
Double stapling method for closure of intraoperative alveolar air leakage adjacent to the staple line: a randomized experimental study on ex vivo porcine lungs
Introduction
Alveolar air leakage from visceral pleural defects is a frequent complication after lung resection. It can occur during the dissection of pleural adhesion, that of incomplete fissures, or stapling of the lung parenchyma (1-3). Prolonged alveolar air leakage can lead to extended hospitalization and postoperative complications such as intrathoracic infection (4,5). Therefore, intraoperative closure of alveolar air leakage is required to avoid these situations. Interlobar fissures dissected with electrocautery or scissors, and dissected areas from pleural adhesions to the chest wall are frequent sites of alveolar leakage during lung surgery. On the other hand, the air leakage adjacent to the staple lines after wedge resection is a challenging air leakage in terms of intraoperative closure (6-10). After closing the pleural defect by conventional suturing with the pledget method, the visceral pleura occasionally tears from the needle holes due to tension around the staple line. We recently introduced a novel closure technique named the double stapling method for the intraoperative closure of pleural defect adjacent to the staple line. Although there is no literature about this technique as far as we searched, it is currently used in some institutions for patients with fragile lung parenchyma that will not withstand the application of sutures. We expected this method to distribute tension around the staple line to prevent further pleural tears. To investigate the efficacy of this method, we established a model of alveolar air leakage adjacent to the staple line using ex vivo porcine lungs, based on a previous ex vivo experimental study on pleural defects after wedge resection (6). This study compared the efficacy of two closure methods; the double stapling method and conventional suturing method with pledgets using ex vivo porcine lungs. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1960/rc).
Methods
Experimental protocol
Ex vivo lungs, freshly excised from slaughtered edible pigs, aged from 6 to 10 months and weighing approximately 100 kg, were used in the study. We used lungs obtained from dead animals; therefore, ethical approval was not required based on our institutional guidelines. Damaged lungs were not included. The left porcine lungs consist of cranial and caudal lobes, similar to humans’ left upper and lower lobes. Before the experiment, the weight of the whole left lung was measured to compare differences in lung size among individual pigs.
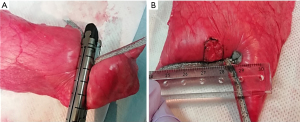
To assume large wedge resection of basal segments of the left lower lobe in humans, wedge resection of the caudal lobe was performed using the following procedure. After connecting the left caudal lobe bronchus to a manual ventilation pump, the lobe was inflated in water at a pressure of 30 cmH2O to ensure permeability and expand the visceral pleura. The thickness of the inflated caudal lobe was measured using a vernier caliper; a point of 60 mm-thickness was marked using ink. Following deflation of the caudal lobe, a wedge resection was performed using two staplers 6 cm long (Endo GIA reinforced black, Medtronic, Dublin, Ireland). The crossing point of the two staple lines was matched with the marked point with a 60 mm thickness. Endo GIA-reinforced black cartridges were designed for 1.95–2.7-mm-thick tissue after compression. When inflated, 60-mm thickness corresponded to almost 2.5-mm thickness at compression in the pilot experiments. Therefore, we selected a thickness of 60 mm. The angles between two staple lines were designed to be 120°. Following wedge resection, a superficial square visceral pleural defect (10 mm × 10 mm) adjacent to the crossing point of the two staple lines was made using a scalpel (Figure 1A,1B).
Assuming intraoperative fixation of the defect in clinical practice, it was closed using the following two methods: (I) suturing with pledgets (n=10); and (II) double stapling method (n=10). The 20 caudal lobes with pleural defects were randomly allocated to the two groups using a computer-generated randomization list to minimize potential cofounders. The weights of lungs were measured to compare differences in lung size between the groups. We are aware of the group allocation during the conduct of the experiment, the following outcome assessment, and the data analysis because it was impossible to perform these operations in a blinded fashion.
