
Effect of hospital case volume on clinical outcomes of patients requiring extracorporeal membrane oxygenation: a territory-wide longitudinal observational study
Introduction
Extracorporeal membrane oxygenation (ECMO) has evolved to become an important form of organ support for patients with severe circulatory or respiratory failure, as a bridge to recovery or to more definitive treatment options. The utilization of ECMO has increased rapidly in the intensive care units (ICU) around the world (1), with the Extracorporeal Life Support Organization reporting a total of 44,043 adult ECMO runs between 2015 and 2020 (2).
Hong Kong has a population of 7.6 million, and with a total land area of 1,110 km2, is one of the most densely populated cities in the world (3). The number of ECMO centers has grown from 4 in 2010 during the H1N1 influenza outbreak to 7 at present (4), to parallel a 24.9% increase in ICU admissions from 2008 to 2018 (5). Some studies have suggested that patient outcomes were better in high case-volume hospitals, but the lack of patient-level data did not permit a comparison of performance by using observed and predicted outcomes (6).
Using observational data from a territory-wide patient registry including all patients who received ECMO at publicly-funded hospitals in Hong Kong over 10 years, the patterns of ECMO utilization were examined, and observed clinical outcomes were compared with predicted outcomes based on standardized risk scores. We hypothesized that patients cared for in high-case volume ECMO centers had better outcomes compared with low-case volume ECMO centers. We present the following article in accordance with the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1512/rc).
Methods
Study population
This was a retrospective observational study of all adult patients (≥18 years old) who received ECMO and were admitted to general ICU of public hospitals under the Hospital Authority in Hong Kong between January 2010 and December 2019. Data were extracted from a territory-wide electronic health record system. Study patients were identified using an International Classification of Disease Clinical Modification (ICD-9 CM) procedure code for ECMO, and an Acute Physiology And Chronic Health Evaluation (APACHE) form which denotes admission to the ICU. These patients were matched to an administrative patient registry governed by the centralized ICU committee. Patients who had incomplete ECMO information such as the ECMO configuration and duration of ECMO were excluded. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority of Hong Kong West Cluster (IRB reference number: UW 20-573) and individual consent for this retrospective analysis was waived.
Data collection
ECMO data including ECMO configuration and start and termination of ECMO were collected from the administrative patient registry, which were entered manually by trained nurses at each ECMO center. All other patient information including baseline characteristics, components of the Charlson Comorbidity Index (CCI), Sequential Organ Failure Assessment (SOFA) score, and Acute Physiologic Assessment and Chronic Health Evaluation IV (APACHE IV) risk of mortality were collected from the Clinical Data Analysis and Reporting System (CDARS), a centralized de-identified data repository including information from all public hospitals in Hong Kong (7-10). Patient outcomes including mortality and length of stay (LOS) were also collected from CDARS.
Study outcomes
The primary outcomes were hospital mortality and ICU LOS, selected because of their validation by APACHE IV risk scores (9), with comparisons made between patients managed in high-case volume ECMO centers and low-case volume ECMO centers. The secondary outcome was ICU mortality. The observed clinical outcomes were compared with predicted outcomes based on the APACHE IV risk score.
Definition of high-volume and low-volume ECMO centers
Up to the time of data collection, ECMO services were routinely provided at 6 publicly-funded ICUs in Hong Kong. For the purposes of subsequent analysis, we divided all ICUs into high-case volume and low-case volume ECMO centers based on the actual number of ECMO cases performed each year over the study period. With reference to an international guideline, “high-volume” centers were those with >20 ECMO cases annually, while “low-volume” centers were those with ≤20 ECMO cases annually (11).
Statistical analysis
Categorical variables were described with frequencies and percentages. Shapiro-Wilk tests were performed for continuous variables to test normality and described as means with standard deviations or medians with interquartile ranges. Categorical variables between groups were compared using the chi-square test, and continuous variables were compared using the t-test or Mann-Whitney-U test as appropriate.
In the primary analysis, clinical outcomes such as hospital mortality and ICU LOS of patients who received ECMO care in high-volume centers were compared with those in low-volume centers. Assuming a hospital mortality of 50% and a previously reported 40% reduction in odds at high-volume centers (6), a sample size of 658 would have 90% power to detect a significant difference between groups with a two-tailed type I error rate of 0.05. The Mann-Kendall test was used to examine significant temporal trends. Multivariable regression including potential confounders chosen a priori based on biological plausibility was performed, these included the type of ECMO, APACHE IV score, and calendar year. Length of stay was analyzed as count data using negative binomial regression.
