
Long-term patency of bilateral internal thoracic artery Y composite coronary artery bypass grafts—determinants and impact on survival
Introduction
Bilateral internal thoracic artery (ITA) Y-composite grafting with sequential anastomoses is a well-established strategy for multi-arterial coronary artery bypass grafting (CABG) (1-3). In combination with the off-pump technique, it makes no-aortic-touch CABG possible. However, its long-term patency rate beyond 5 years after surgery remains unclear (4). Concerns exist regarding the use of bilateral ITA for a Y-composite graft in CABG in relation to the flow competition phenomenon and the technical complexity of sequential anastomoses, especially in case of beating-heart CABG (2). The Arterial Revascularization Trial (ART) results showed no superiority of bilateral ITA over single ITA (5). Other concerns regarding the use of bilateral ITA are an uncertain survival benefit and increased risk of deep sternal wound infection (6). In contrast, even after the publication of the ART results, there have been papers reporting that bilateral ITA use had a survival benefit compared to single ITA in large patient groups, although they were not randomized controlled trials (7,8).
The primary aim of this study was to investigate the long-term patency of bilateral ITA Y-composite grafts and the factors affecting graft patency. The secondary aim was to investigate whether compromised graft patency observed on computed tomography (CT) scans leads to worse survival. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1731/rc).
Methods
Study subjects
Among 2,160 cases of isolated CABG performed from October 2003 to September 2020 at our institution, bilateral ITA Y-composite grafting was used in 1,521 patients. There were 465 patients who underwent CABG due to triple-vessel disease, including left circumflex (LCx) and/or right coronary artery (RCA) territory grafting by a single surgeon (Park K-H). Among them, 415 patients who had at least 1 set of postoperative imaging (coronary CT angiography or conventional angiography) were finally enrolled in the study (Figure 1). All the subjects were Koreans, and their characteristics are shown in Table 1. In addition to the baseline patient characteristics and operative data, patency data and CT imaging were reviewed separately. Patient survival data were obtained from the Ministry of Public Administration and Security of the Republic of Korea. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Seoul National University Bundang Hospital (IRB No. B-2102/667-108) and individual consent for this retrospective analysis was waived.
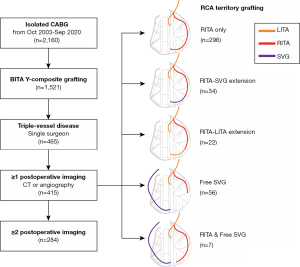
Table 1
| Variables | Beating (n=322) | Non-beating (n=93) | P value | Total (N=415) |
|---|---|---|---|---|
| Sex (male) | 255 (79%) | 69 (74%) | 0.321 | 324 (78%) |
| Age (years) | 66±10 | 64±9 | 0.111 | 66±9 |
| BMI (kg/m2) | 24.9±2.7 | 25.0±3.1 | 0.708 | 24.9±2.8 |
| Smoker | 207 (64%) | 56 (60%) | 0.541 | 263 (63%) |
| Hypertension | 247 (77%) | 71 (76%) | >0.999 | 318 (77%) |
| Diabetes | 161 (51%) | 55 (59%) | 0.127 | 216 (52%) |
| Dyslipidemia | 146 (45%) | 46 (50% | 0.555 | 192 (46%) |
| Previous CVA | 60 (19%) | 21 (23%) | 0.458 | 81 (20%) |
| Chronic renal failure | 32 (10%) | 7 (8%) | 0.317 | 39 (9%) |
| Peripheral vascular disease | 53 (17%) | 13 (14%) | 0.632 | 66 (16%) |
| COPD | 23 (7%) | 1 (1%) | 0.039 | 24 (6%) |
| Preoperative atrial fibrillation | 13 (4%) | 7 (8%) | 0.174 | 20 (5%) |
| Diagnosis | 0.012 | |||
| Unstable angina | 182 (57%) | 53 (57%) | 235 (57%) | |
| Stable angina | 56 (17%) | 7 (8%) | 63 (15%) | |
| NSTEMI | 43 (13%) | 17 (18%) | 60 (15%) | |
| Silent ischemia | 29 (9%) | 6 (7%) | 35 (8%) | |
| STEMI | 12 (4%) | 10 (11%) | 22 (5%) | |
| LV dysfunction (LV EF ≤40%) | 31 (10%) | 28 (30%) | <0.001 | 59 (14%) |
Categorical variables are shown as numbers with percent points, and continuous variables were shown as mean ± standard deviation. BMI, body mass index; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; LV, left ventricle; EF, ejection fraction.
