The impact of age and sex on in-hospital outcomes in acute type A aortic dissection surgery
Introduction
Acute type A aortic dissection (AAAD) is a life-threatening emergency associated with high mortality reaching 1% per hour if not treated (1). Surgical repair is the gold standard as it increases survival and reduces mortality (1). Age, clinical presentation, compromised neurological status, organ malperfusion, pre-operative shock and renal dysfunction have been described as risk factors for death (1,2).
Age remains a determining factor in the outcomes of cardiovascular surgery. Patients aged 75 years or more must be evaluated critically before elective cardiovascular surgery, since outcome and postoperative quality of life may be unsatisfactory. In the setting of AAAD, deep hypothermia, long cardiopulmonary bypass and aortic cross-clamping times, need for circulatory arrest, and selective brain perfusion further increase the risk of adverse outcome in elderly patients (2). However, on-going progress in technology, surgical technique and anesthesiological management resulted in progressively better outcomes of elective and emergency cardiac surgery, leading to an increased number of elderly patients accepted for AAAD repair in the past decades.
Several retrospective studies have tried to shed light on the results of AAAD surgery in the elderly population, reporting quite encouraging outcomes (3). In addition, given the tendency to consider female sex as an important risk factor for morbidity and mortality in AAAD, the aim of the present study was to analyze age- and sex-related differences in outcome in patients undergoing surgery for AAAD. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1863/rc).
Methods
Study design
This is a departmental retrospective study of all consecutive patients who underwent emergency surgery for AAAD between January 1st, 2006 and December 31st, 2018.
Patient selection, data collection and study outcomes
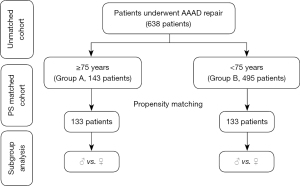
All consecutive patients undergoing surgery for AAAD in the above mentioned period were included and non were excluded. Data on demographics, surgical indications, operative notes were collected. Based on age, patients were divided into two groups; Group A includes patients aged 75 years or older and Group B those younger than 75. In-hospital mortality was the primary study outcome. In addition, Intra operative and post-operative data were compared between the two groups before and after propensity score matching. In each matched group, outcomes were compared based on sex (Figure 1).
Definitions of variables and outcomes
There are some definitions of “elderly” and the World Health Organisation has elaborated on the topic based on the expected projection until 2050 of the worldwide proportion of persons above the age of 65 years. On the other hand, as also stated by the WHO, categorical definitions of the old, elderly, aged and ageing are neither straightforward nor universally applicable (4). Furthermore, ageing and health cannot be understood without a sex perspective. Based on all this, with regard to our European area of influence and surgical literature, we arbitrarily choose the cutoff value of 75 years aiming at identifying a subgroup of individuals in whom frailty and comorbidities would be of impact on surgical outcomes (5).
The Penn classification defines the presence or absence of branch-vessel malperfusion, circulatory collapse, or both (6).
In hospital mortality was defined as procedural mortality which consists of all-cause mortality within 30 days or index procedure hospitalization if the postoperative stay is longer than 30 days (7). Myocardial Infarction (MI) was defined according to the Fourth Universal Definition of Myocardial Infarction (8). Stroke was defined as an acute episode of focal or global neurological dysfunction confirmed by specialized clinical and neurological examination and/or computed tomography (9). Renal impairment was defined as serum creatinine value >200 µmol/L (10). Familiality was defined as a positive family history for aortic pathology, based on collected information about disorders from which the direct blood relatives of the patient have suffered (11).
Replacement of the ascending aorta (RAA) was intended as ascending aorta replacement proximally to the innominate artery with or without open distal anastomosis. Hemiarch replacement (HAR) was defined as resection of the concavity of the aortic arch down to the proximal descending thoracic aorta without arch vessel re-implantation. Total arch replacement (TAR) was intended as replacing the entire aortic arch from the offspring of the Innominate artery to a point beyond the offspring of the left subclavian artery (LSA) (12).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Cantonal Ethics Committee in Zurich (No. 2017-00824) and informed consent was waived for all patients included until December 31st, 2015. From January 1st 2016, all patients signed an informed consent according to the new national data privacy protection.
Statistical analysis
Continuous variables were reported as mean ± standard deviation and compared with analysis of variance (paired Student’s t-test). Categorical variables were expressed as frequencies and compared with fisher exact test or chi quadrat (χ2) test. Normality of data was verified using Quantil-Quantil-Diagramm (Q-Q plots). A two-sided P value of 0.05 was considered significant. Since the patient cohort was chosen in a non-randomized fashion, we used 1:1 propensity score (PS) matching to reduce the confounding impact of variables in this non-randomized study. To estimate the PS, a logistic regression model including all baseline covariates reported in Table 1 (except sex) as main effects was utilized. Matching was performed with the PS matching plug-in for Statistical Product and Service Solutions software (SPSS) using the Nearest-Neighbour Matching algorithm without replacement, as recommended by Austin (13). Good covariate balance and a fair number of matched pairs were achieved with a caliper width of 0.2 standard deviations of the linear predictor. Balance of baseline covariates was assessed by computing the P value by χ2-, fisher exact- and t-test (balance achieved, if P<0.5) and by computing the standardized mean difference (SMD) (balance achieved, if SMD <0.2). All statistical analyses were conducted using SPSS version 26 (SPSS, Chicago, IL, USA).