In secondary analyses, the observed outcomes in high- and low-volume centers were compared with APACHE IV estimated hospital mortality and ICU LOS. Standardized mortality ratios (SMR) with 95% confidence intervals (CIs) were reported. Patients with missing APACHE IV scores were excluded from this analysis. The discrimination and calibration of APACHE IV scores in predicting hospital mortality was examined using the area under receiving operating characteristic (AUROC) curve and the Hosmer-Lemeshow test.
Sensitivity analyses were conducted to address known heterogeneity in survival after different ECMO configurations (12-14), by repeating the primary analyses in subgroups of veno-venous (V-V) ECMO, veno-arterial (V-A) ECMO, and ECMO cardiopulmonary resuscitation (ECPR). Other sensitivity analyses included subgroups excluding patients who received ECMO after cardiotomy procedures, and excluding patients with missing APACHE IV scores.
As an exploratory analysis, the observed hospital mortality was compared to predicted mortality by the survival after veno-arterial ECMO (SAVE) and respiratory ECMO survival prediction (RESP) scores in subcohorts of patients on V-A ECMO and V-V ECMO, respectively, from one hospital (15,16).
All statistical analysis and data visualization were performed in Stata MP, version 16.1 (StataCorp, College Station, TX, USA) (Stata, RRID:SCR_012763). Tests were considered statistically significant with two-tailed P values less than 0.05.
Results
Study population
A total of 923 episodes of ECMO treatment between January 2010 and December 2019 were identified, which accounted for 0.74% of the 125, 101 ICU admissions over the same period. After excluding 12 (1.3%) episodes which were recurrent ECMO runs in the same patient within the same hospitalization, a total number of 911 ICU episodes of ECMO treatment were analyzed (Figure 1).
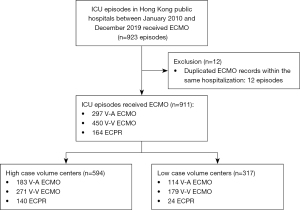
The median age was 54 years (IQR 42–62 years), and 583 (64.0%) were male patients. There were 297 (32.6%) V-A ECMO, 450 (49.4%) V-V ECMO, and 164 (18.0%) ECPR in the cohort. Among the patients on V-A ECMO, 65 (21.9%) patients had myocardial infarction, 50 (16.8%) had acute myocarditis, and 182 (61.3%) required ECMO for other causes. Among the patients on V-V ECMO, 190 (42.2%) had bacterial pneumonia, 105 (23.3%) had viral pneumonia, and 155 (34.4%) patients required V-V ECMO for other causes. Among the patients who received ECPR, 63 (38.4%) patients had myocardial infarction, 19 (11.6%) had myocarditis, and 82 (50.0%) patients were admitted for other causes. Detailed patient characteristics are presented in Table 1.
Table 1
| Patient characteristics | Low-volume centers, N=317 | High-volume centers, N=594 | Total, N=911 |
|---|---|---|---|
| Demographic information | |||
| Age at admission† | 53 [41–61] | 56 [43–63] | 54 [42–62] |
| Age 65 or above | 60 (18.9) | 125 (21.0) | 185 (20.3) |
| Male, gender | 182 (57.4) | 401 (67.5) | 583 (64.0) |
| Comorbid conditions | 54 (17.0) | 110 (18.5) | 164 (18.0) |
| Cardiovascular diseases | 10 (3.2) | 4 (0.7) | 14 (1.5) |
| Respiratory diseases | 7 (2.2) | 32 (5.4) | 39 (4.3) |
| Chronic renal failure/dialysis | 7 (2.2) | 20 (3.4) | 27 (3.0) |
| Cirrhosis | 2 (0.6) | 8 (1.3) | 10 (1.1) |
| Hepatic failure | 1 (0.3) | 3 (0.5) | 4 (0.4) |
| Metastatic carcinoma | 16 (5.0) | 28 (4.7) | 44 (4.8) |
| Lymphoma | 6 (1.9) | 8 (1.3) | 14 (1.5) |
