
A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 1: a guide to decision-making
Introduction
Early-stage non-small cell lung cancer (NSCLC) is changing. Increased use of CT scanning is detecting smaller and more indolent tumors (1-4). The average age of patients diagnosed and treated is increasing, as is the proportion with co-morbidities (2,3,5). Possible reasons include increasing general life expectancy, decreasing treatment-related toxicities, and increasing willingness to be treated.
Treatment options for stage I NSCLC have evolved. This includes minimally invasive surgical techniques [e.g., video-assisted thoracic surgery (VATS)], less extensive resection, and non-surgical treatments like stereotactic body radiotherapy (SBRT) and thermal ablation. It is important to appropriately match the treatment to the patient and tumor, avoiding both overtreatment and undertreatment.
Decision-making regarding stage I NSCLC has become complex. There are several treatment options and a spectrum of patients and tumors. There are multiple aspects to consider, e.g., short-term impact and long-term outcomes. Weighing various considerations is what constitutes clinical judgement. Furthermore, the likelihood and severity of various potential outcomes must be assessed for an individual patient and setting—considering applicability and ambiguities of the available evidence as well as patients’ values and preferences.
Our decisions should be evidence-based, but this is challenging with stage I NSCLC. The spectrum of patients, tumors, treatments and outcomes is wide. The available evidence is extensive, but confusing and confounded. Often outcomes and cohorts aren’t clearly defined, multiple sources of uncertainty exist, and nuances of patients, tumors and settings affect the applicability.
We undertook to sift through the evidence, critically addressing confounders and limitations, seeking to provide as much clarity and confidence in applicability in various circumstances as possible. Furthermore, this initiative aims to assemble the evidence in a concise format that enhances clinicians’ real-life decision-making with individual patients. The project consists of 4 publications: part 1 (this paper) concisely summarizes the evidence and provides a framework to guide clinical decision-making, part 2 reviews the body of evidence regarding surgery in generally healthy patients (6), part 3 addresses specific patients and tumors (7), part 4 focuses on evidence regarding SBRT and ablation (8). We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1823/rc).
Methods
Overarching strategy
The goal of this initiative is to summarize and organize data that is relevant to decision-making for individual patients, recognizing that there are many patient-related, tumor-related, technical/therapy-related and local environment-related factors and values that bear upon the decision for a particular patient. Clinical judgment involves weighing an aggregate of considerations; the impact of various factors, their relevance to the patient and situation at hand, and the degree of uncertainty about these aspects. We avoid defining a generally right answer, aiming instead to assess relevant evidence, ambiguity and uncertainty in a manner that provides a scientific foundation with flexibility to facilitate application to individual patients.
We sought to be as inclusive as possible in gathering relevant data, recognizing that indirect data can be useful when direct data is limited. We also sought to be a critical as possible in assessing the degree of confidence that observed outcomes can be attributed to a treatment intervention as opposed to confounding factors. Additionally, we critically considered nuances of the patients, tumors, settings and interventions to gain insight into applicability and/or limitations of the observations.
Study panel
A study panel was assembled with representatives of relevant specialties, all without relevant conflicts of interest. Most members have a long history of close collaboration and joint decision-making for stage I NSCLC. There was no funding source.
Key questions
Key study questions are described below and listed in Appendix 1-1.
Patients and tumors
The focus of the project is patients with stage cI NSCLC—ideally cIA tumors (i.e., T1N0M0 (≤3 cm)]. To facilitate contemporary applicability and consistency, we use the current 8th edition TNM classification (9) throughout (translating earlier TNM definitions into the 8th edition nomenclature). However, the 8th edition defines size by the solid (imaging) or invasive (histologic) component, ignoring the size of a ground glass (GG) or lepidic component. Because most studies don’t differentiate total vs. solid/invasive size, we had to use the reported size—a minor issue since studies involve predominantly solid tumors. The consolidation to tumor ratio [CTR, meaning the ratio on lung windows of the consolidated (dense) to total (GG) lesion size] provides a surrogate in reports focused on pure or part-solid GG lung cancers.
While the spectrum of patients constitutes a continuum, the available evidence falls into 3 categories: generally healthy patients, older patients, and patients compromised by severely limited pulmonary reserve.
Interventions and comparators
Curative-intent interventions for stage I include surgical resection (specifically lobectomy, segmentectomy and wedge), SBRT (a.k.a. stereotactic ablative body radiotherapy, SABR) and thermal ablation (e.g., radiofrequency, microwave ablation, cryotherapy).
Choice of outcomes of interest
Short-term outcomes deemed most relevant to physicians and patients are treatment-related mortality, toxicity/morbidity, pain, and short-term quality-of-life (QOL). Among long-term outcomes we considered survival, recurrence, long-term functional capacity and QOL to be most relevant. We find lung cancer specific survival (LCSS) to be the cleanest measure of treatment effectiveness. [Assessments of accuracy have documented that cause of death in cancer patients is quite reliably assigned (10-12)]. Overall survival (OS) mixes treatment effect and competing causes of death. We chose freedom-from-recurrence (FFR) as the best measure of recurrence. Disease-free survival (DFS) or recurrence-free survival (RFS) obscures assessment because recurrence and competing deaths are combined. We find it inappropriate to consider any death as probably due to cancer in a context that includes compromised patients and favorable tumors.