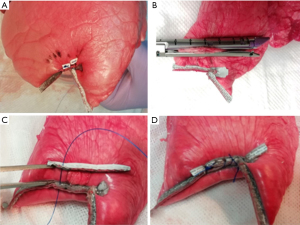
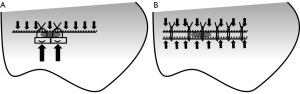
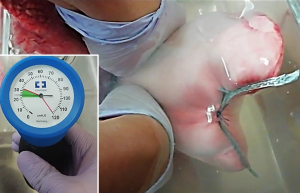
When suturing with pledgets, two pledget pads (3 mm × 7 mm; Medtronic) were attached on the side of the pleural defect opposite to the staple line. The staple line and pledgets were then closely sutured using 2-0 Surgipro V-20 (90 cm; Medtronic) (Figure 2A). The double stapling method was performed as follows. First, stapling of the edges of the pleural defect opposite to the original staple lines was performed using a stapler 6 cm in length and 1.2–1.95 mm thick [Endo GIA reinforced (purple), Medtronic] (Figure 2B). The parallel staple lines on both sides of the pleural defect were then attached and sutured using 2-0 Surgipro V-20 (Figure 2C,2D). The double stapling method was developed to distribute tension along two staple lines (Figure 3). After the closure of the pleural defect, the caudal lobe was inflated in water to confirm the presence or absence of air leakage at airway pressures of 20, 25, and 30 cmH2O (Figure 4), according to the methods described in previous studies (11,12). If air leakage was observed (closure failure), the pressure at the time was recorded. Closure success was defined by the absence of air leakage at a pressure of 30 cmH2O. The experimental procedures for each caudal lobe were completed within 2 hours after defrosting the specimen to avoid tissue deterioration. Finally, the lung specimens containing closed defects were resected and fixed in 15% formalin; cross-sectionally cut. The closure status of the pleura was compared between the two methods macroscopically. The specimens were further embedded in paraffin and processed to obtain sections for hematoxylin-eosin staining. All experiments, including preservation of ex vivo materials and recording study data, were conducted at the laboratory in the Division of Thoracic Surgery, Keio University School of Medicine.
Statistical analysis
The sample size was estimated based on pilot experiments (3 per group). Closure success rates in the pilot experiments were 1/3 (33.3%) in the suturing with pledget group and 3/3 (100%) in the double stapling group. A sample size estimation for Fisher’s exact test, with a power of 0.8 and an alpha of 0.05, indicated that at least nine specimens were required in each group to detect a statistically significant difference (G*Power 3.1.9.2, University of Düsseldorf, Düsseldorf, Germany). Considering a potential dropout rate of 10%, we allocated 10 specimens to each group.
Fisher’s exact test was used to compare the closure success rates between the two methods, while the Mann-Whitney U test was used to compare the average weight of the lungs between the two groups; statistical significance was assumed if P<0.05. All statistical evaluations were performed using JMP®15 (SAS Institute Inc., Cary, NC, USA).
Results
The average weights of the whole left lungs used for suturing with pledgets group (n=10) and double stapling method group (n=10) were 248±16 and 252±15 g, respectively; this difference was not statistically significant (P=0.62). Wedge resection of the left caudal lobe and creation of pleural defects were performed according to the experimental procedures described in the Methods section on 20 lungs; pleural defect closure was performed by either suturing with pledgets (n=10) or via the double stapling method (n=10). During additional stapling in the double stapling method, further tearing of the lung parenchyma beyond the second staple lines was not observed.
Air leak test
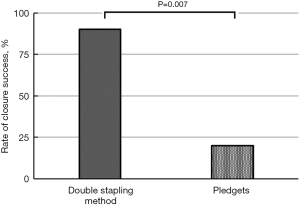
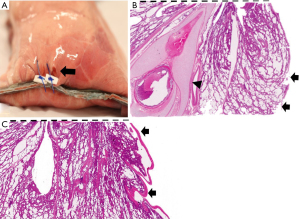
After closing the pleural defects, we performed air leak tests. Closure success was confirmed in two (20%) out of 10 cases in the suturing with pledgets group, and nine (90%) out of 10 in the double stapling method group (P=0.007) (Figure 5). Among eight failed cases in the suturing with pledget group, air leakage was confirmed in one case at a pressure of 20 cmH2O, four cases at 25 cmH2O, three cases at 30 cmH2O. In the double stapling method group, nine cases showed closure success, and one failed case presented air leakage at a pressure of 30 cmH2O. In nine failed cases in both groups, air leakage was observed from the needle holes of suturing; four cases in the suturing with pledget method had needle holes extended more than 3 mm, vertical to the initial staple lines (Figure 6A). In contrast, there were no cases with additional pleural clefts longer than 3 mm in the double stapling group.
Histological examination
In nine failed cases, a discontinuity of visceral pleura and exposure of bare alveoli were confirmed histologically with hematoxylin-eosin staining (Figure 6B). In addition, bronchioles with cartilage components were observed close to the staple lines, suggesting that the wedge resection was sufficiently deep (Figure 6B). In the successful cases, the visceral pleura around the closure sites was not interrupted, suggesting closure of the defect without pleural damage, even microscopically (Figure 6C). In the parenchymal area, a dense concentration of collapsed parenchyma was found near the staple line owing to the influence of suturing in both groups (Figure 6B,6C).