Table 1
| Variable | Unmatched | PS matched | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≥75 years, Group A? | P value | SMD | Age ≥75 years, Group A? | P value | SMD | ||||
| Yes (n=143) | No (n=495) | Yes (n=133) | No (n=133) | ||||||
| Baseline characteristics used for matching | |||||||||
| Age | 80.6±5.5 | 58.7±10.8 | 0.001 | 80.4±5.6 | 58.26±12 | 0.001 | |||
| Female sex, n (%) | 68 (47.6) | 131 (26.5) | 0.001 | 62 (46.6) | 36 (27.1) | 0.001 | |||
| Used for propensity score matching, n (%) | |||||||||
| Penn A | 56 (39.2) | 209 (42.2) | 0.51 | −0.062 | 56 (42.1) | 53 (39.8) | 0.7 | 0.046 | |
| Penn B | 26 (17.8) | 88 (17.8) | 0.9 | 0.010 | 26 (19.5) | 21 (15.8) | 0.4 | 0.097 | |
| Penn C | 38 (26.6) | 48 (9.7) | 0.001 | 0.381 | 28 (21.1) | 33 (24.8) | 0.46 | −0.085 | |
| Penn BC | 22 (15.4) | 145 (29.3) | 0.001 | −0.384 | 22 (16.4) | 24 (18.0) | 0.75 | −0.042 | |
| Arterial hypertension | 101 (70.6) | 328 (67.4) | 0.46 | 0.096 | 95 (71.4) | 87 (65.4) | 0.29 | 0.132 | |
| Preoperative CPR | 3 (2.1) | 23 (4.6) | 0.17 | −0.177 | 3 (2.3) | 5 (3.8) | 0.47 | −0.105 | |
| Diabetes Mellitus | 10 (7.0) | 18 (3.6) | 0.08 | 0.131 | 10 (7.5) | 10 (7.5) | 1.0 | 0.000 | |
| Hypercholesterolemia | 40 (28.0) | 127 (25.7) | 0.58 | 0.051 | 37 (27.8) | 34 (25.6) | 0.68 | 0.050 | |
| Nicotine abuse | 17 (11.9) | 123 (24.9) | 0.001 | −0.399 | 17 (12.8) | 18 (13.5) | 0.86 | −0.023 | |
| Familiality | 8 (5.8) | 59 (12.4) | 0.028 | −0.274 | 8 (6.0) | 7 (5.3) | 0.8 | 0.033 | |
| COPD | 13 (9.1) | 31 (6.3) | 0,79 | 0.098 | 11 (8.3) | 6 (4.5) | 0,21 | 0.130 | |
| Renal dysfunction | 9 (7.8) | 25 (6.3) | 0,58 | 0.051 | 8 (6.0) | 5 (3.8) | 0,4 | 0.093 | |
PS, propensity score; Penn, Penn classification; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; SMD, standardized mean difference.
Results
Between January 1st, 2006 and December 31st, 2018, a total of 638 patients underwent emergency surgery for AAAD, 143 patients (22.4%) were aged 75 years or older (Group A) and 495 patients (77.6%) were younger than 75 (Group B). The propensity score matching yielded 133 patients in each group.
Elderly (Group A) vs. young (Group B)
Patient characteristics and clinical presentation (Table 1)
In the non-matched groups, the rate of female sex was significantly higher in the elderly group (47.6% vs. 25.5%, P=0.001). More patients in Group A presented with circulatory collapse (Penn C, 26.6% vs. 9.7%, P=0.001) while more patients in Group B presented with both circulatory collapse and branch malperfusion (Penn BC, 29.3% vs. 15.4%, P=0.001). After matching, there were no more significant differences between the groups.