Evidence assessment
Literature search and study selection
We performed a systematic search of English literature in PubMed from 2000–2021 according to standards (13). Several searches were conducted, reflecting the patients, tumors, interventions, comparators and outcomes encompassed by the project. Additionally, the reference lists of identified papers, especially systematic reviews, were scrutinized. Details of the search, review and selection process are provided in Appendix 1-2.
Studies were selected if they provided evidence relevant to the primary and secondary questions, focusing on randomized controlled trials (RCTs) and non-randomized comparisons (NRCs). NRCs were required to have adjusted for confounders and have ≥50 patients per arm (reflecting concern about the reliability of adjustment for multiple confounders in smaller studies). Details of inclusion and exclusion criteria for specific evidence tables are listed in table legends in the Part 2–4 papers (6-8). Studies identified by the search were reviewed independently by 2 authors; all studies that contained data relevant to the outcomes of interest and criteria just described and in each table were included.
Data abstraction
Data was abstracted by one panelist and reviewed by others. FCD and UK cross-checked all table entries. We abstracted reported data without imputation for missing data or re-analysis for missing calculations (e.g., hazard ratios, statistical significance).
Assessment of confounders and confidence in attribution of cause and effect
In any complex area multiple factors are at play. It is easy to mistakenly attribute an observation to the intervention of interest; accurate assessment requires critical evaluation of all potential alternative explanations. RCTs (ideally) evenly distribute all known and unknown confounders between the arms; however, few RCTs are available addressing the patients, tumors and treatments in question. To be useful, NRCs must disentangle the impact of confounders from the intervention of interest (14).
Major potential confounding factors were identified at the outset. These included non-medical patient related factors (e.g., age, education, socioeconomic status), medical patient-related factors [e.g., comorbidities, performance status (PS), severity of comorbidities], discrepancies in stage classification (e.g., node assessment), study era (treatments skewed towards different time periods), facility quality (treatments skewed towards particular facilities), discrepancy in treatment quality (e.g., margin adequacy, adjuvant therapy), favorable tumor selection (e.g., smaller, GG or less metabolically active tumors, conversion to lobectomy if upstaging is suspected/encountered). Many confounders are inter-related.
Several statistical methods of adjustment for multiple factors are available, such as multivariable regression (e.g., Cox proportional hazards model) and propensity scoring (e.g., propensity score adjustment, propensity matching, stratification and inverse weighting). These have strengths and weaknesses that can have greater or limited impact depending on the nature of the data, outcomes and question of interest. Most important, however, is which and how many potential confounders are actually accounted for. In fact, a prerequisite for propensity scoring is the inclusion of all relevant factors (15,16); however, this is widely ignored.
Overall risk of bias in relevant NRCs was assessed using a general tool (17) and a tool developed to provide a more detailed assessment of the identified domains of potential confounders relevant to stage I lung cancer. Two reviewers rated each domain in each study and intervention (details provided elsewhere; see app. 2-1 of Part 2) (6).
Interpretation of available evidence
Our approach to interpretation of evidence was to focus on understanding for which patients and situations the evidence is stronger or weaker, instead of seeking a single right answer for most patients. The summary assessments provided in this paper of various factors involved in clinical decisions for stage I patients are based on a detailed look at the available evidence (see Part 2–4 papers) (6-8). This involves thoughtfully organized tables, structured to facilitate understanding relationships between patient and tumor characteristics (e.g., age, stage, size), the degree of confounding and outcomes. This Part 1 paper assembles this into a framework to guide decision-making.
Assessment of ambiguity and nuances of applicability
Ambiguity arises from uncertainty to what degree confounding factors may influence the observed impact, how well we know which patients, tumors, and settings the evidence applies to, and the confidence that we can extrapolate beyond this. Nuances refers to factors that impact the effect of interventions (e.g., VATS vs. open). Exploring similarities and differences among studies (regarding details of the patients, tumors, treatments and confounders) provides insight. In effect, this combines traditional evidence-based medicine with the realist approach to clinical science—asking what works, when, in which patients and how (18).
Framework for decision-making
This project aims to provide a framework that facilitates clinical decision-making, which involves weighing multiple considerations and applying clinical science to an individual. The multitude of considerations and vagaries of the available clinical science make this challenging. We created a framework that allows one to identify and focus on issues with the most impact in a particular setting. This is achieved by assessing differences relative to what can be reasonably considered clinically meaningful (defined in Table S1-1). Individualization requires an understanding of the general evidence, as well as the inherent uncertainties and applicability to a particular patient. Furthermore, individual patients may value particular outcomes differently. Conceptually this can be imagined as shifting the fulcrum to give greater leverage to particular outcomes. We have categorized the considerations into benefits and downsides and short-, intermediate and long-term outcomes (Figure 1). Finally, nuances of patients and treatments that modify the applicability or outcomes should be kept in mind during clinical decision-making.

General framework for decision-making about treatment options in individual patients. Qualitative assessment of the impact of treatment approaches on various key outcome measures and the confidence in the evidence. Differences are categorized by degree of clinically meaningful differences (defined in Table S1-1).
Extpol, extrapolation; SBRT, stereotactic body radiotherapy.
Results
Stepwise approach to individual patients
A stepwise approach promotes efficiency and focus (Table 1). The available evidence falls into several categories—identifying which one(s) apply to an individual patient and tumor is the first step. Assessing the evidence highlights which outcomes impact decision-making (e.g., consistency of clinically meaningful differences). Technical considerations may narrow which treatments are reasonably feasible.
