively few had thoracic stage I–II disease (n=19) and their OS was relatively poor compared to those thoracic stage III disease (Table 2). This likely reflects a limitation of small sample size, although could reflect a unique biology of cancer that metastasizes to the brain early without mediastinal nodal involvement or advanced T stage.
While upfront WBRT reduces the rate of local and distant intracranial recurrences (29), it is well recognized that WBRT does not prolong survival (as was the case in our study) and is associated with worse quality of life and neurocognitive outcomes (29). WBRT is therefore not recommended for patients with limited brain metastases.
In regards to the role of upfront systemic therapy, the lack of appreciable benefit, along with the prolonged extracranial TTP (median 14.2 months) observed in patients treated with UTT without upfront systemic therapy suggests that biology, rather than treatment, may be an important factor in why some patients with brain-only metastases do not experience progression in new extracranial sites. A better ability to predict which patients will experience prolonged extracranial TTP may allow for reservation of palliative systemic therapy until the time of extracranial progression, if it ever occurs.
There are several limitations of our study. These include its retrospective nature which makes ascertaining the selection for, and relative benefit of, different treatment modalities difficult due to underlying bias. Close to one-third of patients did not undergo PET for staging, which would be unlikely to occur presently. There was heterogeneity in the treatment (surgery vs. radiation) and radiotherapy dosing of those undergoing UTT. Another limitation is that EGFR and ALK status was known in only 38 (41.3%) patients with adenocarcinoma histology, as the majority were treated before our institution routinely tested for these mutations. Furthermore, details regarding upfront systemic therapy agents, dosing and number of cycles were not analyzed as these details were not always available. Perhaps most notably, all of our patients were treated in the pre-immunotherapy era and before the era of targeted agents with high intracranial activity that could alter patterns of recurrence as well as TTP and OS. The improved PFS and OS associated with adjuvant immunotherapy after definitely concurrent chemoradiation for Stage III NSCLC (40) suggest that definitive radiotherapy could also benefit patients with limited metastatic disease receiving immunotherapy. In addition, as recurrences may not always be symptomatic, choice of surveillance imaging modalities might affect the timing and location of detectable recurrences. Lastly, patients alive without recurrence at last follow-up may still develop recurrences that we have not accounted for.
Conclusions
Our results show that patients presenting with advanced stage NSCLC and brain-only metastases based on modern staging techniques are unlikely to develop first recurrences at distant sites outside the brain or thorax and nearly one half may not develop new clinically appreciable extracranial metastases prior to death. These results from a single institution may not be generalizable to the general population. As patients treated with SRS have the potential for durable brain control and/or successful salvage of progressive intracranial disease, the use of UTT to control existing thoracic disease is vital to improving long-term survival in patients with synchronous presentation. Future development of randomized prospective trials, especially in the era of newer immune modulated therapies, is crucial to better understanding the disease free and OS benefit added by this treatment approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1640/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1640/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1640/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1640/coif). MTM serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2014 to August 2022. MTM reports royalties from Wolters Kluwer and honorarium from Galera Therapeutics and Astra Zeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Rochester’s Research Studies Review Board (Study #00000862), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Stelzer KJ. Epidemiology and prognosis of brain metastases. Surg Neurol Int 2013;4:S192-202. [Crossref] [PubMed]
- Villano JL, Durbin EB, Normandeau C, et al. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol 2015;17:122-8. [Crossref] [PubMed]
- Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 2017;19:1511-21. [Crossref] [PubMed]
- Kromer C, Xu J, Ostrom QT, et al. Estimating the annual frequency of synchronous brain metastasis in the United States 2010-2013: a population-based study. J Neurooncol 2017;134:55-64. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 2014;90:526-31. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Bergsma DP, Salama JK, Singh DP, et al. The evolving role of radiotherapy in treatment of oligometastatic NSCLC. Expert Rev Anticancer Ther 2015;15:1459-71. [Crossref] [PubMed]
- Li D, Zhu X, Wang H, et al. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. J Thorac Dis 2017;9:310-7. [Crossref] [PubMed]
- StataCorp. Stata Statistical Software: Release 14. College Station TSL; 2015.
- Sperduto PW, Yang TJ, Beal K, et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 2017;3:827-31. [Crossref] [PubMed]
- Parlak C, Mertsoylu H, Güler OC, et al. Definitive chemoradiation therapy following surgical resection or radiosurgery plus whole-brain radiation therapy in non-small cell lung cancer patients with synchronous solitary brain metastasis: a curative approach. Int J Radiat Oncol Biol Phys 2014;88:885-91. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19-23. [Crossref] [PubMed]
- Girard N, Cottin V, Tronc F, et al. Chemotherapy is the cornerstone of the combined surgical treatment of lung cancer with synchronous brain metastases. Lung Cancer 2006;53:51-8. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75. [Crossref] [PubMed]
- Macchiarini P, Buonaguidi R, Hardin M, et al. Results and prognostic factors of surgery in the management of non-small cell lung cancer with solitary brain metastasis. Cancer 1991;68:300-4. [Crossref] [PubMed]
- Mussi A, Pistolesi M, Lucchi M, et al. Resection of single brain metastasis in non-small-cell lung cancer: prognostic factors. J Thorac Cardiovasc Surg 1996;112:146-53. [Crossref] [PubMed]
- Wroński M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg 1995;83:605-16. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Villarreal-Garza C, de la Mata D, Zavala DG, et al. Aggressive treatment of primary tumor in patients with non-small-cell lung cancer and exclusively brain metastases. Clin Lung Cancer 2013;14:6-13. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Kwok Y, et al. Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer 2003;42:327-33. [Crossref] [PubMed]
- Niibe Y, Nishimura T, Inoue T, et al. Oligo-recurrence predicts favorable prognosis of brain-only oligometastases in patients with non-small cell lung cancer treated with stereotactic radiosurgery or stereotactic radiotherapy: a multi-institutional study of 61 subjects. BMC Cancer 2016;16:659. [Crossref] [PubMed]
- Fleckenstein J, Petroff A, Schäfers HJ, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer 2016;16:348. [Crossref] [PubMed]
- Milano MT, Chiang VLS, Soltys SG, et al. Executive summary from American Radium Society's appropriate use criteria on neurocognition after stereotactic radiosurgery for multiple brain metastases. Neuro Oncol 2020;22:1728-41. [Crossref] [PubMed]
- Ha D, Choi H, Chevalier C, et al. Survival in patients with metachronous second primary lung cancer. Ann Am Thorac Soc 2015;12:79-84. [Crossref] [PubMed]
- Iwasaki A, Shirakusa T, Yoshinaga Y, et al. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg 2004;26:488-93. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Chidel MA, Suh JH, Greskovich JF, et al. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Investig 1999;7:313-9. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Su S, Hu Y, Ouyang W, et al. The survival outcomes and prognosis of stage IV non-small-cell lung cancer treated with thoracic three-dimensional radiotherapy combined with chemotherapy. Radiat Oncol 2014;9:290. [Crossref] [PubMed]
- Lopez Guerra JL, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys 2012;84:e61-7. [Crossref] [PubMed]
- Mac Manus MP, Matthews JP, Wada M, et al. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer 2006;106:1110-6. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]