Discussion
Alveolar air leakage is a common complication of lung resection, and its closure can be problematic. Among several types of alveolar air leakage, the leakage adjacent to the staple line after large wedge resection is challenging to close intraoperatively. The dissection of thick tissues using staplers can cause strong tension around the staple line. When attempting to close the pleural defects by conventional suturing with pledgets, we occasionally experience expansion of the cleft from the suturing needle holes. Therefore, we assessed the efficacy of a new closure technique called double stapling method for air leakage adjacent to the staple line in an ex vivo porcine model.
In this study, the double stapling method was found more effective in closing pleural defects adjacent to the staple line than the conventional suturing with pledgets. The closure success was confirmed in two (20%) out of 10 cases in the suturing with pledgets group and nine (90%) out of 10 in the double stapling method group (P=0.007). In four out of 10 cases in the suturing with pledgets group, new pleural clefts longer than 3 mm developed around the needle holes on the side of the pledget, and none developed on the side of the original buttressed staple line. In contrast, there were no cases with additional pleural clefts longer than 3 mm in the double stapling method group. This is evidence of the superiority of the buttressed staple line in preventing pleural tears. The possible mechanism was the distribution of tension over the integrated structure of the lung parenchyma, staples, buttress material, and suture strings, although we could not prove this hypothesis mechanically in this study (Figure 3). There might be a concern that the double stapling method has a negative impact on residual lung volume. We speculate that the loss of pulmonary function derived from the double stapling method should be minimal, although we did not assess the residual lung volume in this study. The reasons for this are as follows. First, the amount of parenchyma resected by additional stapling was small (Figure 2B). Second, the distance between the two parallel staple lines was within 1 cm (Figure 2C).
In previous studies, various surgical techniques and materials have been used to control the intraoperative alveolar air leakage. Attachment of fibrin glue and polyglycolic acid sheets is a widely used method for alveolar air leakage (13). The attachment of human fibrinogen-thrombin sheets (TachoSil®) is also widely used (4,14). The attachment of these biological sealants are easy and fast to perform even in thoracoscopic surgery, compared with suturing. However, these biological materials are associated with the risk of viral infections (15). Moreover, detachment of the sheets owing to airway pressure sometimes occurs during the air leakage test before chest wall closure. The sealant can peel off because of inadequate bonding strength. On the other hand, recent studies have reported that placement of a free subcutaneous/pericardial fat pad was effective in preventing prolonged air leakage (16,17). This method does not involve the risk of viral infection. Moreover, it seems more resistant to airway pressure than the attachment of sheets because the fat pad is fixed to the pleura by stitching. A comparison between the efficacy of the free fat pad method and the double stapling method will be a subject of future research.
Due to the similarities in lung anatomy and physiology between humans and pigs (18), ex vivo porcine lungs were used in this study. The ex-vivo porcine lungs have been used in several studies, including our previous study to investigate optimal alveolar air leakage closure techniques (11,12,19). In this study, we set the maximum thickness of the lung parenchyma to be resected at 60 mm, assuming large wedge resection in routine clinical practice. The histological examination revealed bronchioles with cartilage components near the staple line, suggesting that wedge resection was deep enough. The size of the pleural defect adjacent to the staple line was set to 10 mm square based on our clinical experience of defect sizes that were difficult to close intraoperatively. The maximum airway pressure for the air leak test was set at 30 cmH2O, based on the pressure used in clinical practice and previous studies (12).
This study has three limitations. The major limitation of our experiment is that it was limited to wedge resections of the basal segments of the left caudal lobe. In pleural defects created by wedge resection of parts near the interlobar fissure, the second stapling could be difficult due to an insufficient amount of lung parenchyma between the staple lines and intrapulmonary vessels and bronchus. Therefore, the double stapling method can be applied to selected parts of the lung, distant from the hilar or interlobar portions. The second limitation is the selection of surgical devices in this study. We generally use buttressed staplers for fragile lungs at our institution based on previous studies reporting its efficacy for preventing air leakage (7-10). We sutured the lung parenchyma with 2-0 non-absorbable monofilament sutures, such as Surgipro. However, the selection of staplers or sutures is surgeon-dependent. Therefore, the efficacy of the double stapling method using other materials such as non-buttressed staplers or thinner suture strings should be assessed in the future. The third limitation is the extrapolability of this ex vivo porcine lung experiment to clinical practice. Although this model is well established for assessing air leakage (12,20-22), we could not assess the long-term results of the double stapling method or its efficacy on emphysematous lungs in this model. These assessments will be the subject of future clinical research.
In this ex vivo study investigating a suitable intraoperative closure method for the alveolar air leakage adjacent to the staple line after wedge resection, the double stapling method was found more effective than the conventional suturing method with pledgets. This method is not only effective but also easy to perform using standard surgical equipment.
