
Prognostic value of selected platelet parameters of patients operated for non-small cell lung cancer
Introduction
Lung cancer is the most commonly-diagnosed malignant neoplasm worldwide, with over 1.6 million people dying of lung cancer in 2012. According to WHO statistics, the highest incidence of lung cancer is now observed in the western Pacific region, with Europe in second position (1).
As the disease is asymptomatic in its initial stage, it is mostly diagnosed at the stage of local advancement. However, despite the advance of surgical techniques and application of new-generation anti-neoplastic drugs, prognosis remains poor, with a 5-year survival rate of around 15% (2).
Neoplastic progression is strongly driven by inflammatory processes, which stimulate cellular angiogenesis and proliferation. Indeed, a number of inflammatory markers are known to be associated with neoplasms, including neutrophil, lymphocyte and platelet count, platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), mean platelet volume (MPV) and platelet distribution width (PDW) (3,4).
Most studies on the diagnostic potential of platelet parameters on neoplasms have been performed on groups from eastern Asia. Therefore, the present study was performed to obtain data on the significance of these indicators in a population from a country in Eastern Europe.
The aim of the study is to determine the prognostic value of selected platelet parameters in patients operated on due to non-small cell lung cancer (NSCLC). We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1401/rc).
Methods
Studied population
In the period of 2007–2014, a total of 1,013 patients were operated due to lung malignancy in our centre. Patients in I–IIB stage were qualified for surgery. Patients in IIIA stage were considered for an operation only if positron emission tomography (PET) examination revealed a single mediastinal metastasis. All laboratory tests required prior to the surgery were carried out with use of Sysmex XN 2000 flow cytometer (Sysmex Europe GmbH, Norderstedt, Germany).
Patients’ characteristics
The patients operated on in 2007–2014 due to NSCLC, both those diagnosed with microcellular carcinoma or carcinoid and those who had undergone wedge resections or segmentectomies were excluded from the analysis. Therefore, a total of 532 patients were included in the analysis. The group included 174 female and 358 male patients, aged 36–84 years (mean age: 63.6 years).
The study was granted approval from the Bioethics Committee of the Medical University (No. RNN/83/19/KE). All authors conformed to the principles of the Helsinki Declaration (as revised in 2013) and the personal data of the participants was kept confidential. As the study has a retrospective character, the Bioethics Committee did not require a consent for an insight into medical records from individual patients.
Less than half of patients (45%) negated nicotine addition. The tumor involved the right lung in 311 patients and the left one in 221 patients. The indications for surgical treatment included mainly squamous cell carcinoma and adenocarcinoma (269 and 204 cases respectively), large cell carcinoma and adenosquamous carcinoma were noted in 43 and 16 cases respectively. In 375 patients Charlson Comorbidity Index (CCI) value ranged between 4 and 6 points. The value of 2 and 3 points was observed for 100 patients (Table 1).
Table 1
| Parameter | n [%] |
|---|---|
| Sex | |
| F | 173 [33] |
| M | 358 [67] |
| Nicotinism | |
| Yes | 295 [55] |
| No | 237 [45] |
| Charlson Comorbidity Index (CCI) | |
| 2 | 20 [4] |
| 3 | 80 [15] |
| 4 | 141 [26.5] |
| 5 | 126 [24] |
| 6 | 108 [20] |
| 7 | 43 [8] |
| 8 | 12 [2] |
| 9 | 2 [0.5] |
| Localization: lung | |
| Left | 220 [41] |
| Right | 312 [59] |
| Localization: lobe | |
| Left upper | 119 [22] |
| Left lower | 101 [19] |
| Right upper | 178 [34] |
| Middle | 16 [3] |
| Right lower | 118 [22] |
Surgical treatment
All patients underwent typical anatomical resections, i.e., lobectomies, bilobectomies and pneumonectomies. The surgical interventions were conducted under general anesthesia, with the application of a double-lumen tube. The anterolateral approach was most preferable technique used for surgery. Intraoperatively, all patients were resected at least six N1 and N2 lymph node groups according to the Classification of the Japan Lung Cancer Society (6). The eighth edition of TNM (UICC from 2017) was used to determine the stage of neoplastic disease. Hilar and mediastinal lymph nodes were identified according to the Naruke map (7).