Surgical techniques
The general CABG strategy of our institution has been described in a previous article (9). Both ITA grafts were harvested in a skeletonized manner. The proximal end of the free right ITA graft was anastomosed to the side of the in situ left ITA in an inverted Y configuration. The in situ left ITA of the composite graft was used for the revascularization of the left anterior descending (LAD) artery with or without the diagonal branches. The right ITA was used for the LCx (obtuse marginal branches) and/or RCA territory (posterolateral or posterior descending branches). In patients with moderately stenotic (<75% of the diameter) lesions in the RCA territory or whose right ITAs were too short to reach the end target site, separate aortocoronary bypass using a free saphenous vein graft (SVG) was performed. Occasionally, the right ITA graft was extended with a short segment of the saphenous vein or residual left ITA graft to reach the posterolateral or posterior descending branches (Figure 1).
Operative data are shown in Table 2. In the overall group of study subjects (N=415), the total number of anastomoses was 476 and 1,165 in the left and right ITA grafts, respectively. Sixty-one patients (15%) had sequential anastomoses in the left ITA grafts, and 397 patients (96%) had sequential anastomoses in the right ITA grafts. An additional free saphenous vein graft (aortocoronary) was used in 63 patients (15%).
Table 2
| Variables | Beating (n=322) | Non-beating (n=93) | P value | N=415 |
|---|---|---|---|---|
| Urgency | 0.001 | |||
| Elective | 253 (79%) | 56 (60%) | 309 (75%) | |
| Urgent (<48 hours) | 68 (21%) | 35 (38%) | 103 (25%) | |
| Emergency | 1 (0.3%) | 2 (2%) | 3 (1%) | |
| Pump support | - | |||
| Off-pump CABG | 286 (89%) | 286 (69%) | ||
| On-pump beating CABG | 36 (11%) | 36 (9%) | ||
| Conventional CABG (non-beating) | 93 (100%) | 93 (22%) | ||
| Number of anastomosis | ||||
| LITA | 1.14±0.34 | 1.18±0.39 | 0.303 | 1.15±0.81 |
| RITA | 2.77±0.80 | 2.95±0.98 | 0.072 | 2.81±0.85 |
| LITA + RITA total | 3.90±0.85 | 4.13±1.07 | 0.063 | 3.95±0.91 |
| RITA graft end target | <0.001 | |||
| Obtuse marginal branch | 24 (8%) | 27 (29%) | 51 (12%) | |
| Posterolateral branch | 69 (21%) | 16 (17%) | 85 (21%) | |
| Posterior descending artery | 227 (71%) | 49 (52%) | 275 (66%) | |
| Right coronary artery | 2 (0.6%) | 2 (2.2%) | 4 (1%) | |
| RITA graft type | 0.492 | |||
| RITA only | 275 (85%) | 84 (90%) | 359 (87%) | |
| RITA + SVG | 29 (9%) | 5 (5%) | 34 (8%) | |
| RITA + free LITA segment | 18 (6%) | 4 (4%) | 22 (5%) | |
| Additional free SVG (aortocoronary) used | 28 (9%) | 35 (38%) | <0.001 | 63 (15%) |
| LAD endarterectomy | 7 (2%) | 7 (8%) | 0.020 | 14 (3%) |
Categorical variables are shown as numbers with percent points, and continuous variables are shown as mean ± standard deviation. CABG, coronary artery bypass grafting; LITA, left internal thoracic artery; RITA, right internal thoracic artery; SVG, saphenous vein graft; LAD, left anterior descending.