Table 1
| Steps | Details | Impact |
|---|---|---|
| 1. Identify relevant category(ies) of evidence | • Healthy patients, typical solid tumors • Older patients, typical solid tumors • Compromised patients, solid tumors • Favorable tumor characteristics |
Assembles general evidence into manageable moderately homogeneous cohorts Informs nuances of uncertainty/applicability of general evidence |
| 2. Assess the relevant evidence | • Magnitude of differences • Consistency of confidence in the evidence • Uncertainty • Applicability |
Provides basis for weighing various aspects, considerations |
| 3. Assess technical issues (anatomic, physiologic, specific treatment-related considerations) | • Ability to carry out treatment options without compromise | Eliminates unrealistic treatments; informs nuances of applicability of general evidence |
| 4. Identify what is most important to the individual patient (short-, long-term, benefits, potential harms) | • Identify, address fears • Correct misinformation/assumptions about outcomes • Ensure that patient has full perspective (e.g., short-, mid-, long-term, outcome without treatment) |
Establish patient’s state-of-mind, allows rational evaluation; establishes trust/insight that allows joint decision-making |
| 5. Individualized treatment selection | • Focus on considerations with major impact (set aside those without meaningful impact or not applicable) • Weigh differences, uncertainties in context of what is most important to the patient |
Provides framework to guide physician and patient to a well-founded decision |
A next step is to gain insight into the patient’s attitudes about their life at present and the future (what a normal day is like, what do they enjoy, look forward to). This builds a relationship and provides a stronger understanding of values than explicitly asking about quality vs. quantity of life or short- vs. long-term outcomes (or about treatment preferences, which may be misinformed).
This framework focuses attention on key outcome differences among feasible treatment options and streamlines addressing fears and misinformation. This provides a solid basis for an effective discussion of tradeoffs and uncertainties, and ultimately well-founded joint decision-making.
Summary of outcomes in healthy patients
Resection extent
In healthy patients contemporary RCTs demonstrate equivalent perioperative mortality for segmentectomy or wedge vs. lobectomy (1–4% 90-day mortality) (6). Sublobar resection doesn’t alter the incidence of major complications (5–15% grade ≥3) (6). A significant benefit to VATS over thoracotomy has been demonstrated extensively for lobectomy; this also appears true for segmentectomy. Reasonable extrapolation (direct data being absent) is that robot-assisted resection yields similar outcomes to VATS. Pain and impaired QOL is generally resolved by 3 months after VATS resection (see Tabs. 6,7 and Fig. 1 of Part 2) (6).
Adjusted NRCs with high confidence that outcomes are attributable to the treatment demonstrate worse OS for segmentectomy or wedge resection than lobectomy (see Tabs. 1,2,3 and Figs. S2-1,S2-2,S2-3 of Part 2) (6). Multiple NRCs (with greater residual confounding) mostly favor lobectomy; most clearly for OS and LCSS for wedge, less so for segmentectomy vs. lobectomy. It is unclear if lesser resection increases recurrence risk (due to low locoregional recurrence rates, few NRCs, low confidence that results reflect resection extent; see Tab. 4 of Part 2) (6). We await mature results from 2 RCTs; present aggregate evidence indicates meaningfully worse long-term outcomes after segmentectomy or wedge resection than lobectomy in healthy patients.
VATS resection has little long-term impact on QOL, but open resection persistently impairs QOL. The impact of sublobar resection is unclear due to confounding by VATS/open approach (see Tabs. 6,7 of Part 2) (6). Pulmonary function tests (PFTs) aren’t meaningfully better after segmentectomy (especially multi-segmentectomy) than lobectomy in healthy patients, but might diminish a subjective increase in dyspnea sometimes noted after lobectomy (see Tab. 5 of Part 2) (6).
Evidence suggests no meaningful differences in short-, intermediate- or long-term outcomes for a “lobe-like” multi-segmentectomy (e.g., lingulectomy or left upper division resection) vs. lobectomy. Locoregional recurrence rates are ~25% for margins of ≤1 cm and ~10% with larger margins, with generally similar findings for a margin/tumor size ratio of <1 vs. ≥1 (see Tabs. 8,9 of Part 2) (6). Worse long-term outcomes are reported when a microscopic finding of “spread through air spaces” (STAS) is present (especially after sublobar resection); this is confounded because STAS is associated with many negative prognostic factors (see Tabs. 10,11 of Part 2) (6). Whether converting to a lobectomy mitigates the impact of STAS is unclear.
SBRT/ablation vs. surgery in healthy patients
Short-term mortality is ~1% better after SBRT vs. surgery (8). Grade ≥3 toxicity after SBRT is low initially, but is noted in ~10–20% by 2 years (6). Comparing across studies, average QOL is clearly better after SBRT than surgery, most markedly in the short-term; also long-term after open resection (less so after VATS; see Tabs. 6,7 of Part 2 and Tab. 4 of Part 4) (6,8). On average, PFTs are minimally decreased after SBRT/ablation; differences between SBRT/ablation vs. surgery appear to be clinically relevant only vs. lobectomy. However, 20–40% of SBRT patients experience a meaningful decline in PFTs after 1–2 years (8).
Limited accrual renders completed RCTs inconclusive. Results of ongoing RCTs in good-risk and high-risk patients are anticipated in 2024–2026. Adjusted NRCs quite consistently report meaningfully worse OS and LCSS for SBRT/ablation vs. lobectomy or sublobar resection (see Tabs. 1,2 and Fig. S4-1A,S4-1B of Part 4) (8). Nevertheless, adjustment for confounders when comparing SBRT/ablation vs. surgery is inherently challenging.