Intraoperative characteristics (Table 2)
Table 2
| Variable | Unmatched | PS Matched | |||||
|---|---|---|---|---|---|---|---|
| Age ≥75 years, Group A? | P value | Age ≥75 years, Group A? | P value | ||||
| Yes (n=143) | No (n=495) | Yes (n=133) | No (n=133) | ||||
| Intraoperative data | |||||||
| CPB time (min) | 191.9±81 | 202.1±92 | 0.21 | 191.7±80 | 202.8±93 | 0.30 | |
| Cross-Clamping time (min) | 96.6±58 | 104±60 | 0.20 | 97.1±57 | 102.8±65 | 0.45 | |
| Circulatory arrest time (min) | 29.7±81 | 33.1±91 | 0.70 | 28.3±79 | 41.9+103 | 0.26 | |
| Hypothermia (°C) | 26.4±7 | 26.5±6 | 0.78 | 26.4±7 | 26.1±8 | 0.76 | |
| Operation time (min) | 309.2±152 | 322.7±172 | 0.43 | 310.9±151 | 303.6±186 | 0.76 | |
| Isolated ascending aorta, n (%) | 32 (22.4) | 101 (20.4) | 0.61 | 30 (22.6) | 26 (19.5) | 0.55 | |
| Ascending aorta + hemiarch, n (%) | 60 (42.0) | 144 (29.1) | 0.004 | 55 (41.4) | 38 (28.6) | 0.029 | |
| Isolated hemiarch, n (%) | 25 (17.5) | 102 (20.6) | 0.41 | 23 (17.3) | 29 (21.8) | 0.35 | |
| Isolated aortic arch, n (%) | 7 (4.9) | 46 (9.3) | 0.09 | 7 (5.3) | 14 (10.5) | 0.11 | |
| Ascending aorta + arch, n (%) | 4 (2.8) | 42 (8.5) | 0.02 | 4 (3.0) | 9 (6.8) | 0.16 | |
| Root repair (Glue), n (%) | 22 (15.4) | 88 (18.0) | 0.47 | 21 (15.8) | 24 (18.0) | 0.62 | |
| Root replacement, n (%) | 33 (23.1) | 166 (33.5) | 0.0179 | 31 (23.3) | 50 (37.6) | 0.011 | |
| Mechanical prosthesis, n (%) | 3 (2.1) | 93 (18.8) | <0.001 | 3 (2.3) | 26 (19.5) | <0.001 | |
| Biological prosthesis, n (%) | 30 (21.0) | 69 (13.9) | 0.041 | 28 (21.1) | 21 (15.8) | 0.27 | |
| David procedure, n (%) | 2 (1.4) | 13 (2.6) | 0.39 | 1 (0.8) | 6 (4.5) | 0.055 | |
| Yacoub procedure, n (%) | 5 (3.5) | 31 (6.3) | 0.21 | 4 (3.0) | 8 (6.0) | 0.24 | |
| Coronary bypass grafting, n (%) | 9 (6.3) | 61 (12.3) | 0.042 | 8 (6.0) | 20 (15.0) | 0.017 | |
| Postoperative outcome | |||||||
| Pneumonia, n (%) | 43 (30.1) | 100 (20.2) | 0.013 | 37 (27.8) | 26 (19.5) | 0.11 | |
| Postoperative MI, n (%) | 6 (4.2) | 28 (5.7) | 0.49 | 6 (4.5) | 8 (6.0) | 0.58 | |
| ECMO, n (%) | 9 (6.3) | 21 (4.2) | 0.31 | 8 (6.0) | 7 (5.3) | 0.79 | |
| Stroke, n (%) | 23 (16.1) | 74 (14.9) | 0.74 | 21 (15.8) | 21 (15.8) | 1 | |
| Renal dysfunction, n (%) | 44 (30.8) | 146 (29.5) | 0.77 | 39 (29.3) | 37 (27.8) | 0.79 | |
| Intraoperative mortality, n (%) | 7 (4.9) | 12 (2.4) | 0.13 | 7 (5.3) | 5 (3.8) | 0.56 | |
| In-hospital mortality, n (%) | 26 (18.2) | 72 (14.5) | 0.29 | 25 (18.8) | 16 (12.0) | 0.13 | |
| Ventilation time (h) | 99.9±202 | 94.1±168 | 0.74 | 97.9±201 | 86.1±151 | 0.60 | |
| Days on ICU (days) | 10.7±12 | 11.6±17 | 0.57 | 10..8±13 | 11.3±13 | 0.77 | |
PS, propensity score; CPB, cardiopulmonary bypass; MI, myocardial infarction; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
In the non-matched groups, Group B patients received more TAR in combination with RAA (8.5% vs. 2.8%, P=0.021), this difference disappeared after matching (3% vs. 6.8%, P=0.15). In Group A the combination of RAA und hemiarch replacement (HAR) was higher (42% vs. 29.1%, P=0.004) and persisted after matching (41.4% vs. 28.6%, P=0.029). More Group B patients received aortic root replacement (ARR) (33.6% vs. 23.2%, P=0.019) and needed concomitant bypass surgery (CABG) (12.3% vs. 6.3%, P=0.042) which stayed significant after matching (37.6% vs. 23.3%, P=0.01, 15% vs. 6%, P=0.017, respectively). Cross clamping time and cardiopulmonary bypass (CPB) time were similar.
Morbidity and mortality (Table 2)
Group A showed a higher incidence of pneumonia (31.6% vs. 20.4%, P=0.006), which became comparable after matching. In-hospital mortality was comparable between the groups.
