
Iatrogenic opioid withdrawal syndromes in adults in intensive care units: a narrative review
Introduction
Opioids are used for pain management both during hospitalization and outpatient treatment. Their use in hospitalized patients includes patients on mechanical ventilation and postoperative patients. One large study based on 1.14 million hospital admissions reported that nearly 51% of hospital admissions include orders for opioids (1). These drugs suppress the production of endogenous neurotransmitters by decreasing cAMP levels, thereby reducing pain signals (2,3). For patients that have been taking opioids for a long period of time, drug tolerance and physical dependence can develop and leave the patient at risk for developing opioid withdrawal syndrome (4).
Opioid withdrawal has been described as withdrawal symptoms developing after reducing or terminating opioids prescribed by a physician for either outpatient or inpatient use. Symptoms of withdrawal include but are not limited to rhinorrhea, increased lacrimation, myalgia, diarrhea, nausea, vomiting, pupillary dilation, insomnia, tachycardia, hypertension, sweating, tachypnea, anxiety, irritability, and hyperreflexia (2). Outpatient prescriptions of opioids increased in the early 2000s. According to the Centers for Disease Control and Prevention, the opioid dispensing rate in the United States increased in 2006 and peaked in 2012. At that time 81.3 prescriptions were dispensed per 100 people (4). Although the dispensing rate fell to its lowest rate of 46.7 prescriptions per 100 people in 2019, opiate addiction is still a serious medical concern.
While considerable effort has been devoted to outpatient opiate prescription and subsequent addiction and withdrawal symptoms in adults, opiate use during critical care hospitalizations has been neglected. This review focuses on the frequency and characteristics of the iatrogenic opiate withdrawal syndrome (IOWS) in critically ill hospitalized adults. This syndrome, for this review, is defined as an opioid withdrawal syndrome developed by critically ill inpatient after the abrupt reduction or cessation in opioid administration initially prescribed during hospitalization. This narrative review has the following key questions. (I) How is this syndrome defined, based on initial studies in pediatric and neonatal ICUs? (II) What are the characteristics of these patients in adult ICUs? (III) How frequently does it occur in adults? (IV) What are the factors that seem to predict its development? (V) What is the mechanism underlying this syndrome? (VI) What tools are available to identify this syndrome? (VII) How should it be treated? We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-157/rc).
Methods
The initial literature search used the PubMed MeSH terms “Analgesics”, “Opioids”, and “Iatrogenic Disease”. This search yielded 70 articles which were reviewed. After review, 13 papers were included. Reference lists on these articles were then reviewed, and citation lists were reviewed when available. Excluded papers were not considered relevant to understanding IOWS in critically ill patients. Many excluded papers focused on opiate addiction and withdrawal symptoms in outpatients, were not full papers in peer reviewed journals, or were not in English. Papers involving pediatric populations were included for some comparisons. Additional searches also used the PubMed MeSH term “Opioids” to identify review articles on the background neurobiology of opioid use and opioid use disorders. Google and Google Scholar were also used for article searches. Table S1 provides more detail on the literature searches. Table S2 provides an example of the results with a search.
Neurobiology of opiate addiction
Opiate withdrawal symptoms probably occur through several different pathways. By binding to Mu Opiate Receptors on inhibitory interneurons, opiates decrease GABA-mediated inhibitory effects on dopamine-releasing neurons. This allows large amounts of dopamine to be released in the nucleus accumbens, a major pleasure center of the brain (5,6). Over time, exposure to opioids increases the threshold for these dopaminergic neurons, requiring increased amounts of opioid stimulus to release dopamine. Higher thresholds for dopamine release mean that a lack of high opioid stimulus causes withdrawal symptoms (5). Other proposed pathways include stress-induced withdrawal symptoms, drug craving from increased cortisol and other HPA-related molecules, and genetic predispositions (5,6). Critically ill patients exposed to high doses of opiates for extended periods of time are at risk for developing withdrawal symptoms, but the timeframe and the dose response relationships for the development of withdrawal symptoms are uncertain in these ICU patients. The complex pathogenesis of this syndrome in ICU patients is reviewed in the later section on mechanisms.