Decision-making guide in generally healthy patients
Resection extent
Key outcomes
The focus regarding resection extent is on long-term outcomes (Figure 2A); key points are summarized in Table 2. Short-term outcomes aren’t meaningfully different. Worse OS and LCSS is reported, especially for wedge resection vs. lobectomy (moderate- to low-confidence). QOL is similar; wedge resection may have a marginal advantage in PFTs and possibly in ameliorating an increase in dyspnea vs. lobectomy.
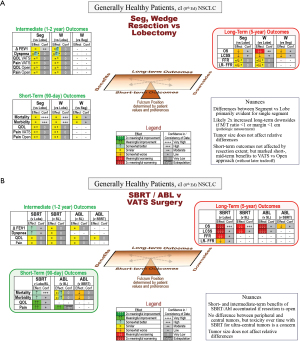
(A) Resection extent; (B) SBRT/ablation vs. VATS surgery. Decision guide for a generally healthy patient with a typical stage I lung cancer. The reference (for improvement or worsening) is the treatment in parentheses.
a, data not parsed by resection extent (segment vs. wedge).
Δ FEV1, change in FEV1 ≥6 months; Abl, ablation (any thermal technique); Conf, confidence in the evidence; FFR, freedom from recurrence (only recurrence counts as an event); LCSS, lung cancer specific survival (only a death due to lung cancer counts as an event); L, lobectomy; LR-FFR, locoregional freedom from recurrence; M/T, margin (distance) to tumor (diameter) ratio; NSCLC, non-small cell lung cancer; OS, overall survival; QOL, quality of life; SBRT, stereotactic body radiotherapy; SL, sublobar resection; Seg, segmentectomy; VATS, video-assisted thoracic surgery; W, wedge.
Table 2
| • NRCs indicate worse OS/LCSS after segmentectomy/wedge vs. lobectomy |
| • Short-term outcomes are equivalent after lobectomy, segmentectomy or wedge resection |
| • Marginally meaningful benefit in PFTs after segmentectomy/wedge vs. lobectomy |
| • A ≤1 cm sublobar resection margin portends a ~25% loco-regional recurrence rate and meaningfully worse RFS |
| • SBRT/ablation has a meaningful benefit in short-term outcomes over surgery |
| • NRCs strongly indicate long-term downsides to SBRT/ablation vs. surgery |
| • Tumor size appears not to modify long-term differences between lesser resection vs. lobectomy or between SBRT/ablation vs. surgery |
NRCs, non-randomized comparisons; OS, overall survival; LCSS, lung cancer specific survival; PFTs, pulmonary function tests; RFS, recurrence-free survival; SBRT, stereotactic body radiotherapy.
Effect modifiers
A sublobar resection margin of ≤1 cm or a margin/tumor ratio of <1 is associated with a higher loco-regional recurrence rate (~25% vs. ~10%) and meaningfully worse RFS (moderate-confidence evidence). STAS correlates with worse long-term outcomes for both lobectomy and sublobar resection. Tumor size appears not to modify the difference between lobectomy vs. lesser resection in OS and LCSS.
PFTs are marginally better after a single segmentectomy vs. lobectomy. However, “lobe-like” multi-segmentectomy (e.g., lingulectomy or left upper division resection) provides no benefit (but also perhaps little long-term downside). Whether long-term outcome differences for sublobar resection vs. lobectomy stem from suboptimal node dissection (and adjuvant chemotherapy) is unclear due to conflicting evidence.
Selection and patient preferences
The long-term downsides of sublobar resection in healthy patients are hard to counter by selection factors or preferences.
SBRT/ablation vs. surgery
Key outcomes
The focus is balancing short-term benefits of SBRT/ablation vs. a detriment in OS and LCSS compared with surgery (high- and low-confidence evidence, Figure 2B). A small advantage in PFTs for SBRT over lobectomy is marginally clinically relevant and a weak argument in treatment selection.
Effect modifiers
Figure 2B addresses VATS resection—deemed to be the appropriate comparator to SBRT/ablation. Thoracotomy accentuates the short-term benefits of SBRT/ablation (Figure S1-1). Short-term outcomes aren’t affected by tumor location (central/peripheral). Tumor size doesn’t appear to modify the long-term differences between SBRT/ablation vs. surgery.
Selection and patient preferences
Preferences affect how outcomes are weighed. However, the long-term downsides to SBRT/ablation vs. VATS resection are a strong counterweight to the short-term benefits. For SBRT/ablation vs. open surgery the balance of short- and intermediate-term benefits vs. long-term downsides is more even. SBRT/ablation should be avoided for ultra-central tumors.
Summary of outcomes in older patients
Resection extent
The average life expectancy (~8–20 years) of older lung cancer patients argues that most should be treated unless there are severe comorbidities well beyond what is typical for these patients.
Reported peri-operative mortality among older patients is consistently low (~2–4%); a slight increase between age 65 and 80 is noted in some series (see Tab. 1 of Part 3) (7). Mortality is minimally lower for sublobar resection vs. lobectomy; in older age cohorts differences are only slightly increased. Most complications are minor; limited data suggests that morbidity may be lower after sublobar resection (see Tab. 1 of Part 3) (7), but a VATS vs. open approach is likely more impactful.
