
Learning for the next pandemic: when high level evidence is not readily available…
Coronavirus disease 2019 (COVID-19) represents the most severe pandemic since the 1918 pandemic of Spanish flu. As of February 11th 2022, there have been 404,910,528 laboratory-confirmed cases of COVID-19, including 5,783,776 deaths reported to the World Health Organization (WHO) (1). Approximately 5% of hospitalised patients with COVID-19 have been classified as critical cases due to the presence of severe respiratory failure and/or multiple organ dysfunction (2), for whom treatment is still supportive rather than definitive.
Lack of high-quality evidence in early response
In this era of evidence-based medicine (EBM), clinicians are accustomed to therapeutic strategies based on solid clinical evidence or, at least, extensive experience. Unfortunately, this was not the case for the rapidly emerging infectious disease that was COVID-19 which presented with a new and highly unusual phenotype predominantly targeting the lung but also causing widespread thromboembolism affecting pulmonary, venous and arterial vessels. The risk to healthcare workers was also palpable yet uncertain.
Higher-quality clinical evidence, especially those from randomized controlled trials (RCTs), would only become available months later. The first clinical cases of COVID-19 and the transmission dynamics were published online in late January 2020 (3,4), about one month after the initial four unusual cases of pneumonia were noticed by local clinicians in Wuhan (2). The results of the first RCT, assessing lopinavir/ritonavir, were published online on March 19th 2020 (5), by which time 207,637 confirmed cases and 8,317 deaths had been notified to the WHO (1). By the time the results of the first RCT of remdesivir were published online on April 29th 2020 (6), laboratory-confirmed cases of COVID-19 had hit 3,036,231 worldwide, including 219,404 deaths (1). Preliminary results of the Randomised Evaluation of COVID-19 thERapY (RECOVERY) Trial of dexamethasone were provided in a press release on June 16th 2020 (7) by when 7,917,172 cases and 434,884 deaths had been reported. In view of the difficulties in access to testing in these early days, these numbers likely represent a considerable under-estimate.
Impressively, there have been a plethora of RCTs however, at the time of writing, 14 months after the first reports, corticosteroids remain the only pharmacological therapy consistently shown to improve clinical outcomes in COVID-19 patients (7). Positive outcomes from some RCTs investigating other anti-viral and immunomodulatory interventions showing benefit have not been replicated by others. National and local recommendations vary, despite reviewing the same evidence. Interleukin-6 receptor blockade has been strongly endorsed by the UK Department of Health and Social Care (8), yet only manages a ‘very low’ certainty of evidence recommendation by the Infectious Diseases Society of America (9) and intermediate support from the Cochrane Collaboration living systematic review (10). There remains a dearth of controlled trial evidence surrounding optimal ventilatory strategies. Even with corticosteroid therapy, outcomes beyond 28 days remain unknown and intensivists still grapple with the uncertainty of treating patients who continue to deteriorate despite 6 mg/day dexamethasone. Should bigger doses be used and, if so, how much and for how long?
A solid evidence base thus remains elusive. Many interventions, often with a dubious underpinning scientific rationale, have been given on a compassionate use basis, with outcomes frequently unrecorded. Retrospective case series suggesting benefit have not been reproduced by subsequent RCTs. Hydroxychloroquine, azithromycin and convalescent plasma are but three examples. There is the urge to do something for the hordes of sick patients, even if fuelled solely by social media bandwagons.
Learning of novel findings
Under this circumstance, the challenge is how to learn to manage this novel disease phenotype in a quick, systematic, safe, and correct manner about how to manage this novel disease phenotype. This applies not just to patient management, but also infection control, surveillance, lockdown measures and so forth.
From past experience with the severe acute respiratory syndrome (SARS) outbreak in 2003, we learnt that the SARS coronavirus (SARS-CoV) could be transmitted by means of droplet and direct contact (11). Notwithstanding the appearance of a novel coronavirus that causes human infection, it was reasonable to assume that SARS-CoV-2 might have similar, if not exactly the same, transmission modes. When we had the opportunity to review the medical records of physicians infected with SARS-CoV-2 in Wuhan in late December 2019, many were found to be ophthalmologists, otorhinolaryngologists and dentists. These specialists, who had limited (if any) training with regard to infection control procedures, often had close contact with their patients without a facemask during physical examination and procedures. On the contrary, very few emergency physicians, pulmonologists and infectious disease specialists were affected, despite similar or even greater chances of close contact with COVID-19 patients, yet they always examined the patients wearing a facemask. Based on these findings and sound clinical reasoning, it was intuitive that a surgical facemask might provide adequate protection for close contacts during non-aerosol-generating procedures, as later recommended by WHO guidance (12).