Gender differences
Patient characteristics and clinical presentation (Table 3)
Table 3
| Variable | Unmatched | PS matched | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age ≥75 years, Group A | Age <75 years, Group B | Age ≥75 years, Group A | Age <75 years Group B | ||||||||||||
| Female (n=68) | Male (n=75) | P value | Female (n=131) | Male (n=364) | P value | Female (n=62) | Male (n=71) | P value | Female (n=36) | Male (n=97) | P value | ||||
| Penn A | 27 (39.7) | 29 (38.7) | 0.899 | 49 (37.4) | 160 (44.0) | 0.193 | 27 (43.5) | 29 (40.8) | 0.753 | 12 (33.3) | 41 (42.3) | 0.350 | |||
| Penn B | 8 (11.8) | 18 (24.0) | 0.058 | 27 (20.6) | 61 (16.8) | 0.323 | 8 (12.9) | 18 (25.4) | 0.071 | 4 (11.1) | 17 (17.5) | 0.367 | |||
| Penn C | 22 (32.4) | 16 (21.3) | 0.136 | 14 (10.7) | 34 (9.3) | 0.655 | 16 (25.8) | 12 (16.9) | 0.209 | 8 (22.2) | 25 (25.8) | 0.674 | |||
| Penn BC | 11 (16.2) | 11 (14.7) | 0.803 | 39 (29.8) | 106 (29.1) | 0.888 | 11 (17.7) | 11 (15.5) | 0.728 | 11 (30.6) | 13 (13.4) | 0.022 | |||
| Arterial hypertension | 54 (79.4) | 47 (62.7) | 0.028 | 94 (71.8) | 234 (64.3) | 0.121 | 49 (79.0) | 46 (64.8) | 0.070 | 25 (69.4) | 62 (63.9) | 0.552 | |||
| Preoperative CPR | 2 (2.9) | 1 (1.3) | 0.503 | 5 (3.8) | 18 (4.9) | 0.599 | 2 (3.2) | 1 (1.4) | 0.481 | 2 (5.6) | 3 (3.1) | 0.507 | |||
| Diabetes mellitus | 5 (7.4) | 5 (6.7) | 0.872 | 5 (3.8) | 13 (3.6) | 0.898 | 5 (8.1) | 5 (7.0) | 0.824 | 2 (5.6) | 8 (8.2) | 0.601 | |||
| Hypercholesterolemia | 22 (32.4) | 18 (24.0) | 0.266 | 28 (21.4) | 99 (27.2) | 0.191 | 20 (32.3) | 17 (23.9) | 0.286 | 10 (27.8) | 24 (24.7) | 0.721 | |||
| Nicotine abuse | 6 (8.8) | 11 (14.7) | 0.281 | 28 (21.4) | 95 (26.1) | 0.283 | 6 (9.7) | 11 (15.5) | 0.316 | 4 (11.1) | 14 (14.4) | 0.619 | |||
| Familiarity | 2 (2.9) | 6 (8.0) | 0.189 | 15 (11.5) | 44 (12.1) | 0.847 | 2 (3.2) | 6 (8.5) | 0.206 | 1 (2.8) | 6 (6.2) | 0.434 | |||
| COPD | 7 (10.3) | 6 (8.0) | 0.634 | 6 (4.6) | 25 (6.9) | 0.354 | 5 (8.1) | 6 (8.5) | 0.936 | 1 (2.8) | 5 (5.2) | 0.557 | |||
| Renal dysfunction | 5 (7.4) | 4 (5.3) | 0.619 | 7 (5.3) | 18 (4.9) | 0.858 | 4 (6.5) | 4 (5.6) | 0.843 | 2 (5.6) | 3 (3.1) | 0.507 | |||
PS, propensity score; Data are shown as number (percentage). Penn, Penn classification; CPR, cardiopulmonary resuscitation; COPD, chronic obstructive pulmonary disease.
In the matched cohorts, females in group B presented a higher rate of circulatory collapse and branch malperfusion when compared to male patients in the same group of age (Penn BC, 30.6% vs. 13.4%, P=0.022).