Statistical analysis
The following platelet parameters were subjected to statistical analysis: platelet count, PDW and MPV. In addition, the ratio of the absolute platelet count to the absolute lymphocyte count PLR was also included. The systemic immune-inflammation index (SII) was calculated (SII = platelet count × neutrophil count/lymphocyte count). These selected parameters were compared to the following clinical characteristics: age, sex, smoking, histopathological diagnosis, T value, N value, Charlson Comorbidity Index—age adjusted CCI, type of surgery. Primary clinical endpoint was overall survival (OS).
The following statistical methods were applied:
- Continuous variables were first verified with the Shapiro-Wilk test. As they were found to not have a normal distribution, they were presented as medians and lower and upper quartiles (25–75%). Further analyses were performed with non-parametric tests;
- Comparisons of two groups were performed using the Mann-Whitney U-test, and comparisons of more than two groups with the Kruskal-Wallis test with post hoc comparisons and the Dunn-Bonferroni test;
- Nominal variables were calculated for the study as the number of observations and percent values. Comparisons between nominal variables were made with the Chi square test.
- Survival analysis was performed using the Kaplan-Meyer curves, the Log-rank test and univariate analysis of nominal parameters;
- Univariate analysis of continuous parameters and multivariate analysis was performed using the Cox proportional hazard model (all effects). Along with Cox Model a proportional hazard was calculated.
Patients or whole variables with missing data were not dropped from the analysis in order to prevent from causing bias. Those data were labelled as missing and not substituted by any imputation models.
Results
Surgical treatment
Lobectomy was the most preferable surgery (400 cases). Pneumonectomy and bilobectomy were carried out in 95 and 37 patients respectively. R0 resection was noted in all patients on the basis of a histopathological analysis conducted after the operation. Stage I was observed in 220 (41%) patients, stage II in 180 (34%) patients and stage III in 132 (25%) patients. Three patients died after the operation during the hospital stay (myocardial infarct, cerebral stroke or gastric ulcer perforation) and another five within 30 days following the surgery. Postoperative complications were revealed in 112 cases (21%). These included prolonged air leak, which occurred most frequently (59 cases), atelectasis requiring interventional bronchoscopy (20 cases) and postoperative anemia requiring blood transfusion (19 cases). Ten patients had to be treated after they demonstrated postsurgical arrhythmia (Table 2). 228 patients underwent complementary treatment based on combined administration of cisplatin with either vinorelbine or gemcitabine; In the event of contraindications for cisplatin the patients were given carboplatin. In total, 76 patients did not receive adjuvant treatment due to their poor health condition or refusal to continue the therapy.
Table 2
| Parameter | n [%] |
|---|---|
| Diagnosis | |
| Squamous cell carcinoma | 269 [50] |
| Adenocarcinoma | 204 [38] |
| Large cell carcinoma | 42 [8] |
| Mixed type carcinoma | 16 [4] |
| Surgical treatment | |
| Lobectomy | 400 [75] |
| Bilobectomy | 37 [7] |
| Pneumonectomy | 95 [18] |
| Stage of the disease | |
| T IA1 | 3 [1] |
| T IA2 | 40 [8] |
| T IA3 | 39 [8] |
| T IB | 138 [25] |
| T IIA | 8 [2] |
| T IIB | 172 [32] |
| T IIIA | 132 [24] |
| Adjuvant chemotherapy | 226 [43] |
| Postoperative complications | |
| All complications | 112 [21] |
| Prolonged air leak | 59 |
| Atelectasis | 20 |
| Postoperative transfusion | 19 |
| Atrial fibrillation | 10 |
| Bronchia fistula | 2 |
| Pleural empyema | 1 |
| Pneumonia | 1 |
Postoperative survival of the patients
The mean survival of the patients was 50 months. The 5-year survival rate was 35%, noted in 185 patients. The results of univariate analysis both clinical and laboratory marker factors is presented in Tables 3,4 in details.