The fear of an increased transmission risk to healthcare workers from presumed aerosol-generating procedures such as noninvasive ventilation (NIV) and high flow nasal oxygen (HFNO) generated considerable concern within hospital staff and governmental bodies. Yet this was based upon an imperfect appreciation of the existing, albeit relatively sparse, literature (13). Nonetheless, this fear generated widely conflicting national recommendations (14). Initial guidance steered towards early intubation of COVID-19 patients with mild-to-moderate acute respiratory failure. In early March 2020 reasonable worst-case planning assumptions indicated that the National Health Service (NHS) could need up to 90,000 beds with ventilators yet there was only an absolute maximum of around 7,400 ventilators, including many not normally used to treat critically ill patients (15). These requirements far exceeded production and supply capability, even in high-income countries. Crucially, consideration had also not been paid to the lack of trained staff to safely operate this number of machines as the UK normally operates on fewer than 4,000 ICU beds.
Early experience from China in January 2020 indicated that intensive care resources were rapidly overwhelmed. Necessity had driven the use of noninvasive respiratory support in non-ICU settings, notwithstanding the potential risks to healthcare workers magnified by issues with personal protective equipment supply. Chinese clinicians caring for these patients bravely accepted this risk and, fortunately, the scant evidence base suggesting safety was borne out as they did not contract severe COVID-19 disease themselves. Rapid dissemination of this learning experience did not occur, neither from China nor, subsequently, Italy. A cautious approach was suggested in interim guidance from the WHO on 28th January 2020 (16) recommending “HFNO or NIV should only be used in selected patients with hypoxemic respiratory failure” and patients receiving a trial of NIV or HFNO... “should be in a monitored setting and cared for by experienced personnel capable of endotracheal intubation in case the patient acutely deteriorates or does not improve after a short trial (about 1 hr)”. This guidance also reiterated recent data that “suggest that newer HFNO and NIV systems with good interface fitting do not create widespread dispersion of exhaled air and therefore should be associated with low risk of airborne transmission”. Governmental healthcare policy makers and clinicians however remained largely unaware so an early intubation strategy remained promulgated while the pandemic spread around Europe and North America.
Informal communication between the authors in early March, backed up by similar email responses from other colleagues in China and Italy, prompted early adoption of non-invasive respiratory support using continuous positive airways pressure (CPAP) at University College London Hospital (17). Other hospitals in London also moved successfully to CPAP during the rapid onset of the first surge when London threatened to be overwhelmed. NHS national guidance was only changed on 24th March 2020 to approve use, with appropriate caveats about patient selection, staff safety, and oxygen supplies. HFNO however remained contraindicated.
We now know that many such patients could have been successfully managed on non-invasive respiratory support alone with no increased risk to healthcare workers taking appropriate precautions. An RCT of NIV or HFNO in COVID-19 patients with moderate-to-severe respiratory failure confirmed multiple case series that approximately 40% of patients could avoid intubation (18). National UK data indicate a 19% reduction in the odds of mortality per 4-week period across the first surge, with 22.2% of this mortality reduction mediated by changes in respiratory support (19). Similar data have been reported over the first wave in 4,244 patients admitted to French ICUs (20).
A similar story applied to prone positioning. Many critically ill COVID-19 patients experienced severe hypoxaemia refractory to standard lung-protective ventilation, i.e., low tidal volume and high positive end-expiratory pressure (PEEP). Chinese clinicians found that prone positioning could often produce dramatic improvements in oxygenation. This procedure is recommended in severe acute respiratory distress syndrome (ARDS) yet there was an ongoing debate as to whether or not COVID-19 pneumonia represented ARDS (21). This early successful experience was well received in China, although the exact mechanism still remains to be elucidated. Some clinicians subsequently implemented a protocol that extended the duration of prone positioning up to 36 hours (22). This practice was quickly adopted by clinicians taking care of less severely ill COVID-19 patients who attempted awake prone positioning during oxygen therapy or NIV (23). Although not supported by high-quality evidence, awake prone positioning in these patients appears efficacious, well tolerated by most patients, and not associated with severe complications. As a result, Chinese guidelines advised almost all COVID-19 patients with hypoxaemia, no matter whether mild or severe, to maintain prone positioning for at least 12 hours per day, especially those experiencing significant improvements in arterial oxygenation. Interestingly, the updated Surviving Sepsis Campaign guideline has not issued a recommendation on the use of awake prone positioning in non-intubated adults due to insufficient evidence, though they acknowledged the benefits may not be captured in clinical trials (24). As with NIV, this learned experience was not rapidly disseminated; use of prone positioning in both awake and intubated patients gathered momentum slowly in other countries, including the UK, rather than being instituted from the outset.