Intraoperative characteristics and outcomes
Group A (Table 4)
Table 4
| Variable | Unmatched cohorts (≥75 years), Group A | PS matched cohorts (≥75 years), Group A | |||||
|---|---|---|---|---|---|---|---|
| Male (n=75) | Female (n=68) | P value | Male (n=71) | Female (n=62) | P value | ||
| Intraoperative data | |||||||
| CPB time (min) | 202.5±78 | 180.1±84 | 0.1 | 203.4±80 | 178.1±80 | 0.074 | |
| Cross-Clamping time (min) | 101.6±57 | 91.0±59 | 0.28 | 102.9±58 | 90.2±55 | 0.21 | |
| Circulatory arrest time (min) | 25.9±66 | 33.7±96 | 0.60 | 26.2±67 | 30.8±90 | 0.76 | |
| Hypothermia (°C) | 26.4±4 | 26.3±9 | 0,95 | 26.5±4 | 26.4±9 | 0.94 | |
| Operation time (min) | 340.0±160 | 276.1±136 | 0.028 | 342.9±164 | 274.9±127 | 0.024 | |
| Isolated ascending aorta, n (%) | 12 (16.0) | 20 (29.4) | 0.06 | 12 (16.9) | 18 (29.0) | 0.10 | |
| Ascending aorta + hemiarch, n (%) | 35 (46.7) | 25 (35.8) | 0.23 | 32 (45.1) | 23 (37.1) | 0.35 | |
| Isolated hemiarch, n (%) | 14 (18.7) | 11 (16.2) | 0.70 | 13 (18.3) | 10 (16.1) | 0.74 | |
| Isolated aortic arch, n (%) | 4 (5.3) | 3 (4.4) | 0.80 | 4 (5.6) | 3 (4.8) | 0.83 | |
| Ascending aorta + arch, n (%) | 3 (4.0) | 1 (1.5) | 0.36 | 3 (4.2) | 1 (1.6) | 0.38 | |
| Root repair (Glue), n (%) | 12 (16.0) | 10 (14.7) | 0.83 | 11 (15.5) | 10 (16.1) | 0.92 | |
| Root replacement, n (%) | 18 (24.0) | 15 (22.1) | 0.78 | 17 (23.9) | 14 (22.6) | 0.85 | |
| Mechanical prosthesis, n (%) | 2 (2.7) | 1 (1.5) | 0.62 | 2 (2.8) | 1 (1.6) | 0.64 | |
| Biological prosthesis, n (%) | 16 (21.3) | 14 (20.6) | 0.91 | 15 (21.1) | 13 (21.0) | 0.98 | |
| David procedure, n (%) | 0 (0) | 2 (2.9) | 0.13 | 0 (0.0) | 1 (1.6) | 0.28 | |
| Yacoub procedure, n (%) | 2 (2.7) | 3 (4.4) | 0.57 | 2 (3.2) | 2 (2.8) | 0.89 | |
| Coronary bypass grafting, n (%) | 6 (8.0) | 3 (4.4) | 0.38 | 6 (8.5) | 2 (3.2) | 0.21 | |
| Postoperative outcome | |||||||
| Pneumonia, n (%) | 27 (36.0) | 16 (23.5) | 0.10 | 25 (35.2) | 12 (19.4) | 0.042 | |
| Postoperative MI, n (%) | 2 (2.7) | 4 (5.9) | 0.34 | 2 (2.8) | 4 (6.5) | 0.31 | |
| ECMO, n (%) | 5 (6.7) | 4 (5.9) | 0.85 | 5 (7.0) | 3 (4.8) | 0.59 | |
| Stroke, n (%) | 15 (20.0) | 8 (11.8) | 0.18 | 14 (19.7) | 7 (11.3) | 0.18 | |
| Renal dysfunction, n (%) | 28 (37.3) | 16 (23.5) | 0.07 | 26 (36.6) | 13 (21.0) | 0.048 | |
| Intraoperative mortality, n (%) | 6 (8.0) | 1 (1.5) | 0.07 | 1 (1.0) | 4 (11.1) | 0.007 | |
| In-hospital mortality, n (%) | 13 (17.3) | 13 (19.1) | 0.78 | 13 (18.3) | 12 (19.4) | 0.88 | |
| Ventilation time (h) | 123.3±255 | 75±119 | 0.17 | 125.5±262 | 67.6±88 | 0.10 | |
| Days on ICU (days) | 11.4±15 | 10.1±9 | 0.54 | 11.9±16 | 9.6±8 | 0.29 | |
CPB, cardiopulmonary bypass; MI, myocardial infarction; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Although significantly longer operation time was noted in male both unmatched (340±160 vs. 276±136, P=0.028) and matched (348.85±163 vs. 274.85±126.9, P=0.024), no differences were noted in proximal and distal repair complexity. A higher rate of pneumonia (35.2% vs. 19.4%, P=0.042) and renal dysfunction (36.6% vs. 21%, P=0.048) were noted. However, mortality was comparable between groups.