Reported 5-year OS in older cI patients is reasonable (40–65%). Several NRCs deemed to have little residual confounding demonstrate somewhat worse OS/LCSS after segmentectomy/wedge vs. lobectomy (see Tab. 2 and Fig. S3-2 of Part 3); less well-adjusted NRCs generally support this (7).
Resected older patients are clearly selected, but how is not well-defined. Most patients had an excellent PS; many had comorbidities (presumably not severe).
SBRT/ablation vs. surgery
Short-term mortality is 1–4% lower after SBRT than surgery in patients age >70 (see Fig. 4 of Part 4) (8,19). This is more pronounced as age increases, and for open resection. Morbidity is higher initially after surgery, but late toxicity after SBRT renders the overall incidence relatively equal after 2 years. Surgery (especially open) impairs QOL; SBRT/ablation has little impact (see Tabs. 6,7 of Part 2 and Tab. 4 of Part 4) (6,8).
Several extensively-adjusted NRCs in older patients demonstrate clinically relevant worse OS/LCSS after SBRT than surgery; other less well-adjusted studies generally support this (see Tab. 5 and Fig. S4-2 of Part 4) (8).
Decision guide in older patients
Resection extent
(I) Key outcomes
The focus of decision-making in older patients is on a long-term detriment for sublobar resection (moderate-confidence, Figure 3A); key points are summarized in Table 3. There is little difference in short- or intermediate-term outcomes (very low-confidence and indirect evidence). This pattern is similar to that in generally healthy patients, although the impact on survival is somewhat attenuated in older patients.
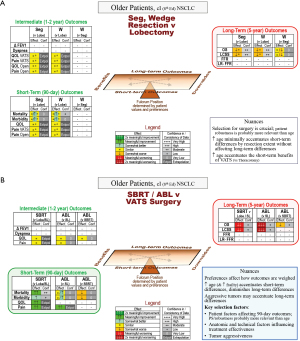
(A) Resection extent; (B) SBRT/ablation vs. VATS surgery. Decision guide for an older patient with a typical stage I lung cancer. The reference (for improvement or worsening) is the treatment in parentheses.
a, data not parsed by resection extent (segment vs. wedge).
Δ FEV1, change in FEV1 ≥6 months; Abl, ablation (any thermal technique); Conf, confidence in the evidence; FFR, freedom from recurrence (only recurrence counts as an event); LCSS, lung cancer specific survival (only a death due to lung cancer counts as an event); L, lobectomy; LR-FFR, locoregional freedom from recurrence; NSCLC, non-small cell lung cancer; OS, overall survival; PS, performance status; QOL, quality of life; SBRT, stereotactic body radiotherapy; SL, sublobar resection; Seg, segmentectomy; VATS, video-assisted thoracic surgery; W, wedge.
Table 3
| • NRCs indicate worse OS/LCSS after segmentectomy/wedge vs. lobectomy |
| • Marginal benefits in short-term outcomes for segmentectomy/wedge vs. lobectomy |
| • SBRT/ablation results in short-term benefits and long-term downsides vs. surgery |
| • Increasing age/frailty accentuates short-term benefits and diminishes long-term downsides of SBRT/ablation vs. surgery |
| • Selection criteria are not well-defined; factors affecting 90-day outcomes, technical success and tumor aggressiveness are probably most useful |
| • Patient preferences affect how outcomes are weighed |
NRCs, non-randomized comparisons; OS, overall survival; LCSS, lung cancer specific survival; SBRT, stereotactic body radiotherapy.
(II) Effect modifiers
Increasing age appears to minimally increase short-term differences and not to substantially modify long-term outcome differences; smaller tumor size doesn’t clearly impact long-term differences. The accentuation of long-term outcome differences associated with a limited resection margin seen in healthy patients likely applies to older patients as well, although the impact of oncologic outcomes is diminished in the face of major competing causes of death.
(III) Selection and patient preferences
The long-term downsides of sublobar resection are hard to counter by selection factors or preferences.
SBRT/ablation vs. surgery
(I) Key outcomes
This decision in older patients involves balancing short-term benefits of SBRT against long-term downsides (Figure 3B). Intermediate-term QOL is similar for SBRT vs. VATS but SBRT is clearly better vs. open resection (Figure S1-2). Ablation offers little short-term advantage over sublobar resection but is associated with a major detriment in OS.
(II) Effect modifiers
Increasing age probably magnifies short-term benefits and diminishes long-term downsides of SBRT/ablation vs. VATS resection (moderate-confidence evidence). The same is probably true with increasing frailty and comorbidities (speculative extrapolation). Indirectly-supported rationale suggests that long-term downsides of SBRT/ablation vs. surgery are accentuated with aggressive tumors (e.g., faster growth, greater PET-avidity).
(III) Selection and patient preferences
Patient preferences can easily affect the balance when considering surgical vs. non-surgical treatment in older patients.
Characteristics favoring selection for SBRT/ablation vs. surgery are not well-defined but are probably those affecting short- and long-term outcome differences.
Summary of outcomes in patients with limited pulmonary reserve
Resection extent
Most patients with major comorbidities and early-stage lung cancer die of lung cancer, suggesting that treatment is generally warranted. The available data focuses on patients with severely limited pulmonary reserve; extrapolation is needed for less severe pulmonary compromise or other major comorbidities.