Reverse transcription polymerase chain reaction (RT-PCR), the current gold-standard laboratory test for the identification of SARS-Cov-2 in the clinical setting, may not be readily available in the early days of the pandemic. This labor-intensive, skill- and resource-demanding technique is subject to false-negative results, which may be attributed to the timeline of PCR positivity in different sampling approaches (nasopharyngeal swabs, nasal swabs, saliva samples, throat swabs, and pooled nasal and throat swabs), sampling time in relation to illness onset, and inappropriate sampling technique (25). In comparison, chest CT, as a noninvasive imaging approach, may help differentiate a variety of etiologies of lung injury by depicting certain characteristics within minutes (26). Interestingly, artificial intelligence using deep learning technology has demonstrated great success to detect and differentiate bacterial and viral pneumonia among pediatric patients (27). Differentiation between COVID-19 and non-COVID-10 (bacterial, fungal, viral) pneumonia by similar deep learning-based algorithm has been associated with high accuracy, with area under the receiver operating characteristic curve up to 0.98 (26,28,29). These findings indicate that, with the assistance of artificial intelligence model, early chest CT scan may serve as an adjunct to detection of highly suspected COVID-19 patients, who should be isolated while awaiting the RT-PCR results.
The challenge
Effective dissemination is important to close the gap between discovery of novel findings and application in clinical practice. Traditional platforms for disseminating information, i.e., the 3 Ps (paper, podium and poster), may not be applicable in a rapidly spreading disease such as the COVID-19 pandemic. This is due in part to time delays and in part to global strategies of infection prevention and control that significantly decrease opportunities for traditional face-to-face contact. As a consequence, multiple platforms such as social media, e-mails, preprints, media interviews, podcasts, blogs, press releases and online webinars are used to disseminate novel yet non-peer reviewed information to (un)targeted audiences.
As part of the national response to the overwhelmed healthcare resource in Hubei province, the Chinese government deployed 344 medical rescue teams (including 11,416 physicians and 28,679 nurses), 90% of whom worked in the twelve designated hospitals in Wuhan. Attending physicians working in the same hospital would convene at a daily multidisciplinary round, to discuss the most difficult cases and share personal experiences. In addition, social media and telemedicine quickly gained popularity. The official WeChat account of Chinese Society of Critical Care Medicine (CSCCM) has released updated information daily from the onset of the outbreak. CSCCM hosted its first webinar on the prevention and management of COVID-19 in pregnant women on January 24th 2020, with 36,478 attendees from all over China. CSCCM subsequently organized webinars on COVID-19-related topics on a daily basis, with microbiologists/virologists, intensivists, infectious diseases specialists, pulmonologists, infection control specialists, and nurses as invited speakers, discussing diagnosis, respiratory support, immunomodulatory therapy, infection control, and so on. More time within these webinars was devoted to panel discussions, questions and answers. By April 30th 2020, the CSCCM had organized 133 webinars on COVID-19, with 1,329,127 participants (Figure 1).
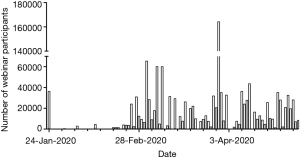
The challenge remains as to how to sift out useful from pointless or even injurious advice, to discriminate true from fake news, and to rapidly learn from others’ experiences rather than needing to go through the same learning curve (30). This applies to policymakers and clinicians alike. Living evidence networks provide useful distillations of published trial data (31).