Group B (Table 5)
Table 5
| Variable | Unmatched cohorts (<75 years), Group B | PS matched cohorts (<75 years), Group B | |||||
|---|---|---|---|---|---|---|---|
| Male (n=364) | Female (n=131) | P value | Male (n=97) | Female (n=36) | P value | ||
| Intraoperative data | |||||||
| CPB time (min) | 211.2±94 | 176.8±82 | <0.001 | 208.8±94 | 186.5±88 | 0.22 | |
| Cross-Clamping time (min) | 111.2±62 | 83.6±46 | <0.001 | 107.5±69 | 90.0±49 | 0.17 | |
| Circulatory arrest time (min) | 31.2±87 | 38.8±100 | 0.45 | 45.4±105 | 31.3±97 | 0.52 | |
| Hypothermia (°C) | 26.7±6 | 26.1±6 | 0.33 | 26.9±8 | 24.1±8 | 0.09 | |
| Operation time (min) | 338.5±168 | 279.4±168 | 0.003 | 304±182 | 299±200 | 0.9 | |
| Isolated ascending aorta, n (%) | 66 (18.1) | 35 (26.7) | 0.037 | 14 (14.4) | 12 (33.3) | 0.015 | |
| Ascending aorta + hemiarch, n (%) | 97 (26.6) | 47 (35.9) | 0.046 | 24 (24.7) | 14 (38.9) | 0.11 | |
| Isolated hemiarch, n (%) | 90 (24.7) | 12 (9.2) | <0.001 | 27 (27.8) | 2 (5.6) | 0.006 | |
| Isolated aortic arch, n (%) | 40 (11.0) | 6 (4.6) | 0.030 | 13 (13.4) | 1 (2.8) | 0.08 | |
| Ascending aorta + arch, n (%) | 27 (7.4) | 15 (11.5) | 0.16 | 5 (5.2) | 4 (11.1) | 0.22 | |
| Root repair (Glue), n (%) | 66 (18.1) | 23 (17.6) | 0.88 | 18 (18.6) | 6 (16.7) | 0.80 | |
| Root replacement, n (%) | 147 (40.4) | 19 (14.5) | <0.001 | 46 (47.4) | 4 (11.1) | <0.001 | |
| Mechanical prosthesis, n (%) | 86 (23.6) | 7 (5.3) | <0.001 | 25 (25.8) | 1 (2.8) | 0.003 | |
| Biological prosthesis, n (%) | 59 (16.2) | 10 (7.6) | 0.015 | 19 (19.6) | 2 (5.6) | 0.049 | |
| David procedure, n (%) | 11 (3.0) | 2 (1.5) | 0.36 | 6 (6.2) | 0 (0) | 0.13 | |
| Yacoub procedure, n (%) | 19 (5.2) | 12 (9.2) | 0.11 | 6 (6.2) | 2 (5.6) | 0.89 | |
| Coronary bypass grafting, n (%) | 43 (11.8) | 18 (13.7) | 0.57 | 15 (15.5) | 5 (13.9) | 0.82 | |
| Postoperative outcome | |||||||
| Pneumonia, n (%) | 82 (22.5) | 18 (13.7) | 0.032 | 20 (20.6) | 6 (16.7) | 0.61 | |
| Postoperative MI, n (%) | 23 (6.3) | 5 (3.8) | 0.29 | 5 (5.2) | 3 (8.3) | 0.49 | |
| ECMO, n (%) | 14 (3.8) | 7 (5.3) | 0.47 | 5 (5.2) | 2 (5.6) | 0.93 | |
| Stroke, n (%) | 52 (14.3) | 22 (16.8) | 0.49 | 14 (14.4) | 7 (19.4) | 0.48 | |
| Renal dysfunction, n (%) | 106 (29.1) | 40 (30.5) | 0.76 | 27 (27.8) | 10 (27.8) | 1.00 | |
| Intraoperative mortality, n (%) | 7 (1.9) | 5 (3.8) | 0.23 | 1 (1.0) | 4 (11.1) | 0.007 | |
| In-hospital mortality, n (%) | 46 (12.6) | 26 (19.8) | 0.045 | 8 (8.2) | 8 (22.2) | 0.028 | |
| Ventilation time (h) | 88.0±146 | 111.8±218 | 0.17 | 71.5±114 | 128.1±223 | 0.17 | |
| Days on ICU (days) | 11.2±17 | 12.6±16 | 0.42 | 11.0±12 | 12.1±16 | 0.67 | |
CPB, cardiopulmonary bypass; MI, myocardial infarction; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
When comparing the two genders, there were significant differences in the distal repair technique: the rate of RAA was higher in female (26.7% vs. 18.1%, P=0.037), while HAR was higher in men (9.2% vs. 24.7%, P=0.001) which was still present after matching (P=0.015 and 0.006, respectively). Further significant differences such as TAR or combination of RAA and HAR were eliminated after matching (P=0.076 and 0.1, respectively). Regarding proximal repair, men received more frequently ARR before (40.4% vs. 14.5%, P=0.001), and after matching (47.4% vs. 11.1%, P=0.001). CPB time (211.2±94 vs. 176.8±82, P<0.001), cross clamping time (111.2±62 vs. 83.6±46, P<0.001), and operation time (338.5±168 vs. 279.4±168, P=0.003) were significantly shorter in young women in the unmatched group. The in-hospital mortality was significantly higher in females before (19.8% vs. 12.6%, P=0.045) and after matching (22.2% vs. 8.2%, P=0.028).