Off-pump techniques were used whenever possible, but cardiopulmonary bypass including extracorporeal membrane oxygenation was used when necessary. In this study, we categorized the patients into beating and non-beating CABG groups; beating CABG included off-pump and on-pump beating CABG, whereas non-beating CABG referred to conventional CABG utilizing aortic cross-clamping.
Postoperative follow-up and imaging studies to evaluate graft patency
Our policy of follow-up examinations in patients who underwent CABG was summarized in a previous article (9). To evaluate graft patency, a 64-slice electrocardiography-gated multidetector computed tomography (MDCT) coronary angiography was utilized. Based on the 3-dimensional reconstruction images, the CT scans were interpreted by two experienced radiologists. All the patients underwent MDCT between 3 to 6 months after CABG, except for those who chose not to receive a follow-up study or had decreased renal function. When early MDCT showed good patency of all grafts and distal anastomoses, the next follow-up MDCT was performed at the fourth year postoperatively unless contraindicated (e.g., progression of chronic renal failure). Patients who showed abnormal findings (e.g., no visualization of the left ITA graft to the LAD or the entire right ITA graft) underwent conventional coronary angiography at 1 month after CT if the patient had angina. Patients who experienced recurrent angina or major cardiovascular events underwent conventional coronary angiography or MDCT. The patients visited the outpatient clinic at least once a year. The mean follow-up duration was 8.0±4.0 years [interquartile range (IQR): 4.8–11.5 years].
In evaluating MDCT for patency, we divided the grafts into three segments: (I) the left ITA graft, (II) the proximal right ITA graft, and (III) the distal right ITA graft. In addition to widely patent and occluded, diffuse narrowing in the shape of a string without significant stenosis of anastomosis was defined as the ‘string sign’, which represented an intermediate finding between widely patent and occlusion (10,11). A finding of newly detected string sign or occlusion on CT was considered to be a patency event. Among 415 patients, 284 patients (68%) had at least two postoperative imaging examinations (including MDCT and conventional coronary angiography). The mean interval between the operation and the last imaging examination was 6.7±3.3 years (IQR: 3.8–9.4 years).
Statistical analyses
Statistical analyses were performed using SPSS 24 (IBM Inc., Armonk, NY, USA) and R (R 3.6.1; The R Foundation for Statistical Computing, Vienna, Austria). Kaplan-Meier survival curves were drawn, and the log-rank test was used for univariable analysis. In the analysis of multiple time-events in a single subject, clustered Cox regression was used. For multivariable analyses on survival, a Cox proportional hazard ratio model was used. A P value less than 0.05 was considered to indicate statistical significance.
Results
Graft type and patency
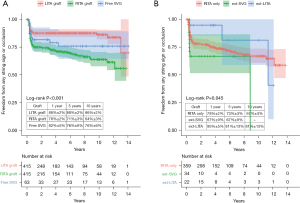
According to the protocol, initial MDCT was scheduled for postoperative 3 to 6 months. However, for reasons such as patient compliance, 44 out of 415 patients (11%) underwent initial MDCT during postoperative 6 to 12 months. There were 56 patients (13%) who underwent conventional angiography at least once postoperatively, and conventional angiography was the last patency evaluation in 38 patients (9%). Initially, long-term patency was compared among three groups: the left ITA graft (n=415), the right ITA graft (n=415), and free SVG (n=63) (Figure 2A). Freedom from patency events (string sign or occlusion) was significantly different among these three groups (P<0.001). The left ITA graft showed the best patency. In the right ITA graft, the patency was compared among subgroups (Figure 2B): right ITA graft only (n=296), right ITA extended with SVG (n=34), and right ITA extended with the remaining left ITA graft (n=22). In this comparison, the right ITA graft extended with remaining left ITA graft showed the highest freedom from patency events (P=0.045).