Iatrogenic opioid withdrawal syndrome in pediatric patients
The initial studies on IOWS focused on pediatric or neonate patients. Pediatric and neonate patients present with IOWS symptoms which include inconsolable crying, irritability, grimacing, tremors, poor feeding, vomiting, diarrhea, fever, sweating, yawning, nasal congestion, and increased muscle tone (7). Critically ill pediatric and neonate patients are at a higher risk of IOWS based on cumulative opioid dose, for example, a dose of over 2.5 mg/kg of fentanyl for pediatric patients. Other risk factors include prolonged exposure to opioids for at least five days, the type of opioid used, the use of synthetic opioids, and critical illness requiring extracorporeal membrane oxygenation (7).
In critically ill pediatric patients, withdrawal is assessed using two tools. The Withdrawal Assessment Tool-Version 1 (WAT-1) was derived from the Opioid and Benzodiazepine Withdrawal Score tool, and the Sophia Observation Withdrawal Symptoms Scale (SOS) was developed from the Sophia Benzodiazepine and Opioid Withdrawal Checklist (7). Both were created to robustly assess a patient’s severity of withdrawal symptoms; the WAT-1 assesses eleven symptoms, and the SOS assesses fifteen. The WAT-1 was found to be 87% and 88% sensitive and specific in children, respectively, but was unable to differentiate benzodiazepine from opioid withdrawal (7). The SOS had a similar deficiency, being designed for opioids and benzodiazepine withdrawal. The SOS had a sensitivity and specificity of 83% and 93%, respectively (7). The lack of distinction between opioid withdrawal and benzodiazepine withdrawal reflects the fact that many critically ill pediatric patients studied received both opioids and benzodiazepines to assist with mechanical ventilation.
Iatrogenic opioid withdrawal syndrome in adults
In contrast to the pediatric literature, there are only a few studies in adults on IOWS. A 2018 analysis of available data concluded that “no adult study has properly examined withdrawal nor developed a screening tool” (8). The following clinical studies report the frequency of IOWS, the characteristics of patients with this syndrome, the drugs associated with this syndrome, and treatment approaches (Table 1).
Table 1
| Author, year, location | Study type, number of patients ICU type | Number of patients with IOWS | Opioids, other drugs | Criteria | Treatment | Predictive factors |
|---|---|---|---|---|---|---|
| Taesotikul, 2021, Thailand (9) | Prospective/55/ICU | 13 (23.6%) | Fentanyl monotherapy (84.9%), midazolam (11.7%), propofol (3.5%), Dex (1.2%) | DSM-5 | Self-limited, Haloperidol, Dex, Restart fentanyl |
Weaning rate >50 µg/h, increased BMI >26.4 kg/m2, increased IOWS |
| Hyun, 2020, Korea (10) | Retrospective/126/medical ICU | 37 (29.4%) | Remifentanil, fentanyl, morphine | Pediatric tools, DSM-5 | NR* | Morphine and prolonged infusion reduced IOWS |
| Arroyo-Novoa, 2020, Puerto Rico (11) | Prospective/50/trauma ICU | 22 (44%) | Fentanyl, morphine, midazolam, lorazepam | DSM-5, ICD-10, previous studies | NR | Multiple models* |
| Zerrouki**, 2019, Canada (12) | Prospective/29/ICU | 20.7% | NR | DSM-5 | NR | Median-3 days to onset |
| Brown, 2000, US (13) | Retrospective/27 on MV/burn ICU | 11 | Fentanyl, morphine, lorazepam, midazolam | Check list | No treatment needed | IOWS related to the rate of weaning |
| Cammarano, 1998, US (14) | Retrospective/28/SICU | 9 (32.1%) | Fentanyl, morphine midazolam, lorazepam, diazepam |
Check list | IOWS related to mean daily dose of fentanyl & lorazepam, NMB, propofol, duration of lorazepam, duration of MV | |
| Wang, 2017, Canada (15) | Prospective/54/trauma ICU | 9 (16.7%) | DSM-5 | NR | No definite relations to dose or duration of opioids | |
| Capilnean, 2019, Canada (16) | Prospective/52/Trauma ICU | 8 (15.4%) | NR | DSM-5 | NR | WAT-1 less sensitive and specific than DSM-5 |
*, Model 6-Duration of mechanical ventilation, opioid cumulative dose, previous drug use RASS score, and delirium predicted IOWS; **, abstract only. BMI, body mass index; Dex, dexmedetomidine; DSM-5, Diagnostic and Statistics Manuel 5th edition; ICD, International Statistical Classification of Disease and Related Health Problems; ICU, intensive care unit; IOWS, iatrogenic opioid withdrawal syndrome; MV, mechanical ventilation; NMB, neuromuscular blocking drug; NR, not reported; SICU, surgical intensive care unit; WAT-1, Withdrawal Assessment Tool-1.