Discussion
As a main finding of this study, we could show that morbidity and mortality are comparable between young and old patients undergoing surgical repair for AAAD. Certainly, this result must be interpreted with great caution and may appear in contrast to previous studies reporting age as an independent factor for mortality in AAAD (2,3). However, in contrary to our study, they did not take into account the preoperative characteristics of young and old patients undergoing surgery for AAAD.
Several factors may have contributed to this relatively low mortality in elderly patients: there is no data available about the number of patients who died before reaching a hospital or were denied surgery. And it is also a practice choosing conservative therapy in older patients due to high surgical risk and expected adverse outcomes (14). This results in a selection bias in patients who receive surgery, and therefore may underestimate the true surgical mortality rate if the number accepted for surgery was comparable to that of younger patients. Furthermore, in elderly patients, surgery tends to be less complex in order to decrease CPB time and ischemia time, as noted in our study, by the lower frequency of aortic replacement surgery, which presumably reduces the postoperative mortality.
Similar to the findings of Kreibich et al. we confirmed that Group A patients with AAAD had more frequently cardiovascular collapse classified as Penn class C (15). This tendency to develop shock in elderly patients was interpreted as a lower compensatory capacity with increasing age. On the other hand, Group B patients had a higher frequency of Penn Class BC presentation with greater extent of dissection with organ malperfusion in association with cardiovascular collapse. This observation concurs with the observations of other groups, Malvindi et al. reported that the extension to the coronary sinus, descending thoracic aorta and abdominal aorta and the presence of an intimal tear at the level of the aortic root correlated inversely with age (16). The reason for this finding might be that younger patients are more likely to have connective tissue diseases or bicuspid aortic valve, which facilitate extension of the dissection proximally and distally. However, information regarding these disorders is not available in our database, one of the limitations of this study.
Despite our results confirm a tendency to conduct more complex surgery at the distal level, intended as TAR in Group B, this tendency was not confirmed after matching. This finding can be interpreted as follows: first, in younger patients the site of intimal tear tends to be more proximal to the aortic root as reported in the International Registry of Aortic Dissection (IRAD), which often results in complex root replacement surgery rather than reparative surgery as will be discussed later (17). Second, the development of interventional techniques to treat the aortic arch in recent years may have contributed to reduce surgical aggressiveness at the level of the aortic arch, even in younger patients. Interestingly, the combination of RAA and HAR was frequent in older patients and this may be attributed to a higher frequency of distal localization of intimal tear in the older population as reported by the IRAD analysis.
Furthermore, our results show that Group B patients had a higher rate of complex proximal surgery intended as ARR and need of concomitant CABG surgery. This is our experience when specifically looking at patients requiring concomitant CABG in the setting of AAAD (18). As above mentioned, the site of intimal tear tends to be more proximal to the aortic root in young patients, which requires more aggressive and durable surgery at the level of the aortic root rather than conservative approaches as recommended by Castrovinci et al. (19), who found that a more extensive root intervention appeared to be protective against aortic reintervention. In addition, Group B patients presented often with Penn class BC. Myocardial malperfusion could not be excluded in these patients and could explain the higher rate of concomitant CABG surgery.
The data reported in the literature regarding gender and sex difference in AAAD are controversial. In a sub-analysis of the IRAD study (20), the surgical mortality rate for acute Type A dissection was 31.9% in women and 21.9% in men (P=0.013). Older age at onset, delayed transfer to hospital and more complications including tamponade, shock, heart failure and coma are cited as potential reasons for higher surgical mortality. Fukui et al. (21) analyzed the impact of sex on preoperative characteristics and outcomes in patients undergoing surgery for AAAD. The operative mortality was found to be similar in Japanese male and female patients (4.5% vs. 5.8%; P=0.6463) and no difference in pre-operative conditions was found.
In a recent analysis from the German Registry for Acute Aortic Dissection Type A (GERAADA), Rylski et al. (22) reported a comparable operative mortality in both sexes, despite a more complicated clinical presentation in males. Compared to the IRAD data, the authors of this analysis believed that a better understanding of aortic pathology, together with advances in surgical therapy underlie a decrease in surgical mortality in AAAD and consequently an improvement in survival in females as well. The three previous studies agreed on 2 elements: an older age by presentation, and a less complex surgery in female patients. The last point was precisely attributed to the older age of the female patients.
In the present study, we observed no sex related difference in the older group (Group A). In the younger group (Group B), female patients underwent less complex surgery with shorter cross clamp time and duration when compared to their male counterpart, perhaps because of smaller aortic diameters, which facilitate the surgeon’s choice of more conservative reparative approaches of the aortic root. However, the mortality in female patients in group B was higher.