| Leukemia/myeloma | 5 (1.6) | 4 (0.7) | 9 (1.0) |
| Immunosuppression | 16 (5.0) | 20 (3.4) | 36 (4.0) |
| Type of ICU admission | |||
| Elective post-operative | 17 (5.4) | 12 (2.0) | 29 (3.2) |
| Emergency post-operative | 21 (6.6) | 27 (4.5) | 48 (5.3) |
| Medical | 279 (88.0) | 555 (93.4) | 834 (91.5) |
| Principal diagnosis | |||
| V-A ECMO | |||
| Myocardial infarction | 18 (15.8) | 47 (25.7) | 65 (21.9) |
| Myocarditis | 19 (16.7) | 31 (16.9) | 50 (16.8) |
| Decompensated heart failure | 15 (13.2) | 19 (10.4) | 34 (11.4) |
| Valvular heart disease | 14 (12.3) | 18 (9.8) | 32 (10.8) |
| Resuscitated cardiac arrest | 4 (3.5) | 13 (7.1) | 17 (5.7) |
| Septic cardiomyopathy | 5 (4.4) | 13 (7.1) | 18 (6.1) |
| Aortic dissection | 7 (6.1) | 8 (4.4) | 15 (5.1) |
| Pulmonary embolism | 6 (5.3) | 1 (0.5) | 7 (2.4) |
| Cardiac tamponade | 0 | 4 (2.2) | 4 (1.3) |
| Refractory arrhythmia | 4 (3.5) | 0 | 4 (1.3) |
| Others: V-A | 22 (19.3) | 29 (15.8) | 51 (17.2) |
| V-V ECMO | |||
| Bacterial pneumonia | 71 (39.7) | 119 (43.9) | 190 (42.2) |
| Viral pneumonia | 47 (26.3) | 58 (21.4) | 105 (23.3) |
| Asthma | 1 (0.6) | 7 (2.6) | 8 (1.8) |
| Others: V-V | 60 (33.5) | 87 (32.1) | 147 (32.7) |
| ECPR | |||
| Myocardial infarction | 10 (41.7) | 53 (37.9) | 63 (38.4) |
| Myocarditis | 6 (25.0) | 13 (9.3) | 19 (11.6) |
| Pulmonary embolism | 0 | 9 (6.4) | 9 (5.5) |
| Sepsis | 3 (12.5) | 4 (2.9) | 7 (4.3) |
| Decompensated heart failure | 0 | 6 (4.3) | 6 (3.7) |
| Valvular heart disease | 0 | 7 (5.0) | 7 (4.3) |
| Cardiac tamponade | 1 (4.2) | 4 (2.9) | 5 (3.0) |
| Others: ECPR | 4 (1.3) | 44 (7.4) | 48 (5.3) |
Data are presented as number (percentage). †, presented with median [IQR]. ICU, intensive care unit; V-A ECMO, veno-arterial ECMO; V-V ECMO, veno-venous ECMO; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation.
The hospital mortality was 456 (50.1%) and ICU mortality was 382 (41.9%). The median (IQR) ICU LOS was 10.2 (4.8–20.1) days and median hospital LOS was 26.8 (10.7–55.6) days. There were a total of 19 (2.1%) patients who were bridged from V-A ECMO to heart transplantation or mechanical assistive devices, while no patients were bridged from V-V ECMO to lung transplantation. There was 1 patient who had lung transplantation more than 1.5 years after the initial ECMO episode. Detailed ECMO characteristics and patient outcomes are presented in Table 2.
Table 2
| ECMO characteristics and outcomes | Low-volume centers, N=317 | High-volume centers, N=594 | Total, N=911 | P value |
|---|---|---|---|---|
| ECMO type | ||||
| V-A ECMO | 114 (36.0) | 183 (30.8) | 297 (32.6) | 0.11 |
| V-V ECMO | 179 (56.5) | 271 (45.6) | 450 (49.4) | 0.002 |
| ECPR | 24 (7.6) | 140 (23.6) | 164 (18.0) | <0.001 |
| Outcomes | ||||
| Hospital mortality | 146 (46.1) | 310 (52.2) | 456 (50.1) | 0.078 |
| ICU mortality | 125 (39.4) | 257 (43.3) | 382 (41.9) | 0.26 |
| 28-day mortality | 109 (34.4) | 249 (41.9) | 359 (39.4) | 0.027 |
| ECMO duration (days)† | 6.1 (3.1–11.0) | 5.1 (2.4–8.8) | 5.4 (2.7–9.4) | 0.005 |
| ICU LOS (days)† | 12.0 (6.3–24.6) | 9.0 (4.1–17.9) | 10.2 (4.8–20.1) | <0.001 |
| Hospital LOS (days)† | 32.0 (14.7–57.2) | 24.1 (8.7–52.9) | 26.8 (10.7–55.6) | 0.003 |
| Heart transplantation/mechanical assistive device | 3 (0.9) | 16 (2.7) | 19 (2.1) | 0.079 |
| Lung transplantation | 0 | 0 | 0 | |
| Risk scores | ||||
| CCI†,‡ | 1 [0–2] | 1 [0–3] | 1 [0–2] | 0.11 |
| CCI ≥2 | 63 (19.9) | 152 (25.6) | 215 (23.6) | 0.053 |