Table 3
| Parameter | Median | Range | HR | 95% CI | P |
|---|---|---|---|---|---|
| Neutrophils [×10³] | 5.8 | 1.4–31.0 | 1.03 | 1.01–1.04 | 0.02 |
| Lymphocytes [×10³] | 1.9 | 0.5–9.3 | 0.84 | 0.72–0.97 | 0.02 |
| Platelets [×10³] | 262.5 | 30.0–674.0 | 1.00 | 1.00–1.00 | 0.16 |
| MPV [fL] | 46.6 | 36.6–88.6 | 1.00 | 0.98–1.02 | 0.03 |
| PDW [%] | 2.7 | 0.5–12.3 | 1.05 | 1.02–1.09 | 0.00 |
| PLR | 148.0 | 17.6–570 | 1.00 | 1.00–1.00 | 0.00 |
| SII | 713.5 | 479.2–1,100.5 | 1.00 | 1.00–1.00 | 0.00 |
NSCLC, non-small cell lung cancer, HR, hazard ratio; 95% CI, 95% confidence interval; MPV, mean platelet volume; PDW, platelet distribution width; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Table 4
| Parameter | HR | HR 95% CI upper | HR 95% CI lower | P level |
|---|---|---|---|---|
| Age | 1.0 | 1.0 | 1.0 | 0.0 |
| CCI | 1.2 | 1.1 | 1.3 | 0.0 |
| Sex (M) | 1.5 | 1.2 | 1.9 | 0.0 |
| Stage I | 0.5 | 0.4 | 0.6 | 0.0 |
| Stage II | 0.9 | 0.7 | 1.1 | 0.0 |
| T1 | 0.6 | 0.3 | 1.1 | 0.0 |
| T2 | 1.0 | 0.5 | 2.2 | 0.7 |
| T3 | 1.3 | 0.6 | 2.7 | 0.0 |
| N0 | 0.5 | 0.4 | 0.7 | 0.0 |
| N1 | 0.7 | 0.5 | 1.0 | 1.0 |
| Lobectomy | 0.7 | 0.5 | 0.9 | 0.1 |
| Bilobectomy | 0.7 | 0.5 | 1.1 | 0.5 |
| Non-smokers | 0.8 | 0.6 | 0.9 | 0.0 |
| Adenocarcinoma | 1.3 | 0.7 | 2.4 | 0.3 |
| Squamous cell | 1.1 | 0.6 | 2.1 | 0.7 |
| Mixed | 1.0 | 0.5 | 2.1 | 0.6 |
HR, hazard ratio; 95% CI, 95% confidence interval; CCI, Charlson Comorbidity Index; T, tumor stage; N, nodal stage.
Significantly shorter lifespan were observed for men compared to women (3.91 versus 4.87; P=0.00117) and for nicotine smokers compared to non-smokers (3.88 versus 4.63; P=0.006) The conducted analysis confirmed a relationship between the advancement stage of neoplasia, identified with T3 and T4, as well as N1 and N2 and a shorter survival period (P=0.0009 and P=0.0000, respectively). In stages T1 and T2, the mean survival period was 4.605, whereas in stages T3 and T4, the value was 3.934; these values were 4.80, 3.66 and 2.76 for stages N0, N1 and N2, respectively (Figure 1).
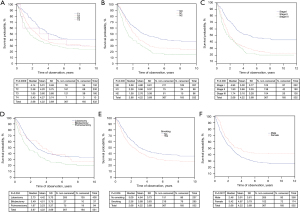
Patients who underwent pneumonectomy demonstrated significantly shorter survival than patients after lobectomy or bilobectomy: 3.23 versus 4.42 and 4.51 (P=0.0046) respectively. In addition, patients with a CCI value 4 or less demonstrated significantly longer survival than those with a higher value (5.02 vs. 3.55; P=0.0000). Patients’ age also was significant in teams of influence on OS. The analysis did not show any significant relationship between the type of tumor and patient OS (P=0.7) (Figure 2).