The tendency to have a high mortality in young women has been noted after other cardiac surgery procedures. In a landmark study, Vaccarino et al. (23) analyzed the outcomes of 51,187 patients undergoing CABG. Younger women undergoing CABG surgery were at higher risk of in-hospital death than men, but this difference in risk decreased with advancing age. Similar results were reported from Enger et al. (24) in patients undergoing combined valve and CABG surgery. Genetic and hormonal mechanisms have been cited in both studies to explain these findings.
This paradox between complexity of surgery and mortality in young female patients in our study may be attributed in part to a more complex clinical presentation, similar to that reported in the IRAD analysis, since the incidence of a Penn BC class was more significant in female patients in Group B. However, other genetic and pathological factors cannot be ruled out and more in-depth studies are needed to shed light on the histological and genetic features of young females with AAAD.
Limitations
The limitations of our study are the following: it is single-center, retrospective and observational. Furthermore, data regarding connective tissue disorder and aortic morphology are missing. In addition, patients with AAAD who died before reaching the hospital or were denied for surgery are not reported, which could result in an underestimation of the real mortality in old patients.
Conclusions
After propensity matching of our large cohort of AAAD patients, we were unable to show a significant difference in mortality and morbidity comparing patients under and over 75 years of age. When comparing both sexes after matching, female patients younger than 75 seem to have experienced a higher rate of in-hospital mortality than their male counterparts.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1863/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1863/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1863/coif). CAM reports that he received consulting fees from CytoSorbent for online presentation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Cantonal Ethics Committee in Zurich (No. 2017-00824) and informed consent was waived for all patients included until December 31st, 2015. From January 1st, 2016, all patients signed an informed consent according to the new national data privacy protection act.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200-6. [Crossref] [PubMed]
- Trimarchi S, Eagle KA, Nienaber CA, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg 2010;140:784-9. [Crossref] [PubMed]
- Men, ageing and health. WHO 2001.
- McKneally MF. "We didn't expect dementia and diapers": reflections on the Nihon experience with type A aortic dissection in octogenarians. J Thorac Cardiovasc Surg 2008;135:984-5. [Crossref] [PubMed]
- Augoustides JG, Geirsson A, Szeto WY, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med 2009;6:140-6. [Crossref] [PubMed]
- Overman DM, Jacobs JP, Prager RL, et al. Report from the Society of Thoracic Surgeons National Database Workforce: clarifying the definition of operative mortality. World J Pediatr Congenit Heart Surg 2013;4:10-2. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HDESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237-69. [Crossref] [PubMed]
- Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. J Am Coll Cardiol 2018;71:1021-34. [Crossref] [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [Crossref] [PubMed]
- "Family Health History: The Basics". Centers for Disease Control and Prevention. Retrieved 24 October 2020.
- Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019;55:133-62. [Crossref] [PubMed]
- Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057-69. [Crossref] [PubMed]
- Hata M, Sezai A, Niino T, et al. Should emergency surgical intervention be performed for an octogenarian with type A acute aortic dissection? J Thorac Cardiovasc Surg 2008;135:1042-6. [Crossref] [PubMed]
- Kreibich M, Rylski B, Czerny M, et al. Influence of Age and the Burden of Ischemic Injury on the Outcome of Type A Aortic Dissection Repair. Ann Thorac Surg 2019;108:1391-7. [Crossref] [PubMed]
- Malvindi PG, Votano D, Ashoub A, et al. Age-related presentation of acute type A aortic dissection. Asian Cardiovasc Thorac Ann 2018;26:659-66. [Crossref] [PubMed]
- Januzzi JL, Isselbacher EM, Fattori R, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol 2004;43:665-9. [Crossref] [PubMed]
- Morjan M, Reser D, Savic V, et al. Concomitant Coronary Artery Bypass in Patients with Acute Type A Aortic Dissection. Semin Thorac Cardiovasc Surg 2022;34:410-6. [Crossref] [PubMed]
- Castrovinci S, Pacini D, Di Marco L, et al. Surgical management of aortic root in type A acute aortic dissection: a propensity-score analysis. Eur J Cardiothorac Surg 2016;50:223-9. [Crossref] [PubMed]
- Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation 2004;109:3014-21. [Crossref] [PubMed]
- Fukui T, Tabata M, Morita S, et al. Gender differences in patients undergoing surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;150:581-7.e1. [Crossref] [PubMed]
- Rylski B, Georgieva N, Beyersdorf F, et al. Gender-related differences in patients with acute aortic dissection type A. J Thorac Cardiovasc Surg 2021;162:528-535.e1. [Crossref] [PubMed]
- Vaccarino V, Abramson JL, Veledar E, et al. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation 2002;105:1176-81. [Crossref] [PubMed]
- Enger TB, Pleym H, Stenseth R, et al. Reduced Long-Term Relative Survival in Females and Younger Adults Undergoing Cardiac Surgery: A Prospective Cohort Study. PLoS One 2016;11:e0163754. [Crossref] [PubMed]