| SOFA ICU score†,§ | 9 [7–12] | 11 [8–14] | 10 [7–13] | <0.001 |
| SOFA ECMO score†,¶ | 9 [7–12] | 10 [7–12] | 10 [7–12] | 0.051 |
| APACHE†† [N] | 307 [97] | 574 [97] | 881 [97] | 0.86 |
| APACHE IV risk of death† | 0.3 (0.2–0.7) | 0.6 (0.2–0.9) | 0.5 (0.2–0.8) | <0.001 |
| APACHE IV score† | 91 (68–123) | 107 (77–140) | 102 (73–134) | <0.001 |
| APACHE IV estimated LOS† | 7.4 (5.8–9.1) | 6.4 (4.2–8.6) | 6.9 (4.6–8.8) | <0.001 |
Data are presented as number (percentage). †, presented with Median, IQR; ‡, CCI: CCI score was calculated using documented comorbidities before hospital admission; §, SOFA ICU score: SOFA score was calculated using components collected on the day of ICU admission; ¶, SOFA ECMO score: SOFA score was calculated using components collected on the day of starting ECMO; ††, APACHE IV: 30 (1.3) patients were excluded from the calculation of the APACHE IV score due to missing data in one or more of the APACHE components, therefore n=881. ECMO, extracorporeal membrane oxygenation; APACHE IV, Acute Physiology And Chronic Health Evaluation IV; CCI, Charlson Comorbidity Index; ECPR, extracorporeal cardiopulmonary resuscitation; ICU, intensive care unit; LOS, length of stay; SOFA, Sequential Organ Failure Assessment; V-A ECMO, veno-arterial ECMO; V-V ECMO, veno-venous ECMO.
Primary analysis: comparison between high- and low-volume centers
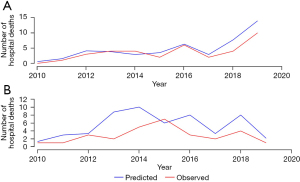
Amongst 6 ECMO centers, high-volume centers contributed 594 (65.2%) cases with an average of 29 per center per year, while low-volume centers accounted for 317 (34.8%) cases with an average of 11 per center per year. Figure 2 shows the annual number of ECMO cases in high- and low-volume centers. Of the cases managed in high-volume centers, 183 (30.8%) were V-A ECMO, 271 (45.6%) were V-V ECMO, and 140 (23.6%) were ECPR. Of the cases managed in low-volume centers, 114 (36.0%) were V-A ECMO, 179 (56.5%) were V-V ECMO, and 24 (7.6%) were ECPR (Table 2). The median APACHE IV scores for patients managed in high-volume centers were significantly higher compared with low-volume centers [107 (IQR 77–140) vs. 91 (IQR 68–123), P<0.001].
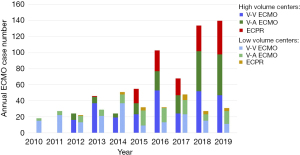
The overall hospital mortality in high- and low-volume centers were 310 (52.2%) and 146 (46.1%), respectively (P=0.08). The median ICU LOS was 9.0 days (IQR, 4.1–17.9) in high-volume and 12.0 days (IQR, 6.3–24.6) in low-volume centers (P<0.001). After adjusting for confounders, management in high-volume centers was not significantly associated with hospital mortality [adjusted odds ratio (OR) 0.86, 95% CI: 0.61–1.21, P=0.38], but with shorter ICU length of stay (P=0.02), compared with low-volume centers. There was no significant difference in ICU mortality [257 (43.3%) vs. 125 (39.4%); adjusted OR 0.76, 95% CI: 0.54–1.06, P=0.10].
Secondary analysis: comparison between observed and predicted outcomes
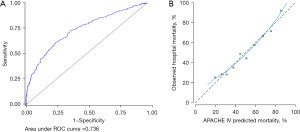
There were 30 (3.3%) patients who were excluded from the calculation of the APACHE IV score due to missing data in one or more of the APACHE components. Patient characteristics of the cohort after exclusion were similar to the original cohort (Table 3). The median APACHE IV score was 102 (IQR 73–134). The APACHE IV score had good discriminatory performance and was well-calibrated in predicting hospital mortality (AUROC 0.736; Hosmer-Lemeshow test P=0.373, Figure 3).