Limited data suggests little difference in short-term outcomes between segmentectomy vs. lobectomy. However, while post-operative morbidity and mortality increases with decreasing pulmonary reserve, this is markedly ameliorated by VATS (see Fig. 3 of Part 3) (7). Thirty-day mortality after lobectomy in patients below criteria cited as contraindications to surgery is 2–3% for VATS and 3–8% for thoracotomy; pulmonary complication rates are ~10–20% for VATS vs. ~20–40% for thoracotomy (see Tab. 3 and Fig. 3 of Part 3) (7).
Lobectomy has less impact on PFTs in patients with severely limited pulmonary reserve, and in a substantial proportion FEV1 is unchanged or even improved. Whether sublobar resection provides a functional benefit over lobectomy is unclear. Limited data suggests resection has little average impact on QOL in patients with limited pulmonary reserve—some are better, some worse and many are unchanged. A QOL benefit for lesser resection vs. lobectomy has not been demonstrated, but data is limited.
Whether there is a difference in long-term outcomes by resection extent in compromised patients is unclear (conflicting results, few NRCs, small study sizes, residual confounding and similar unadjusted outcomes; see Tab. S3-3 of Part 3) (7).
Selection is crucial in compromised patients, but not well-defined. Good short- and long-term outcomes are reported despite severely limited PFTs, but these patients are presumably otherwise robust.
SBRT/ablation vs. surgery
Extrapolation (general evidence, older patients) suggests meaningfully better short-term outcomes for SBRT over surgery. This may be accentuated in more compromised patients and slightly diminished with VATS resection, less clearly diminished by sublobar resection.
Long-term outcomes in compromised patients are consistently worse for SBRT vs. surgery (10–20% 5-year OS difference; see Tab. 6 and Fig. S4-4 of Part 4) (8). However, studies are limited and only partially adjusted. These patients are undoubtedly carefully selected; no specific characteristics have emerged on which to base treatment selection.
Decision guide in compromised patients
Resection extent
(I) Key outcomes
Short-, intermediate- and long-term outcomes are similar after lesser resection vs. lobectomy (Figure 4A); key points are summarized in Table 4. However, this applies to a selected minority of compromised patients. The conclusion should be that, when carefully selected, good outcomes can be anticipated even in compromised patients. The key driver is patient selection.
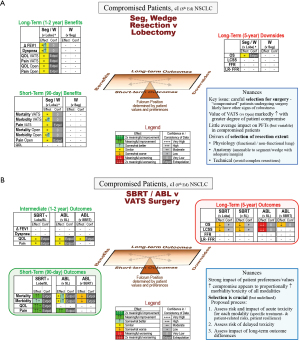
(A) Resection extent; (B) SBRT/ablation vs. VATS surgery. Decision guide for a compromised patient with a typical stage I lung cancer. The reference (for improvement or worsening) is the treatment in parentheses.
a, data not parsed by resection extent (segment vs. wedge).
Δ FEV1, change in FEV1 ≥6 months; Abl, ablation (any thermal technique); Conf, confidence in the evidence; FFR, freedom from recurrence (only recurrence counts as an event); LCSS, lung cancer specific survival (only a death due to lung cancer counts as an event); L, lobectomy; LR-FFR, locoregional freedom from recurrence; NSCLC, non-small cell lung cancer; OS, overall survival; PFT, pulmonary function tests; QOL, quality of life; SBRT, stereotactic body radiotherapy; SL, sublobar resection; Seg, segmentectomy; VATS, video-assisted thoracic surgery; W, wedge.
Table 4
| • Surgery with good short- and long-term outcomes is feasible in patients with severe pulmonary compromise when carefully selected |
| • VATS markedly ameliorates 90-day morbidity and mortality over thoracotomy |
| • There is no clear difference in short- and long-term outcomes between sublobar resection vs. lobectomy |
| • Resection extent is determined primarily by physiologic, anatomic and technical factors |
| • SBRT has the least short-term toxicity but some risk of late toxicity |
| • Ablation may have higher short-term toxicity than SBRT, but little risk of long-term toxicity |
| • Increasing compromise appears to proportionally increase morbidity/toxicity of all modalities |
| • Treatment selection (SBRT, ablation, surgery) should be individualized, key drivers are: |
| • How the patient weighs acute vs. intermediate toxicity and long-term outcomes |
| • Anticipated morbidity/mortality for each treatment option for the individual patient (first short- then intermediate-term) |
| • Long-term OS/LCSS is worse for SBRT/ablation than resection |
VATS, video-assisted thoracic surgery; SBRT, stereotactic body radiotherapy; OS, overall survival; LCSS, lung cancer specific survival.
(II) Effect modifiers
The benefit of VATS increases with greater degrees of compromise (for all types of resection).
(III) Selection and patient preferences
How to select patients for resection is poorly defined. Supported rationale suggests that patients selected for surgery have characteristics that counter a “compromised” categorization—e.g., good PS, normal daily activities, good cardiopulmonary exercise test performance.
Supported rationale suggests anatomic and physiologic factors drive selection of the type of resection. Resection of a non-functioning lobe (e.g., with severe emphysema) may even improve pulmonary function. Anatomic location impacts the feasibility of segment or wedge resection with adequate margins. Technical factors are important—a straightforward lobectomy or large wedge may be better than a complex segmentectomy.
These physiologic, anatomic and technical considerations generally overshadow patient preferences.