Severity of target vessel stenosis and patency
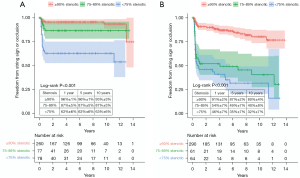
The degree of target vessel stenosis was categorized into two groups according to the severity of terminal target vessel stenosis: ≥90% stenotic versus <90% stenotic (based on measurements of the diameter). In the left ITA graft, the group with high-degree stenosis target vessels (n=260) showed significantly better freedom from patency events (string sign or occlusion) than the less stenotic group (n=155) (Figure 3A). Likewise, in the right ITA graft, the high-degree stenosis group (n=290) also showed significantly better freedom from patency events than the less stenotic group (n=125) (Figure 3B). In target vessels with ≥90% stenosis, the 1-, 5-, and 10-year graft patency was 96%±1%, 96%±1%, and 93%±3% in the left ITA grafts, and 91%±2%, 87%±2%, and 80%±4% in the right ITA grafts, respectively.
Patency by beating versus non-beating CABG
For evaluation of graft patency in sequential grafting, the left ITA, proximal right ITA (LCx), and distal right ITA (RCA) territory were separately analyzed. The patency of the left ITA graft was not significantly different between the beating and non-beating CABG groups (P=0.053 by the log-rank test) (Figure 4A). There were 624 and 500 anastomoses to the LCx and RCA territory, respectively. Clustered Cox regression for multiple time-events in a single subject was performed to evaluate the association of beating or non-beating CABG with graft patency in LCx and RCA territories. Non-beating CABG was associated with a significantly decreased risk of patency events (string sign or occlusion) in both LCx and RCA territories [LCx: hazard ratio (HR) 0.308, 95% confidence interval (CI): 0.119–0.796, P=0.015 and RCA: HR 0.563, 95% CI: 0.336–0.945, P=0.030] (Figure 4B,4C).

Initial patency and long-term survival
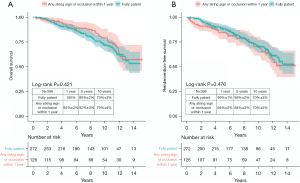
Patients who showed any string sign or occlusion at any site on the initial CT (<1 year) (n=126) were compared with the full-patency group (n=272). Overall survival and reintervention-free survival were not significantly different between the two groups (Figure 5A,5B). The 10-year overall survival was 79%±4% and 73%±3% in the groups with any string sign or occlusion and full patency, respectively (P=0.421 by the log-rank test). The 10-year reintervention-free survival rate was 70%±5% in the group with any string sign or occlusion and 70%±3% in the fully-patent group (P=0.476 by the log-rank test).
Multivariable Cox regression analysis was performed. Non-beating CABG, 1-year patency in each ITA graft, and the other variables that showed P values less than 0.2 in a comparison between the beating and non-beating CABG groups were analyzed (Table 3). In this analysis, beating versus non-beating CABG and 1-year patency in each ITA graft were not independent predictors of overall mortality, whereas age and preoperative atrial fibrillation were significant risk factors.