For continuous variables that significantly affected survival the Receiver Operating Characteristics (ROC) curve and Youden method was used to determinate cut-off point—Kaplan-Meyer curves with cut-of points are presented on Figures 1-3.

Platelet parameters as prognostic factors
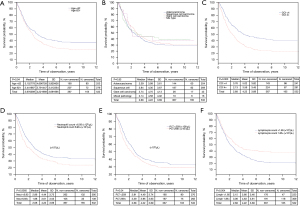
The authors calculated cut-off points for platelets markers (platelet count, PDW and MPV values and calculated PLR and SII) (details data is presented on Figure 3).
The maximum sensitivity and specificity of the studied parameters were determined with the use of the ROC and was calculated for platelet count, PDW and MPV values, and the calculated PLR. The ROC curve was then used to calculate the area under curve (AUC) values. The AUC values for platelet count, PDW, MPV and PLR were respectively: 0.568 [95% confidence interval (95% CI): 0.509–0.627, P=0.0232]; 0.488 (95% CI: 0.429–0.547, P=0.689); 0.570 (95% CI: 0.513–0.627, P=0.0659); 0.567 (95% CI: 0.509–0.624, P=0.0238). Optimal limit values were: 259×10³ for platelets, 11.9% for PDW, 10.9 fL for MPV and 144.77 for PLR.
The univariate analysis revealed a relationship between PDW (P=0.0002), PLR (P=0.00) and MPV (P=0.03) values and patient survival. No significant relationship was observed for platelet count (P=0.16). More detailed data are presented in Table 3.
The multivariate analysis including patient clinical data, identified the following independent prognostic factors for the patients’ survival: age, sex, advancement stage of the neoplastic disease and CCI. Considering age, CCI, PDW and PLR with cut-off points in Cox model only male sex, stage of disease, PLR lower than 144.77 and CCI lower than 4 occurred as independent prognostic factor (Tables 5,6).
Table 5
| Parameter | HR | HR 95% CI upper | HR 95% CI lower | P level | PH Chi-square | PH P level |
|---|---|---|---|---|---|---|
| Age | 0.97 | 0.95 | 0.99 | 0.00 | 6.38 | 0.01 |
| CCI | 1.44 | 1.26 | 1.63 | 0.00 | 7.18 | 0.01 |
| PDW | 0.92 | 0.87 | 0.97 | 0.00 | 4.67 | 0.03 |
| PLR | 1.00 | 1.00 | 1.00 | 0.01 | 0.01 | 0.94 |
| Sex (male) | 1.32 | 1.05 | 1.67 | 0.02 | 1.28 | 0.26 |
| Stage I | 0.52 | 0.40 | 0.67 | 0.00 | 1.20 | 0.27 |
| Stage II | 0.81 | 0.63 | 1.06 | 0.27 | 0.03 | 0.87 |
| 15.05 (general) | 0.04 |
Cox proportional hazard analysis of factors including continuous variables. Goodness of fit R2=0.233. HR, hazard ratio; 95% CI, 95% confidence interval; PH, proportional hazard; CCI, Charlson Comorbidity Index; PDW, platelet distribution width; PLR, platelet-to-lymphocyte ratio.
Table 6
| Parameter | HR | HR 95% CI upper | HR 95% CI lower | P level | PH Chi-square | PH P level |
|---|---|---|---|---|---|---|
| Sex(Male) | 1.31 | 1.04 | 1.65 | 0.02 | 1.31 | 0.25 |
| Stage I | 0.51 | 0.40 | 0.67 | 0.00 | 1.52 | 0.22 |
| Stage II | 0.83 | 0.64 | 1.07 | 0.19 | 0.01 | 0.92 |
| PLR <144.77 | 0.65 | 0.53 | 0.80 | 0.00 | 0.22 | 0.64 |
| CCI <4 | 0.63 | 0.51 | 0.77 | 0.00 | 0.18 | 0.67 |
| 3.63 (general) | 0.60 |
Cox proportional hazard analysis including blood biomarkers with cut-off points Goodness of fit R2=0.198. HR, hazard ratio; 95% CI, 95% confidence interval; PH, proportional hazard; PLR, platelet-to-lymphocyte ratio; CCI, Charlson Comorbidity Index.