SBRT/ablation vs. surgery
(I) Key outcomes
In compromised patients, short-term outcome differences become prominent and long-term differences are diminished relative to healthy patients (Figure 4B). SBRT has clear short-term advantages over surgery (less so for ablation) but worse long-term outcomes (low-confidence evidence). PFTs and QOL are similar after SBRT/ablation vs. VATS resection (indirect evidence).
(II) Effect modifiers
Short- and intermediate-term outcomes increasingly favor SBRT/ablation when surgery involves a thoracotomy instead of VATS (Figure S1-3). Increasing degrees of pulmonary compromise appear to proportionately increase the morbidity/toxicity of all treatment options—i.e., increasing risk generally without accentuating the difference of one modality over another.
Interstitial lung disease (ILD) is particularly challenging. Differentiating between non-progressive interstitial abnormalities and ILD may require specialized pulmonary consultation. ILD is associated with limited survival, but also a high incidence of lung cancer and death from lung cancer—and an increased risk of SBRT toxicity.
(III) Selection and patient preferences
Individualized treatment selection is challenging; data is limited, the time course and nature of morbidities/toxicities of treatment modalities vary, and reported markers of a compromised patient don’t capture actual frailty/resilience. We propose first estimating the risk and impact of acute morbidity/toxicity with each modality. Specific patient- or treatment-related issues may emerge that weigh heavily—e.g., how precariously a patient is functioning in their living environment. SBRT generally has the least acute problems; this is less clear for ablation. Next, consider intermediate-term morbidity/toxicity. SBRT has a low but ongoing risk of late toxicity. It is unclear which SBRT patients experience a decline in lung function. The patient’s valuation of an acute risk vs. possible gradual intermediate-term impairment is important. Finally, consider how long-term outcomes modify the appeal of treatment options emerging from the first steps. Long-term treatment differences have diminished impact as competing risks of death increase.
Summary of outcomes for potentially favorable tumors
Certain tumor characteristics are presumed to correlate with a favorable oncologic biology, suggesting alternative treatments over lobectomy. These include GG, screen-detected, small (≤1 cm), and slow-growing or low PET-avidity tumors. These tumors likely affect long-term oncologic outcomes; general data on short- and intermediate-term outcomes should apply equally to favorable tumors.
GG and screen-detected tumors have excellent long-term outcomes regardless of resection extent (see Tabs. 5,6 of Part 3) (7). However, late recurrence (>5-year) of GG tumors after sublobar resection may occur (20). Speculative extrapolation suggests that tumors exhibiting low PET-avidity or slow progression may also have excellent long-term outcomes regardless of resection extent. However, outcomes are worse after sublobar resection vs. lobectomy for small solid tumors (<1 cm; see Tab. S3-4 and Fig. S3-6 of Part 3) (7).
No data is available on SBRT/ablation in favorable tumor types. For <1 cm tumors rationale suggests worse outcomes for SBRT vs. sublobar resection (considering that small tumors are not consistently favorable, fare worse after sublobar resection vs. lobectomy, and generally worse long-term outcomes with SBRT vs. sublobar resection).
Decision guide in patients with favorable tumors
Decision-making for tumors with favorable characteristics centers on long-term outcomes (Figure 5A,5B); key points are summarized in Table 5. For predominantly GG tumors, sublobar resection is reasonable (no major benefit or downside vs. lobectomy). Arguments for lesser resection are the potential development of additional GG lesions, and that a limited resection margin may have little impact. Arguments against lesser resection are the question about late staple line recurrence and that scarring from resection or SBRT hamper identification of recurrence.
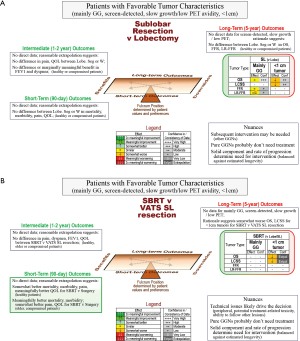
(A) Resection extent; (B) SBRT/ablation vs. VATS surgery. Decision guide for a patient with a stage I lung cancer with favorable tumor characteristics. The reference (for improvement or worsening) is the treatment in parentheses.
Δ FEV1, change in FEV1 ≥6 months; Abl, ablation (any thermal technique); betw, between; Conf, confidence in the evidence; FFR, freedom from recurrence (only recurrence counts as an event); GG, ground glass; GGN, ground glass nodule; LCSS, lung cancer specific survival (only a death due to lung cancer counts as an event); L or Lobe, lobectomy; LR-FFR, locoregional freedom from recurrence; NSCLC, non-small cell lung cancer; OS, overall survival; PET, positron emission tomography; QOL, quality of life; SBRT, stereotactic body radiotherapy; SL, sublobar resection; Seg, segmentectomy; VATS, video-assisted thoracic surgery; W, wedge.
Table 5
| • Mainly GG tumors have excellent long-term outcomes regardless of resection extent; speculation suggests this may extend to screen-detected and low PET-avidity tumors |
| • Scarring after resection or SBRT/ablation may hamper the ability to identify recurrence |
| • Many GGNs remain stable; pure GGNs probably do not need treatment |
| • Small solid tumors (<1 cm) have worse long-term outcomes after sublobar resection than lobectomy, and probably after SBRT/ablation vs. surgery |
GG, ground glass; PET, positron emission tomography; SBRT, stereotactic body radiotherapy; GGN, ground glass nodule.