Table 3
| Variables | P value | Hazard ratio | 95% confidence interval |
|---|---|---|---|
| Non-beating heart surgery | 0.168 | 0.683 | 0.397–1.175 |
| LITA graft fully patent (1-year) | 0.163 | 1.697 | 0.807–3.569 |
| RITA graft fully patent (1-year) | 0.492 | 1.185 | 0.731–1.922 |
| Age | <0.001 | 1.077 | 1.048–1.108 |
| Diabetes | 0.067 | 1.491 | 0.973–2.285 |
| COPD | 0.185 | 1.759 | 0.763–4.054 |
| Preoperative AF | 0.002 | 2.983 | 1.477–6.024 |
| Diagnosis | |||
| Unstable angina (ref.) | – | – | – |
| Silent angina | 0.307 | 1.495 | 0.691–3.237 |
| Stable angina | 0.452 | 1.244 | 0.705–2.197 |
| NSTEMI | 0.586 | 1.192 | 0.633–2.246 |
| STEMI | 0.370 | 0.574 | 0.171–1.929 |
| LV dysfunction (LV EF ≤40%) | 0.150 | 1.502 | 0.863–2.614 |
| Elective surgery | 0.473 | 0.844 | 0.532–1.340 |
| Number of anastomoses | 0.755 | 1.040 | 0.814–1.327 |
| RITA graft target | |||
| Obtuse marginal branch (ref.) | – | – | – |
| Posterolateral branch | 0.851 | 0.897 | 0.287–2.797 |
| Posterior descending artery | 0.820 | 1.148 | 0.349–3.775 |
| Right coronary artery | 0.596 | 1.571 | 0.296–8.342 |
| LAD endarterectomy | 0.544 | 1.460 | 0.431–4.947 |
| Additional free SVG | 0.599 | 1.308 | 0.480–3.564 |
Non-beating surgery, each graft patency, and variables with P values less than 0.2 in the comparison between non-beating and beating groups were evaluated using a Cox proportional hazard ratio model. LITA, left internal thoracic artery; RITA, right internal thoracic artery; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; LV, left ventricle; EF, ejection fraction; LAD, left anterior descending; SVG, saphenous vein graft.
Later change of early poor patency
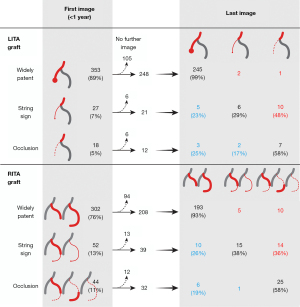
The string sign on the initial CT scan progressed to occlusion in 10 of 21 (48%) cases in the left ITA graft and in 14 of 39 (36%) cases in the right ITA graft. On the contrary, 24 of 104 (23%) ITA grafts showing occlusion (no visibility) or the string sign on the initial CT scan became widely patent in the later images (Figure 6).
Discussion
We analyzed the long-term patency and outcomes of our bilateral ITA Y composite graft strategy by a single experienced surgeon. Initially, we had four questions: (I) whether the severity of target vessel stenosis influences the graft patency, (II) whether beating or non-beating CABG influences the graft patency, (III) whether compromised initial patency leads to worse long-term survival, and (IV) whether the string sign or occlusion demonstrated on the initial MDCT is an immutable finding.
To date, only one principle has been firmly established with respect to CABG: left ITA to LAD anastomosis is the best. Other than that, surgeons perform CABG using their own strategies. According to a recent report, single-arterial CABG is still used in 85% of patients in the US (12). There have been trials that compared the outcomes of single versus multi- arterial grafts, but the results are tight for both sides (3,12-15). A large-scale trial is currently underway to compare the outcomes of single versus multiple arterial grafts in CABG (14). For surgeons who perform multi-arterial CABG, the use of the bilateral ITAs is a good option to achieve total arterial revascularization (2,3). However, contrary to what has been described in the literature, the bilateral in situ ITA strategy has the drawback of graft length limitation, as the in situ right ITA frequently cannot reach the LCx territory or even the distal LAD. Therefore, the bilateral ITA composite Y graft strategy can be an alternative (16,17). With this strategy, access to the ascending aorta can still be avoided under off-pump technique (no-aortic-touch CABG).