Taesotikul et al. prospectively studied 55 patients with a mean age of 60.4±15.5 years admitted to an ICU in Thailand who required mechanical ventilation and opioid infusion for at least 24 h (9). Each patient underwent at least 1 observation period for opioid withdrawal. These included 6 time points of monitoring at baseline, 1, 3, 6, 24, and 72 h after an opioid rate reduction or discontinuation. All patients had received a continuous infusion of fentanyl for a median duration of 3.8 days and a median cumulative dose of 19.6 mcg/kg/day. Most patients (80%) were not on concomitant sedative drugs. Thirteen out of the 55 study patients (23.6%) developed IOWS. The diagnosis was based on Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) criteria, and the most common withdrawal symptoms were mydriasis (9/13), agitation (5/13), insomnia (5/13), and muscle pain (5/13). Patients in the withdrawal group had a daily weaning rate of fentanyl greater than 50 mcg/h and an increased BMI (26.4 vs. 22.1 kg/m2). There was no difference in the number of ventilator-free days or ICU free-days between the patients on fentanyl who had withdrawal and the patients on fentanyl who did not.
Hyun et al. analyzed the frequency of opioid withdrawal syndrome in 126 patients treated with remifentanil (n=58), fentanyl (n=47), or morphine (n=21) during mechanical ventilation (10). These patients had a mean age of 68.3±14.1 years. The diagnosis was based on the presence of at least 3 central nervous system or autonomic nervous system symptoms. This syndrome was more frequent in patients on remifentanil (18, 31.0%) and fentanyl (17, 36.2%) than on morphine (2, 9.5%). The most common symptom identified in these patients was an increase (defined by >20% of the mean in the preceding 4 h) in respiratory rate or heart rate. Multivariable analysis indicated the use of morphine and longer duration of infusions were associated with less frequent withdrawal syndromes. This latter association may reflect the possibility that longer infusions were associated with tapering of the drug dose.
Arroyo-Novoa et al. prospectively studied 55 patients in a trauma intensive care unit who were expected to receive opioids and/or benzodiazepines for 5 or more days (11). These investigators used a check list based on the DSM-5, the International Classification Diseases, 10th edition classification of mental and behavior disorders, and previous research on this syndrome. Patients with probable opioid withdrawal needed to have 3 or more symptoms that developed following the reduction or cessation of the opioid or benzodiazepine drug. The median age of these patients was 37, 88% were male, and 90% required mechanical ventilation. The median opioid dose per day was 109 mg in morphine equivalents, and the median benzodiazepine dose per day was 72 mg in lorazepam equivalents. Probable opioid withdrawal occurred in 22 patients (44%), questionable opioid withdrawal occurred in 10 patients (20%), and no withdrawal occurred in 18 patients (36%). Patients with probable opioid withdrawal had more frequent agitation, restlessness, diarrhea, fever, high blood pressure, lacrimation, tachypnea, and hyperactive delirium. These patients required mechanical ventilation for a longer period and had longer lengths of stay in the ICU and hospital. The investigators used 6 models to analyze the potential predictors of the development of probable withdrawal syndrome. In the model which included 9 parameters (age, benzodiazepine dose, opioid dose, days on benzodiazepines, days on opioids, previous drug use, duration of mechanical ventilation, Richmond Agitation Sedation Scale Score, and the presence of delirium), opioid cumulative dose, days on opioids, previous drug use, duration of mechanical ventilation, Richmond Agitation Sedation Scale score, and the presence of delirium all predicted the development of probable withdrawal syndrome. However, days on opioids was negative predictor for the development of this syndrome.