Table 3
| Patient characteristics | Patients with APACHE IV score, N=881 | Complete cohort, N=911 |
|---|---|---|
| ICU Admissions | ||
| ECMO type | ||
| V-A ECMO | 286 (32.5) | 297 (32.6) |
| V-V ECMO | 439 (49.8) | 450 (49.4) |
| ECPR | 156 (17.7) | 164 (18.0) |
| Demographic information | ||
| Age at admission† | 54 (43-62) | 54 (42-62) |
| Age 65 or above | 182 (20.7) | 185 (20.3) |
| Male, gender | 567 (64.4) | 583 (64.0) |
| Comorbid conditions | 162 (18.4) | 164 (18.0) |
| Cardiovascular diseases | 14 (1.6) | 14 (1.5) |
| Respiratory diseases | 39 (4.4) | 39 (4.3) |
| Chronic renal failure/dialysis | 26 (3.0) | 27 (3.0) |
| Cirrhosis | 10 (1.1) | 10 (1.1) |
| Hepatic failure | 4 (0.5) | 4 (0.4) |
| Metastatic carcinoma | 44 (5.0) | 44 (4.8) |
| Lymphoma | 13 (1.5) | 14 (1.5) |
| Leukemia/myeloma | 8 (0.9) | 9 (1.0) |
| Immunosuppression | 36 (4.1) | 36 (4.0) |
| Type of ICU admission | ||
| Elective post-operative | 29 (3.3) | 29 (3.2) |
| Emergency post-operative | 46 (5.2) | 48 (5.3) |
| Medical | 806 (91.5) | 834 (91.5) |
| Principal Diagnosis | ||
| V-A ECMO | ||
| Myocardial infarction | 65 (22.7) | 65 (21.9) |
| Myocarditis | 48 (16.8) | 50 (16.8) |
| Decompensated heart failure | 33 (11.5) | 34 (11.4) |
| Valvular heart disease | 31 (10.8) | 32 (10.8) |
| Resuscitated cardiac arrest | 15 (5.2) | 17 (5.7) |
| Septic cardiomyopathy | 17 (5.9) | 18 (6.1) |
| Aortic dissection | 15 (5.2) | 15 (5.1) |
| Pulmonary embolism | 7 (2.4) | 7 (2.4) |
| Cardiac tamponade | 4 (1.4) | 4 (1.3) |
| Refractory arrhythmia | 3 (1.0) | 4 (1.3) |
| Others: V-A | 48 (16.8) | 51 (17.2) |
| V-V ECMO | ||
| Bacterial pneumonia | 184 (41.9) | 190 (42.2) |
| Viral pneumonia | 100 (22.8) | 105 (23.3) |
| Asthma | 8 (1.8) | 8 (1.8) |
| Others: V-V | 147 (33.5) | 147 (32.7) |
| ECPR | ||
| Myocardial infarction | 59 (37.8) | 63 (38.4) |
| Myocarditis | 17 (10.9) | 19 (11.6) |
| Pulmonary embolism | 9 (5.8) | 9 (5.5) |
| Valvular heart disease | 7 (4.5) | 7 (4.3) |
| Sepsis | 7 (4.5) | 7 (4.3) |
| Decompensated heart failure | 6 (3.8) | 6 (3.7) |
| Cardiac tamponade | 4 (2.6) | 5 (3.0) |
| Others: ECPR | 47 (5.3) | 48 (5.3) |
| Outcomes | ||
| Hospital mortality | 437 (49.6) | 456 (50.1) |
| ICU mortality | 366 (41.5) | 382 (41.9) |
| 28-day mortality | 342 (38.8) | 359 (39.4) |
| ECMO duration (days)† | 5.6 (2.8–9.5) | 5.4 (2.7–9.4) |
| ICU LOS (days)† | 10.7 (5.0–20.6) | 10.2 (4.8–20.1) |
| Hospital LOS (days)† | 27.1 (10.8–55.8) | 26.8 (10.7–55.6) |
| Heart transplantation/mechanical assistive device | 19 (2.2) | 19 (2.1) |
| Lung transplantation | 0 | 0 |
| Risk scores | ||
| CCI†,‡ | 1 [0–2] | 1 [0–2] |
| CCI ≥2 | 211 (24.0) | 215 (23.6) |
| SOFA ICU score†,§ | 10 [8–13] | 10 (7–13) |
| SOFA ECMO score†,¶ | 10 [7–12] | 10 [7–12] |
| APACHE IV (N) | 881 (100.0) | 881 (96.7) |
| APACHE IV risk of death† | 0.5 (0.2–0.8) | 0.5 (0.2–0.8) |
| APACHE IV score†,†† | 102 [73–134] | 102 [73–134] |
| APACHE IV estimated LOS† | 6.9 (4.6–8.8) | 6.9 (4.6–8.8) |
Data are presented as number (percentage). †, presented with median; IQR. ‡, CCI: CCI was calculated using documented comorbidities before hospital admission; §, SOFA ICU score: SOFA score was calculated using components collected on the day of ICU admission; ¶, SOFA ECMO score: SOFA score was calculated using components collected on the day of starting ECMO; ††, APACHE IV: 30 (1.3) patients were excluded from the calculation of the APACHE IV score due to missing data in one or more of the APACHE components, therefore n=881. APACHE IV, Acute Physiology And Chronic Health Evaluation IV; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; V-A ECMO, veno-arterial ECMO; V-V ECMO, veno-venous ECMO; ECPR, extracorporeal cardiopulmonary resuscitation; LOS, length of stay; CCI, Charlson Comorbidity Index; SOFA, Sequential Organ Failure Assessment.