Nevertheless, concerns remain regarding the bilateral ITA composite Y graft strategy. As there is only one source of blood flow, the chance of flow competition can be higher. Sequential anastomoses might increase the possibility of flow competition even further (18). Our results on the patency rate by the severity of target vessel stenosis are consistent with those of previous studies. The increased chance of flow competition might have led to inferior patency in non-severe target vessel stenosis (1,19). We tried to use a separate blood flow source to the posterolateral/posterior descending region in cases of non-severe target vessel stenosis, due to the concern of flow competition. However, this policy could not be followed in all circumstances. For the determination of whether or not to revascularize, assessing the severity of target vessel stenosis with quantitative coronary analysis (QCA) and/or fractional flow reserve (FFR) may be more helpful than subjective grading (20). Meanwhile, in real-world practice, QCA or FFR assessment is rarely performed in patients in whom CABG is planned. Our results support the argument that revascularization of non-severe lesions using a bilateral Y-composite graft needs to be performed with caution.
Another concern is the technical complexity that can be encountered during sequential anastomoses with the off-pump approach (21). Some articles have reported that there was no difference in survival after off-pump versus on-pump or conventional CABG (22-24). Several recent articles have reported that there was no difference in patency between off-pump and on-pump CABG (25,26). However, it is doubtful whether it is appropriate to differentiate CABG into off-pump versus on-pump in evaluating graft patency, as on-pump beating CABG is technically similar to off-pump CABG. To assess patency, it is more reasonable to divide procedures into beating and non-beating approaches, given the common-sense viewpoint that surgery on a still organ is easier than surgery on a moving organ. Our results on the difference in patency according to beating versus non-beating CABG are noteworthy. A significant difference in patency between the beating and non-beating groups was demonstrated in anastomoses to LCx and RCA regions, while there was no clear patency difference in the LAD region. As the LAD is located in the uppermost front surface of the heart, the difference between the beating and non-beating approaches could have had a negligible impact. We think that the difference between the beating and non-beating approaches influenced the patency of anastomoses in the LCx and RCA territories because those regions are located deeper in the pericardium. Beating-heart CABG under cardiopulmonary bypass or extracorporeal membrane oxygenation may be advantageous in that the maintenance of vital signs during heart positioning is easier, but the anastomosis technique itself is similar to that used in the off-pump approach. Considering that cardiopulmonary bypass and myocardial protection are becoming more sophisticated and stable, it may be reasonable to consider conventional CABG instead of solely performing on-pump beating or off-pump CABG.
The third question is whether differences in patency lead to differences in long-term survival rates (4). There is a paucity of data about the influence of initial graft patency evaluated with MDCT on long-term outcomes. We compared long-term survival between patients who showed full patency versus those with any string sign or occlusion of the grafts on the initial MDCT scan, but there was no significant difference in the long-term survival.
These results should be interpreted considering the characteristics of MDCT. Currently, the gold standard for evaluating graft patency after CABG is conventional angiography. However, due to its invasiveness, MDCT is the most frequently used method for evaluating graft patency. The reliability of MDCT has been documented through a number of studies, and its reliability is being improved with technical advances (27,28). Nevertheless, unlike conventional angiography, MDCT does not allow a functional evaluation of dynamic findings such as flow competition, stagnation, and reversal (29). This limitation might account for our finding that about 23% of patients who initially showed the string sign or occlusion in the grafts showed wide patency in later follow-up (30). We have published a study on the restoration of graft patency in MDCT follow-up after CABG (9). Our results indicate that it is not necessary to ascribe excessive clinical significance to the asymptomatic string sign or occlusion shown on MDCT during follow-up.
There are limitations of this study. First, we maintained a consistent surgical strategy throughout the study period, but this is a small retrospective study of procedures performed by a single surgeon. There were conditions that made it difficult to adhere to this strategy, and various adaptions resulting from those conditions were reflected in the study results. Clearly, the existence of such variability in surgical procedures is a weakness in terms of homogeneity. In a future study, an analysis of the results of almost identical surgical procedures without exceptions would be warranted. Second, unlike conventional coronary angiography, evaluating graft patency with MDCT is not a gold-standard approach, although MDCT is currently the most frequently used method for post-CABG patency follow-up. Third, we had an MDCT follow-up protocol for patients who had undergone CABG, but MDCT follow-up was not performed regularly in a number of patients for various reasons. Considering that decreased renal function prevented several patients from receiving MDCT, this irregular follow-up might have been a source of bias.