Zerrouki et al. prospectively studied 29 patients (median age 65 years) who required mechanical ventilation and opioid administration for at least 72 h (12). Six patients (20.7%) developed withdrawal syndrome within a median of 3 days from opioid weaning. These patients had a wide spectrum of withdrawal symptoms which were similar to the symptoms reported in the study by Arroyo-Novoa (11).
Wang et al. prospectively studied 54 patients admitted to a level 1 trauma center in Canada (15). These patients required mechanical ventilation and narcotic infusions for at least 72 h. The mean age was 50 years. Opioid withdrawal syndrome was identified using the criteria in the DSM-5 criteria for opioid withdrawal. Nine patients (16.7%) developed opioid withdrawal syndrome which occurred 1 to 11 days after reduction or cessation of opioid infusions. When compared to the patients who did not develop opioid withdrawal syndrome, there were no differences in the cumulative opioid dose prior to weaning, the duration of opioid infusion, the duration of mechanical ventilation, or the weaning of rate. One hundred percent of the patients in the opioid withdrawal syndrome group had received benzodiazepines.
Cammarano et al. reviewed the medical records of 28 patients who required mechanical ventilation and intensive care unit hospitalization for >7 days (14). Nine patients (32.1%) developed acute withdrawal syndrome; the mean age was 39.9±4.6 years in the patients who developed withdrawal. All of these patients had received neuromuscular blockers and propofol. The signs and symptoms associated with withdrawal were determined from medical record review. The median duration of propofol infusion, the amount of lorazepam, and the length of mechanical ventilation were all greater in patients who developed opioid withdrawal. The patient characteristics in this study seems to differ from other studies since they required long periods of mechanical ventilation (40 days), long periods of propofol infusion (20 days), and relatively high doses of lorazepam.
Brown et al. retrospectively reviewed the medical records of 11 patients in a burn unit who developed opioid withdrawal syndrome (13). The mean age was 37; the mean percent burn area was 39.7%±4.9%. All patients required mechanical ventilation and received opioids and benzodiazepines. The signs and symptoms of opioid withdrawal were collected from the medical record. In this study the rate of weaning of benzodiazepines and opioids during the drug withdrawal phase appeared to influence the development of this syndrome. The patients in this study differed from the patients in other studies since they were burn patients who probably required different types of care during their intensive care unit management.
Capilnean et al. prospectively studied 52 ICU patients who required mechanical ventilation and received continuous intravenous infusions or intermittent doses of opioids for at least 72 h (16). They used DSM-5 criteria to identify patients with opioid withdrawal. The median age was 51.5 years, 73% were men, and 83% were white. The median ICU length of stay was 18 days. Eight patients (15.4%) developed opioid withdrawal syndrome. These investigators compared to the Withdrawal Assessment Tool-1 with the DSM-5 criteria. These results are discussed below.
These eight studies indicate that IOWS occurs in 15% to 40% of patients in intensive care unit who required opioid infusions (Table 1) (9-16). These reports included patients in medical ICUs, trauma ICUs, surgical ICUs, and burn ICUs; many patients had received an opioid with a sedative agent. Most of the studies used DSM-5 criteria to identify these patients. Factors which predicted the development of this syndrome varied from study to study. Important considerations include the weaning rate for the opioid, the duration of opioid infusion, and the concomitant infusion of benzodiazepines. Treatment approaches included the reinstitution of the opioid infusion with a slower reduction in the rate and the use of an alpha-2 agonist, such dexmedetomidine or clonidine. Many patients appeared to recover without specific treatment.
These studies have used different criteria for classifying a patient as having IOWS. For example, Wang et al. used 3 criteria outlined in the DSM-5, such as dysphoric mood, nausea or vomiting, diaphoresis, or rhinorrhea; Hyun et al. used more neurological criteria, such as an increased pupil size, a Glasgow Coma Scale score increase, or increased respiratory or pulse rates (10,15). This indicates that at present there is no uniform assessment tool for adult IOWS (7). Although both these criteria tools seem adequate, the lack of a uniform assessment tool, especially when compared to current pediatric tools, limits comparisons between studies and should be addressed. Tables 2,3 summarize the diagnostic tools which been used to identify opioid withdrawal syndromes (7,16-19).