However, predominantly GG lesions generally don’t warrant treatment—prospective evidence demonstrates that most don’t progress and surveillance with delayed intervention if needed maintains nearly universal cure rates (21-25). Development or growth of a solid component >2 mm (mediastinal windows, thin-slice CT) or consolidation >5 mm (lung windows) justifies intervention (not growth of the GG component) (21).
Speculation suggests sublobar resection is an alternative to lobectomy for screen-detected, low PET-avidity and slow-growing tumors. However, confirmatory data is unavailable and margin distance may be important. In healthy patients, current long-term outcomes for tumors <1 cm support lobectomy over sublobar resection; speculation suggests an advantage for resection over SBRT.
Discussion
Using the decision guides
The decision guides are designed to enhance judgement by summarizing relevant evidence and uncertainties. Clinicians should decide how relevant specific evidence categories are for an individual (e.g., primarily older, less so compromised patients). The guides highlight the outcomes with relevant differences and the confidence in the evidence. Nuances, ambiguities and particular aspects about the patient and tumor impact the weight given to particular considerations. The availability and expertise with interventions in the local setting also affect decision-making. We think this summary of available, albeit imperfect evidence enhances clinical judgment.
Limitations
Clearly the evidence encompassed in this project leaves uncertainty and hampers drawing definitive conclusions. However, we are forced to make decisions in daily care; therefore, we sought to make the most of the evidence despite limitations. Moreover, we strove to illuminate the weaknesses.
A limitation of the overall approach is that assessments inherently involve a degree of subjectivity. We think this is minimized by requiring extensive discussion and consensus of the entire panel. Furthermore, while consistency among studies supports attribution of an outcome to an intervention, it can also stem from consistent residual confounding. We think this is unlikely because we could not find relationships to the overall degree of or to particular domains of residual confounding.
Nevertheless, we hope that the effort to be comprehensive, critical, transparent and nuanced enhances decision-making for individual patients.
Conclusions
Assessment of evidence for treatment options for cI NSCLC identifies key areas (e.g., short- or long-term outcomes) where relevant differences are manifest in various settings. A graphic tool facilitates focusing on the key areas and weighing multiple outcomes and uncertainties. This promotes applying the available evidence in individualized clinical decision-making.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1823/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1823/coif). The series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation” was commissioned by the editorial office without any funding or sponsorship. FCD served as the unpaid Guest Editor of the series. HSP serves as an unpaid editorial board member of Journal of Thoracic Disease. HSP reports research funding from RefleXion Medical; consulting fees from AstraZeneca; honoraria and speaking fees from Bristol Myers Squibb; and advisory board fees from Galera Therapeutics; all unrelated to current work. BCB reports in the past 36 months, he receives grants from Veterans Affairs Central Office, American Cancer Society, Yale SPORE in Lung Cancer. DCM reports that he is the lead for an early career educational course on microwave ablation that is sponsored by Johnson & Johnson. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Detterbeck FC. Maintaining aim at a moving target. J Thorac Oncol 2011;6:417-22. [Crossref] [PubMed]
- Farjah F, Wood DE, Yanez D 3rd, et al. Temporal trends in the management of potentially resectable lung cancer. Ann Thorac Surg 2008;85:1850-5; discussion 1856. [Crossref] [PubMed]
- Detterbeck F, Mase VJ Jr, Li A, et al. A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation. Part 2: Evidence Regarding Resection Extent in Generally Healthy Patients. J Thorac Dis 2022;
- Bade B, Blasberg J, Mase VJ Jr, et al. A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation. Part 3: Evidence Regarding Surgery in Compromised Patients or Specific Tumors. J Thorac Dis 2022;
- Park H, Detterbeck F, Madoff D, et al. A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation. Part 4: Evidence involving SBRT and Ablation. J Thorac Dis 2022;
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Satariano WA, Ragland KE, Van Den Eeden SK. Cause of death in men diagnosed with prostate carcinoma. Cancer 1998;83:1180-8. [Crossref] [PubMed]
- Penson DF, Albertsen PC, Nelson PS, et al. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst 2001;93:1822-3. [Crossref] [PubMed]
- Huntington JT, Butterfield M, Fisher J, et al. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am J Cancer Res 2013;3:518-22. [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Detterbeck FC, Gould MK, Lewis SZ, et al. Extending the Reach of Evidence-Based Medicine: A Proposed Categorization of Lower-Level Evidence. Chest 2018;153:498-506. [Crossref] [PubMed]
- Freemantle N, Marston L, Walters K, et al. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013;347:f6409. [Crossref] [PubMed]
- Elze MC, Gregson J, Baber U, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol 2017;69:345-57. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Pawson R, Greenhalgh T, Harvey G, et al. Realist review--a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Nakao M, Yoshida J, Goto K, et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [Crossref] [PubMed]
- Mase VJ Jr, Detterbeck FC. Approach to the Subsolid Nodule. Clin Chest Med 2020;41:99-113. [Crossref] [PubMed]
- Sawada S, Yamashita N, Sugimoto R, et al. Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. Chest 2017;151:308-15. [Crossref] [PubMed]
- Kobayashi Y, Fukui T, Ito S, et al. How long should small lung lesions of ground-glass opacity be followed? J Thorac Oncol 2013;8:309-14. [Crossref] [PubMed]
- Lee SW, Leem CS, Kim TJ, et al. The long-term course of ground-glass opacities detected on thin-section computed tomography. Respir Med 2013;107:904-10. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]