Discussion
Our findings indicate that thrombocytes greatly contribute to the occurrence and progression of neoplasm. Hypercoagulopathy is a known marker of an aggressive course, and venous thromboembolic disease increases neoplasia-related mortality (3,8).
The mechanisms causing neoplasia-related thrombocytosis remain unclear. In malignant neoplasms, platelet count increases directly by the paracrine mechanism by activation of megakaryocytes, and indirectly by their induction and platelet activation (8). The most common measures of platelet size are MPV and PDW. The former refers to the mean volume of platelets and is a marker of their activity. The presence of thrombocytosis with low MPV levels indicates an inflammatory or neoplastic process (3).PDW is an indicator of the degree of variability of mean platelet volume, with elevated PDW values indicating that the mean platelet volume is higher than normal (2); however, the role of PDW in the neoplastic process remains unclear. Finally, PLR is another important indicator of the relationship between platelets and the inflammatory process, and it is a known prognostic factor in NSCLC (4).
Our present study evaluated the prognostic role of platelet count, PDW and MPV as well as PLR in the study group. What should be pointed out is the fact that the conducted analysis did not reveal a significant relationship between the platelet count and survival rate (P=0.16). However, this observation is contradictory to those of previous studies, which found thrombocythemia to be an independent preoperative prognostic factor of NSCLC (9,10).
In the present study, PDW was found to have the high prognostic value. Patients with PDW values <11.9% demonstrated significantly shorter life expectancy (P=0.004) than those with lower PDW values. A similar result was obtained by Liu et al., who found that among patients operated due to NSCLC, those who demonstrated preoperative PDW ≤12.65% lived significantly shorter than those with higher values (11). In addition, Cui et al. report that RDW ≤16.3% significantly reduced the survival of patients with NSCLC (12).
Our analysis showed a significant relationship between MPV values and the survival rate of patients operated on due to NSCLC, with MPV <10.9 fl being associated with a lower survival rate (P=0.03). Similarly, Kumagai et al. report significantly shorter survival among patients with MPV <8.5 fl, operated on due to NSCLC (P=0.001) (13), and Gao et al. among those with MPV <11.0fl (P=0.001) (14).
PLR, being a platelet-related ratio, proved to be an independent prognostic factor in our study, with patients with PLR >144 living significantly shorter (P=0.004). In addition, PLR >135 has previously been found to be an independent prognostic factor for T value and a marker for N value (15).
In the present study, the multivariate analysis confirmed that older age, male sex, advancement stage of the neoplastic disease, CCI >4 as well as PDW ≤11.9% and PLR >144 all shortened survival time. Similar results were obtained by Lee et al. The authors have found that preoperative PLR values 180 and TNM stages II and III are unfavourable independent prognostic factors of survival rate, together with higher age, male sex and postoperative radiotherapy (16). In addition, a multifactor analysis by Cui et al. found PDW, age, sex and TNM advancement stage to be independent prognostic factors among patients operated due to NSCLC (12), and similarly to our present study, that the PDW value was higher than the PLR value.
Our work has some limitations. Firstly, the study was retrospective in nature and only included patients with operable NSCLC from a single center. Secondly, some patients were characterized by postoperative stages IIB and IIIA and so received complementary treatment, which would have impacted on the analyzed survival time. Finally, only incomplete molecular profile data (ALK, BRAF, EGFR, KRAS MET, NTRK, PDL1, RET, ROS1) was available for the studied patients, and could not be included in the analysis.