Conclusions
In CABG using bilateral ITA composite Y graft strategy, the patency rate was suboptimal in case of non-severe (<90%) target vessel stenosis. Beating-heart CABG was associated with inferior long-term patency of anastomoses to the LCx and RCA territories. Observation of the string sign or occlusion observed within 1 year postoperatively on MDCT was not associated with inferior long-term survival.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1731/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1731/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1731/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1731/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Seoul National University Bundang Hospital (IRB No. B-2102/667-108) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakajima H, Kobayashi J, Toda K, et al. A 10-year angiographic follow-up of competitive flow in sequential and composite arterial grafts. Eur J Cardiothorac Surg 2011;40:399-404. [Crossref] [PubMed]
- Nakajima H, Kobayashi J, Toda K, et al. Safety and efficacy of sequential and composite arterial grafting to more than five coronary branches in off-pump coronary revascularisation: assessment of intra-operative and angiographic bypass flow. Eur J Cardiothorac Surg 2010;37:94-9. [Crossref] [PubMed]
- Nasso G, Coppola R, Bonifazi R, et al. Arterial revascularization in primary coronary artery bypass grafting: Direct comparison of 4 strategies--results of the Stand-in-Y Mammary Study. J Thorac Cardiovasc Surg 2009;137:1093-100. [Crossref] [PubMed]
- Gaudino M, Bakaeen FG, Benedetto U, et al. Arterial Grafts for Coronary Bypass: A Critical Review After the Publication of ART and RADIAL. Circulation 2019;140:1273-84. [Crossref] [PubMed]
- Taggart DP, Benedetto U, Gerry S, et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N Engl J Med 2019;380:437-46. [Crossref] [PubMed]
- Sef D, Raja SG. Bilateral internal thoracic artery use in coronary artery bypass grafting in the post-ART era - Perspective. Int J Surg 2021;86:1-4. [Crossref] [PubMed]
- Pevni D, Mohr R, Kramer A, et al. Are two internal thoracic grafts better than one? An analysis of 5301 cases. Eur J Cardiothorac Surg 2019;56:935-41. [Crossref] [PubMed]
- Zhu Y, Lingala B, Wang H, et al. Bilateral vs Single Internal Mammary Artery Grafts for Coronary Artery Bypass in the United States. Ann Thorac Surg 2021;111:629-35. [Crossref] [PubMed]
- Lim C, Park KH, Kim TH, et al. Computerized tomography may underestimate the patency of internal thoracic artery composite grafts. Heart Surg Forum 2012;15:E73-8. [Crossref] [PubMed]
- Villareal RP, Mathur VS. The string phenomenon: an important cause of internal mammary artery graft failure. Tex Heart Inst J 2000;27:346-9. [PubMed]
- Siebenmann R, Egloff L, Hirzel H, et al. The internal mammary artery 'string phenomenon'. Analysis of 10 cases. Eur J Cardiothorac Surg 1993;7:235-8. [Crossref] [PubMed]
- Chikwe J, Sun E, Hannan EL, et al. Outcomes of Second Arterial Conduits in Patients Undergoing Multivessel Coronary Artery Bypass Graft Surgery. J Am Coll Cardiol 2019;74:2238-48. [Crossref] [PubMed]
- Janiec M, Dimberg A, Nazari Shafti TZ, et al. No improvements in long-term outcome after coronary artery bypass grafting with arterial grafts as a second conduit: a Swedish nationwide registry study. Eur J Cardiothorac Surg 2018;53:448-54. [Crossref] [PubMed]