Acknowledgments
Funding: This study was funded by Keio University School of Medicine Departmental Teaching and Research Allowance. The funder had no role in the study design, analysis, and reporting of the study.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1960/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1960/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1960/coif). All authors received scholarship donation from Eli Lilly Japan K.K., Shionogi & Co., Ltd., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kyowa Kirin Co., Ltd., Ethicon, Inc., and Covidien Japan Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We used lungs obtained from dead animals; therefore, ethical approval was not required based on our institutional guidelines.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abolhoda A, Liu D, Brooks A, et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest 1998;113:1507-10. [Crossref] [PubMed]
- Bardell T, Petsikas D. What keeps postpulmonary resection patients in hospital? Can Respir J 2003;10:86-9. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Belda-Sanchís J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010;CD003051. [Crossref] [PubMed]
- Bonnet B, Tabiai I, Rakovich G, et al. Air leaks: Stapling affects porcine lungs biomechanics. J Mech Behav Biomed Mater 2022;125:104883. [Crossref] [PubMed]
- Juettner FM, Kohek P, Pinter H, et al. Reinforced staple line in severely emphysematous lungs. J Thorac Cardiovasc Surg 1989;97:362-3. [Crossref] [PubMed]
- Shigeeda W, Deguchi H, Tomoyasu M, et al. The utility of the Stapler with PGA sheet for pulmonary wedge resection: a propensity score-matched analysis. J Thorac Dis 2019;11:1546-53. [Crossref] [PubMed]
- Vaughn CC, Wolner E, Dahan M, et al. Prevention of air leaks after pulmonary wedge resection. Ann Thorac Surg 1997;63:864-6. [Crossref] [PubMed]
- Zhang D, Miao J, Hu X, et al. A clinical study of efficacy of polyglycolic acid sleeve after video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. J Thorac Dis 2017;9:1093-9. [Crossref] [PubMed]
- Bures M, Zardo P, Länger F, et al. Improved application technique of albumin-glutaraldehyde glue for repair of superficial lung defects. J Cardiothorac Surg 2016;11:149. [Crossref] [PubMed]
- Asakura K, Izumi Y, Kohno M, et al. Effect of cutting technique at the intersegmental plane during segmentectomy on expansion of the preserved segment: comparison between staplers and scissors in ex vivo pig lung. Eur J Cardiothorac Surg 2011;40:e34-8. [Crossref] [PubMed]
- Ueda K, Tanaka T, Jinbo M, et al. Sutureless pneumostasis using polyglycolic acid mesh as artificial pleura during video-assisted major pulmonary resection. Ann Thorac Surg 2007;84:1858-61. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Sandri A, et al. Efficacy and safety of human fibrinogen-thrombin patch (TachoSil®) in the treatment of postoperative air leakage in patients submitted to redo surgery for lung malignancies: a randomized trial. Interact Cardiovasc Thorac Surg 2013;16:661-6. [Crossref] [PubMed]
- Kawamura M, Gika M, Izumi Y, et al. The sealing effect of fibrin glue against alveolar air leakage evaluated up to 48 h; comparison between different methods of application. Eur J Cardiothorac Surg 2005;28:39-42. [Crossref] [PubMed]
- Ikeda T, Sasaki M, Yamada N, et al. Controlling air leaks using free pericardial fat pads as surgical sealant in pulmonary resection. Ann Thorac Surg 2015;99:1170-5. [Crossref] [PubMed]
- Shintani Y, Inoue M, Funaki S, et al. Clinical usefulness of free subcutaneous fat pad for reduction of intraoperative air leakage during thoracoscopic pulmonary resection in lung cancer cases. Surg Endosc 2015;29:2910-3. [Crossref] [PubMed]
- Kotoulas C, Panagiotou I, Tsipas P, et al. Experimental studies in the bronchial circulation. Which is the ideal animal model? J Thorac Dis 2014;6:1506-12. [PubMed]
- Zhang R, Bures M, Höffler K, et al. In vitro comparison of two widely used surgical sealants for treating alveolar air leak. Thorac Cardiovasc Surg 2014;62:705-9. [Crossref] [PubMed]
- Eckert CE, Harris JL, Wong JB, et al. Preclinical quantification of air leaks in a physiologic lung model: effects of ventilation modality and staple design. Med Devices (Auckl) 2018;11:433-42. [Crossref] [PubMed]
- Klassen C, Eckert CE, Wong J, et al. Ex Vivo Modeling of Perioperative Air Leaks in Porcine Lungs. IEEE Trans Biomed Eng 2018;65:2827-36. [Crossref] [PubMed]
- Nomori H, Abe M, Sugimura H, et al. Triple-layer sealing with absorptive mesh and fibrin glue is effective in preventing air leakage after segmentectomy: results from experiments and clinical study. Eur J Cardiothorac Surg 2014;45:910-3. [Crossref] [PubMed]