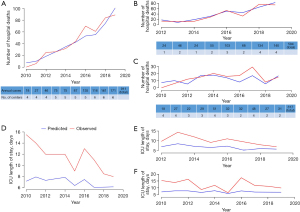
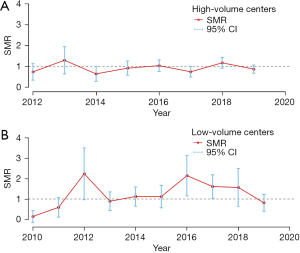
We compared the differences between the observed and APACHE IV-predicted hospital mortality over time. Throughout the 10 years, the observed hospital mortality for the whole cohort was similar to the predicted mortality (Figure 4). The SMR for high-volume centers was 0.93 (95% CI: 0.82–1.03) compared with 1.26 (95% CI: 1.06–1.47) for low-volume centers (P=0.46; Figure 5). The SMR in high-volume centers remained stable over the study period (P for trend=0.22), while in low-volume centers the SMR was variable (P for trend<0.001). The observed median ICU LOS was consistently longer than the APACHE IV-predicted LOS over the entire study period (10.2 vs. 6.9 days, P<0.001).
Sensitivity analysis
High-volume centers had a lower proportion of V-V ECMO compared with low-volume centers [271 (45.6%) vs. 179 (56.5%), P=0.002]. There was no significant association between case volume and hospital mortality or ICU mortality in V-V ECMO (adjusted OR 0.89, 95% CI: 0.56–1.41, P=0.63 and adjusted OR 0.92, 95% CI: 0.57–1.46, P=0.71). For patients requiring V-A ECMO including ECPR, management in high-volume centers was not associated with hospital mortality (adjusted OR 0.82, 95% CI: 0.49–1.36, P=0.44) but with decreased ICU mortality (adjusted OR 0.60, 95% CI: 0.36–0.98, P=0.043).
Other sensitivity analyses including subcohorts after excluding post-cardiotomy ECMO and excluding patients with incomplete APACHE IV data were performed and presented in the Appendix 1.
Exploratory analysis
The observed mortality of subcohorts of patients receiving V-A ECMO and V-V ECMO was similar to that predicted by the SAVE and RESP scores (Figure 6).
Discussion
In this observational study including almost all patients treated with ECMO in Hong Kong, we did not find the association between center-level ECMO volumes and hospital mortality previously reported in other cohorts. It is possible that the relatively low overall numbers even in high-volume centers, partially a result of the high ECMO center-to-population ratio, biased the results towards the null. Although the APACHE IV score was not developed specifically for ECMO patients, it had a satisfactory discriminatory value in predicting hospital mortality across a broad spectrum of patients managed in centers with different organizational and resource settings.
ECMO has evolved to be an important form of organ support for refractory circulatory and respiratory failure in the ICU. However, the administration of this form of therapy is highly complex, resource-intensive, and costly. In a recent systematic review of 2019 values, hospital costs ranged from US$22,305 to US$334,608 across centers in the US, Europe, Japan, Australia, and Taiwan (17). The variability in costs was related to the indication for ECMO support and location, which likely reflects fundamental differences in the healthcare infrastructure across geographical localities. It is therefore important for the provision of ECMO services be organized with territory-wide monitoring of patient outcomes in order to optimize the cost-effectiveness of this expensive therapy.