Acknowledgments
Funding: This work was supported, in part, by research grant Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-062 and 2020-I2M-2-005 from Chinese Academy of Medical Sciences and Peking Union Medical College, and National Key R&D Program of China 2020YFC0841300, 2021YFC0863100, and 2021YFC2500801 from Ministry of Science and Technology of the People’s Republic of China.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-201/coif). BD reports that he received COVID-19-related research funding Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-062 and 2020-I2M-2-005 from Chinese Academy of Medical Sciences and Peking Union Medical College, and National Key R&D Program of China 2020YFC0841300, 2021YFC0863100, and 2021YFC2500801 from Ministry of Science and Technology of the People’s Republic of China. He sits on advisory boards of National Health Commission of the People’s Republic of China for management of COVID-19. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available online: https://COVID19.who.int
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. Erratum in: Lancet 2020;395:496. [Crossref] [PubMed]
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020;382:1787-99. [Crossref] [PubMed]
- Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569-78. Erratum in: Lancet 2020;395:1694. [Crossref] [PubMed]
- Anonymous. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. Available online: https://www.ox.ac.uk/news/2020-06-16-low-cost-dexamethasone-reduces-death-one-third-hospitalised-patients-severe
- Medicines & Healthcare products Regulatory Agency. Interleukin-6 inhibitors (tocilizumab or sarilumab) for hospitalised patients with COVID-19 pneumonia (adults). Available online: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103144
- Bhimraj A, Morgan RL, Shumaker AH, et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/COVID-19-guideline-treatment-and-management/
- Ghosn L, Chaimani A, Evrenoglou T, et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev 2021;3:CD013881. [PubMed]
- Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med 2003;348:1995-2005. [Crossref] [PubMed]
- World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief. Available online: https://apps.who.int/iris/rest/bitstreams/1286634/retrieve
- Greenland JR, Michelow MD, Wang L, et al. COVID-19 Infection: Implications for Perioperative and Critical Care Physicians. Anesthesiology 2020;132:1346-61. Erratum in: Anesthesiology 2020;133:693. [Crossref] [PubMed]
- Raoof S, Nava S, Carpati C, et al. High-Flow, Noninvasive Ventilation and Awake (Nonintubation) Proning in Patients With Coronavirus Disease 2019 With Respiratory Failure. Chest 2020;158:1992-2002. [Crossref] [PubMed]
- National Audit Office, UK Government. Investigation into how government increased the number of ventilators available to the NHS in response to COVID-19. Available online: https://www.nao.org.uk/report/increasing-ventilator-capacity-in-response-to-COVID-19/
- World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. Interim guidance, 28 January 2020. Available online: https://apps.who.int/iris/handle/10665/330893
- Singer M, Shipley R, Baker T, et al. The UCL Ventura CPAP device for COVID-19. Lancet Respir Med 2020;8:1076-8. [Crossref] [PubMed]
- Grieco DL, Menga LS, Cesarano M, et al. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA 2021;325:1731-43. [Crossref] [PubMed]
- Docherty AB, Mulholland RH, Lone NI, et al. Changes in in-hospital mortality in the first wave of COVID-19: a multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir Med 2021;9:773-85. [Crossref] [PubMed]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 2021;47:60-73. [Crossref]
- Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care 2020;24:154. [Crossref] [PubMed]
- Carsetti A, Damia Paciarini A, Marini B, et al. Prolonged prone position ventilation for SARS-CoV-2 patients is feasible and effective. Crit Care 2020;24:225. [Crossref] [PubMed]
- Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 2020;8:765-74. [Crossref] [PubMed]
- Alhazzani W, Evans L, Alshamsi F, et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med 2021;49:e219-34. [Crossref] [PubMed]
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020;323:2249-51. [Crossref] [PubMed]
- Li L, Qin L, Xu Z, et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020;296:E65-71. [Crossref] [PubMed]
- Kermany DS, Goldbaum M, Cai W, et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018;172:1122-1131.e9. [Crossref] [PubMed]
- Long C, Xu H, Shen Q, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 2020;126:108961. [Crossref] [PubMed]
- Harmon SA, Sanford TH, Xu S, et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat Commun 2020;11:4080. [Crossref] [PubMed]
- Lipworth W, Gentgall M, Kerridge I, et al. Science at Warp Speed: Medical Research, Publication, and Translation During the COVID-19 Pandemic. J Bioeth Inq 2020;17:555-61. [Crossref] [PubMed]
- The COVID-NMA initiative: a living mapping and living systematic review of Covid-19 trials. Available online: https://COVID-nma.com/