Conclusions
PDW, PLR, SII appear to be independent prognostic factors of patients operated on for NSCLC. However, multivariate analysis indicates that age, the advancement stage of the disease and the presence of comorbidities, expressed as CCI Index and PLR <144.77 have the highest prognostic value.
More research is needed to consider use of platelet parameters such as PDW and PLR as a prognostic factor. Moreover the cut-off level of these parameters has not yet been fully verified and standardization is a major need.
Acknowledgments
We would like to thank Dove Medical Press Limited for permitting to reuse the tables from the publication: Cancer Manag Res 2021;13:7795-802.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1401/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1401/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1401/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was granted approval from the Bioethics Committee of the Medical University (No. RNN/83/19/KE). All authors conformed to the principles of the Helsinki Declaration (as revised in 2013) and the personal data of the participants was kept confidential. As the study has a retrospective character, the Bioethics Committee did not require a consent for an insight into medical records from individual patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Didkowska J, Wojciechowska U, Mańczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150. [Crossref] [PubMed]
- Oncel M, Kiyici A, Oncel M, et al. Evaluation of Platelet Indices in Lung Cancer Patients. Asian Pac J Cancer Prev 2015;16:7599-602. [Crossref] [PubMed]
- Şahin F, Aslan AF. Relationship between Inflammatory and Biological Markers and Lung Cancer. J Clin Med 2018;7:160. [Crossref] [PubMed]
- Diem S, Schmid S, Kraft M, et al. Neutrophil-to-lymphocyte ratio (NLR) and Platelet-to-lymphocyte ratio (PLR) as a prognostic markers in patients in non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Łochowski M, Chałubińska-Fendler J, Zawadzka I, et al. The Prognostic Significance of Preoperative Platelet-to-Lymphocyte and Neutrophil-to-Lymphocyte Ratios in Patients Operated for Non-Small Cell Lung Cancer. Cancer Manag Res 2021;13:7795-802. [Crossref] [PubMed]
- Itazawa T, Tamaki Y, Komiyama T, et al. The Japan Lung Cancer Society-Japanese Society for Radiation Oncology consensus-based computed tomographic atlas for defining regional lymph node stations in radiotherapy for lung cancer. J Radiat Res 2017;58:86-105. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Plantureux L, Mège D, Crescence L, et al. Impacts of Cancer on Platelet Production, Activation and Education and Mechanisms of Cancer-Associated Thrombosis. Cancers (Basel) 2018;10:441. [Crossref] [PubMed]
- Yu D, Liu B, Zhang L, et al. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp Ther Med 2013;5:1351-4. [Crossref] [PubMed]
- Maráz A, Furák J, Varga Z, et al. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res 2013;33:1725-9. [PubMed]
- Liu C, Zhang H, Qi Q, et al. The preoperative platelet distribution width: predictive factor of the prognosis in patients with non-small cell lung cancer. Thorac Cancer 2020;11:918-27. [Crossref] [PubMed]
- Cui MM, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep 2017;7:3456. [Crossref] [PubMed]
- Kumagai S, Tukuno J, Ueda Y, et al. Prognostic significance of preoperative mean platelet volume in resected non-small cell lung cancer. Mol Clin Oncol 2015;3:197-201. [Crossref] [PubMed]
- Gao L, Zhang H, Zhang B, et al. Prognostic value of combination preoperative platelet count and platelet mean volume in patients with respectable non-small cell lung cancer. Oncotarget 2017;8:15632-41. [Crossref] [PubMed]
- Xu F, Xu P, Cui W, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios may aid and identifying patients with non-small cell lung cancer and predicting tumor-node-metastases stage. Oncol Lett 2018;16:483-9. [Crossref] [PubMed]
- Lee BM, Rodrigeuz A, Mena G, et al. Platelet-to-lymphocyte ratio and use of NSIADs during perioperative period as prognostic indicators in patients with NSCLC undergoing surgery. Cancer Control 2016;23:284-94. [Crossref] [PubMed]