Data from the Extracorporeal Life Support Organization (ELSO) registry up to 2013 showed that patients receiving ECMO at hospitals with more than 30 adult ECMO cases per year had significantly lower odds of hospital mortality (6). A nationwide study in Japan demonstrated an inverse volume-outcome relationship for respiratory ECMO, where in-hospital mortality was 62.5%, 54.7% and 50.4% for low, medium-, and high-volume centers, respectively (18). More recently during the coronavirus disease 2019 (COVID-19) pandemic, better 90-day survival were demonstrated in French ECMO centers who had higher annual volumes in the preceding year (19). Based on the totality of the evidence, international guidelines recommend that ECMO centers have an annual volume of at least 20 cases per year with a minimum of 12 cases of respiratory ECMO (11). Considering the background rate of potential indications for ECMO, the same guideline recommends that one center cover a catchment area of 2–3 million population. At the time of data collection, Hong Kong had 6 publicly-funded ECMO centers for a population of 7.6 million, equating to an average of 1 ECMO center per 1.3 million population. In our cohort, the average annual number of ECMO cases in high- and low-volume centers were 29 and 11, respectively, but there was no significant reduction in hospital mortality or ICU mortality in high-volume centers. In our system where the distribution of ECMO machines across the territory are centrally-coordinated, the average number of ECMO consoles available at ECMO centers ranged from 1 to 4. This finding of comparable hospital mortality amongst centers with different resource settings and case volumes is worthy of consideration, as there is evidence to support that structured simulated training in low-volume centers may help to maintain volume-dependent standards of proficiency (20). A potential benefit in maintaining a higher center to population ratio is the preparedness to respond to sudden surges in demand, such as during outbreaks of emerging infectious diseases (21).
At the same time, it remains possible that the high ECMO center-to-population ratio may have resulted in an evened out distribution of cases across the territory, and with the annual case volume of high-volume centers at a modest number of 29, the beneficial effect of high case volumes did not readily manifest. Data in the COVID-19 pandemic utilized a cut-off of 30 annual cases of V-V ECMO, and demonstrated that treatment at these centers were associated with a three-fold increase in 90-day survival (19). It can be extrapolated that the total number of cases including all ECMO configurations at these centers well exceed 30. Moreover, annual case volume is a simplified metric which does not account for other characteristics in an ECMO center, such as staff expertise and auxiliary medical services, that are conducive to favorable patient outcomes. The higher APACHE IV scores in high-volume centers also suggest that increased hospital mortality in high-volume institutions may represent a selection of patients who are sicker, but would otherwise not have been supported with ECMO in low-volume centers (22).
We capitalized on the comprehensive electronic health records of the public healthcare system in Hong Kong where de-identified patient-level data allowed accurate computation of the APACHE IV score. Data in the literature validating the performance of risk prediction scores in real-world ECMO cohorts especially in the Asian population are sparse and most population sizes are modest (23,24). We showed that the APACHE IV score had satisfactory predictive performance for hospital mortality after ECMO, while the ICU LOS of our cohort was consistently longer than predicted. There may be characteristics in the Hong Kong healthcare infrastructure that lead to systematic over- or under-performance of actual outcomes, such as the relative lack of destination therapy including organ transplantation after end-stage organ failure (25), the finding that no patient over 10 years was bridged from V-V ECMO to lung transplantation affirms this observation (26). The low costs of hospital stay in publicly funded healthcare institutions may skew length of stay data. It is also possible that other ECMO-specific risk models, such as the SAVE and RESP scores, may provide better estimates of patient outcomes (15,16), although data collection is less straightforward given the granularity of patient-level physiological parameters required.
This study had some limitations. First, some patient data such as APACHE IV components were entered manually with possible errors in entry. However, data collection was performed by a designated team of trained ICU nurses, and the primary outcomes of hospital mortality and length of stay were automatically drawn from hospital administrative data with minimal risk of error. Second, we had only included patients managed in medical ICUs and did not examine outcomes of surgical ICUs or coronary care units. Third, we could not fully delineate the underlying factors of the volume-outcome association, as other data such as ECMO provider experience and resource allocation were not systematically recorded. Fourth, it was not possible to accurately compute and assess the performance of other published ECMO mortality risk scores due to the lack of territory-wide patient-level physiology data (15,16,27). Lastly, the overall cohort size was modest and it is possible we did not have adequate power to detect a significance difference.