- Gaudino M, Alexander JH, Bakaeen FG, et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial-rationale and study protocol. Eur J Cardiothorac Surg 2017;52:1031-40. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Shih BC, Chung S, Kim H, et al. Outcomes and Patency of Complex Configurations of Composite Grafts Using Bilateral Internal Thoracic Arteries. Korean J Thorac Cardiovasc Surg 2020;53:64-72. [Crossref] [PubMed]
- Kawajiri H, Grau JB, Fortier JH, et al. Bilateral internal thoracic artery grafting: in situ or composite? Ann Cardiothorac Surg 2018;7:673-80. [Crossref] [PubMed]
- Raza S, Blackstone EH, Bakaeen FG, et al. Long-Term Patency of Individual Segments of Different Internal Thoracic Artery Graft Configurations. Ann Thorac Surg 2019;107:740-6. [Crossref] [PubMed]
- Tinica G, Chistol RO, Enache M, et al. Long-term graft patency after coronary artery bypass grafting: Effects of morphological and pathophysiological factors. Anatol J Cardiol 2018;20:275-82. [Crossref] [PubMed]
- Glineur D, Boodhwani M, Hanet C, et al. Bilateral Internal Thoracic Artery Configuration for Coronary Artery Bypass Surgery: A Prospective Randomized Trial. Circ Cardiovasc Interv 2016;9:e003518. [Crossref] [PubMed]
- Nakano J, Okabayashi H, Noma H, et al. Early angiographic evaluation after off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2013;146:1119-25. [Crossref] [PubMed]
- Yang L, Lin S, Zhang H, et al. Long-Term Graft Patency After Off-Pump and On-Pump Coronary Artery Bypass: A CORONARY Trial Cohort. Ann Thorac Surg 2020;110:2055-61. [Crossref] [PubMed]
- Sajja LR, Sarkar K, Mannam G, et al. One-year outcomes of off- and on-pump coronary artery bypass grafting: PROMOTE patency trial. Indian J Thorac Cardiovasc Surg 2020;36:469-75. [Crossref] [PubMed]
- Puskas JD, Williams WH, O'Donnell R, et al. Off-pump and on-pump coronary artery bypass grafting are associated with similar graft patency, myocardial ischemia, and freedom from reintervention: long-term follow-up of a randomized trial. Ann Thorac Surg 2011;91:1836-42; discussion 1842-3. [Crossref] [PubMed]
- Sajja LR, Sarkar K, Mannam G, et al. Graft patency at 3 months after off- and on-pump coronary bypass surgery: a randomized trial. Indian J Thorac Cardiovasc Surg 2020;36:93-104. [Crossref] [PubMed]
- Seki T, Yoshida T. Comparison of Mid-Term Graft Patency between On-Pump and Off-Pump Coronary Artery Bypass Grafting. Ann Thorac Cardiovasc Surg 2017;23:141-8. [Crossref] [PubMed]
- Chan M, Ridley L, Dunn DJ, et al. A systematic review and meta-analysis of multidetector computed tomography in the assessment of coronary artery bypass grafts. Int J Cardiol 2016;221:898-905. [Crossref] [PubMed]
- Barbero U, Iannaccone M, d'Ascenzo F, et al. 64 slice-coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: A meta-analysis. Int J Cardiol 2016;216:52-7. [Crossref] [PubMed]
- Gabriel J, Klimach S, Lang P, et al. Should computed tomography angiography supersede invasive coronary angiography for the evaluation of graft patency following coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg 2015;21:231-9. [Crossref] [PubMed]
- Kim JB, Kang JW, Song H, et al. Late improvement in graft patency after coronary artery bypass grafting: Serial assessment with multidetector computed tomography in the early and late postoperative settings. J Thorac Cardiovasc Surg 2011;142:793-9. [Crossref] [PubMed]