Conclusions
In a territory-wide longitudinal study, we demonstrated that in the setting of a high center-to-population ratio, ECMO in high-volume centers was not associated with hospital mortality. Factors that are important for maintaining standards of care in low-volume centers should be well-delineated, as they improve preparedness of a healthcare system to respond to surges in demand.
Acknowledgments
Funding: This work was supported by an unrestricted philanthropic donation from Mr. and Mrs. Laurence Tse.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1512/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1512/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1512/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority of Hong Kong West Cluster (IRB reference number: UW 20-573) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. Extracorporeal Membrane Oxygenation Use and Outcomes: 2002-2012. Semin Thorac Cardiovasc Surg 2015;27:81-8. [Crossref] [PubMed]
- ELSO Annual Report 2020. 2021:22/33. Available online: https://www.elso.org/Portals/0/Files/pdf/ELSO%20Annual%20Report%202020%20PRODUCED.pdf
- Goverment HK. Health Facts of Hong Kong (2020 Edition). 2020. Available online: https://www.dh.gov.hk/english/statistics/statistics_hs/files/2020.pdf.
- Sin SW, Young K. Development of extracorporeal membrane oxygenation in Hong Kong: current challenges and future development. Hong Kong Med J 2017;23:216-7. [Crossref] [PubMed]
- Ling L, Ho CM, Ng PY, et al. Characteristics and outcomes of patients admitted to adult intensive care units in Hong Kong: a population retrospective cohort study from 2008 to 2018. J Intensive Care 2021;9:2. [Crossref] [PubMed]
- Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 2015;191:894-901. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 2006;34:1297-310. [Crossref] [PubMed]
- Zimmerman JE, Kramer AA, McNair DS, et al. Intensive care unit length of stay: Benchmarking based on Acute Physiology and Chronic Health Evaluation (APACHE) IV. Crit Care Med 2006;34:2517-29. [Crossref] [PubMed]
- Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014;190:488-96. [Crossref] [PubMed]
- Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 2019;7:163-72. [Crossref] [PubMed]
- Richardson AS, Schmidt M, Bailey M, et al. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 2017;112:34-40. [Crossref] [PubMed]
- Smith M, Vukomanovic A, Brodie D, et al. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care 2017;21:45. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246-56. [Crossref] [PubMed]
- Oude Lansink-Hartgring A, van Minnen O, Vermeulen KM, et al. Hospital Costs of Extracorporeal Membrane Oxygenation in Adults: A Systematic Review. Pharmacoecon Open 2021;5:613-23. [Crossref] [PubMed]
- Muguruma K, Kunisawa S, Fushimi K, et al. Epidemiology and volume-outcome relationship of extracorporeal membrane oxygenation for respiratory failure in Japan: A retrospective observational study using a national administrative database. Acute Med Surg 2020;7:e486. [Crossref] [PubMed]
- Lebreton G, Schmidt M, Ponnaiah M, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med 2021;9:851-62. [Crossref] [PubMed]
- Puślecki M, Ligowski M, Dąbrowski M, et al. BEST Life-"Bringing ECMO Simulation To Life"-How Medical Simulation Improved a Regional ECMO Program. Artif Organs 2018;42:1052-61. [Crossref] [PubMed]
- Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med 2020;8:518-26. [Crossref] [PubMed]
- Bailey KL, Downey P, Sanaiha Y, et al. National trends in volume-outcome relationships for extracorporeal membrane oxygenation. J Surg Res 2018;231:421-7. [Crossref] [PubMed]
- Ng WT, Ling L, Joynt GM, et al. An audit of mortality by using ECMO specific scores and APACHE II scoring system in patients receiving extracorporeal membrane oxygenation in a tertiary intensive care unit in Hong Kong. J Thorac Dis 2019;11:445-55. [Crossref] [PubMed]
- Lin CY, Tsai FC, Tian YC, et al. Evaluation of outcome scoring systems for patients on extracorporeal membrane oxygenation. Ann Thorac Surg 2007;84:1256-62. [Crossref] [PubMed]
- Number of organ/tissue donation & patient waiting for transplantation under Hong Kong Hospital Authority (1.1.2011 - 31.3.2021). Department of Health, The Government of the Hong Kong Special Administrative Region. Accessed May 26, 2021. Available online: https://www.organdonation.gov.hk/eng/statistics.html.
- Hsin MKY, Wong CF, Yan SW, et al. The history of lung transplantation in Hong Kong. J Thorac Dis 2018;10:S1899-904. [Crossref] [PubMed]
- Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016;42:370-8. [Crossref] [PubMed]