Table 2
| Assessment tool | Withdrawal Assessment Tool-1 (7,16) | Opioid and Benzodiazepine Withdrawal Score tool (7) | Sophia Benzodiazepine and Opioid Withdrawal Checklist (7) | Sophia Observation Withdrawal Symptoms Scale (7) |
|---|---|---|---|---|
| Intended patient population | Critically ill pediatric patients, primarily in the PICU | Critically ill pediatric patients | Critically ill pediatric patients admitted to the ICU | Critically ill pediatric patients admitted to the ICU |
| Tool description | Eleven item tool, often performed by the patient’s nurse, that reviews the patient’s medical record from the last twelve hours of admission then observes the patient for two minutes. During direct observation the patient’s responsiveness is compared under different stimuli. This tool is performed twice a day. A positive response for a symptom is assigned one point, and a negative response no points. The sum of points at the end of the assessment is scored between a 0–12. Assessment takes an average of seven minutes to complete | Twenty-one item checklist adapted from a prior 1995 Children’s Hospital flowsheet created by the same authors. It assesses the frequency and severity of withdrawal symptoms among CICU patients receiving opioid and/or benzodiazepine therapy. In its founding study it was used for patients that received at least five days of drug therapy, and was performed by the patient’s nurse every four hours until two days after discontinuation of drug therapy | A twenty-four-item tool centered around signs and symptoms associated with the CNS, GI, and ANS. Assessment completed by nurses monitoring the patient within twenty-four hours of tapering or terminating opioid use | An abbreviated version of the SBOWC that focuses on signs and symptoms deemed most relevant by physicians and nurses consulted for the Ista et al. study in 2008. Assessment takes an average of two minutes to complete. Lists only fifteen-items |
| Symptoms recorded | ||||
| Restlessness/agitation | No | Yes | Yes | Yes |
| Yawning | Yes | Yes | Yes | No |
| Tremor | Yes | Yes | Yes | Yes |
| Anxiety | No | No | Yes | Yes |
| Pupil size | No | Yes | Yes | No |
| Fever | Yes | No | Yes | Yes |
| Sleep/changes | No | Yes | Yes | Yes |
| Startle to touch | Yes | Yes | No | No |
| Time to gain calm | Yes | Yes | No | No |
| Inconsolable crying | No | Yes | Yes | Yes |
| Grimacing | No | No | Yes | Yes |
| Hallucinations | No | Yes | Yes | Yes |
| Seizure | No | No | No | Yes |
| Tachycardia | No | No | Yes | Yes |
| Hypertension | No | No | Yes | No |
| Tachypnea | No | Yes | Yes | Yes |
| Lacrimation/rhinorrhea | No | Yes | No | No |
| Sneezing | Yes | Yes | Yes | No |
| Frequent suction | No | Yes | No | No |
| N/V/D | Yes | Yes | Yes | Yes |
| Feeding retention | No | No | Yes | No |
| Sweating | Yes | Yes | Yes | Yes |
| Piloerection | No | No | No | No |
| Hot/cold flushes | No | No | No | No |
| Mottling | No | No | Yes | No |
| Bone/joint/muscle aches | No | No | No | No |
| Uncoordinated movement | Yes | Yes | Yes | Yes |
| Muscle tone | Yes | Yes | Yes | Yes |
| Effectiveness with intended patient population | With a WAT score of 3 or more it is 87% sensitive and 88% specific. Higher scores (i.e., 4 and up) was found to correlate with longer opioid treatment prior to tapering, a longer opioid weaning period, longer mechanical ventilation, and longer PICU stays | Inter-rater reliability of >80% among nurses completing the assessment. However, only half of the 4-hourly assessments expected were completed | ||
| Effectiveness with adult critically ill patients with IOWS | With adult critically ill patients that need mechanical ventilation and regular narcotics for over seventy-two hours the sensitivity and specificity of WAT-1 when assessing IOWS was 50% and 65.9% respectively | Not applied to critically ill adults at time of writing | Not applied to critically ill adults at time of writing | Not applied to critically ill adults at time of writing |
ANS, autonomic nervous system; CICU, cardiac intensive care unit; CNS, central nervous system; GI, gastrointestinal; ICU, intensive care unit; N/V/D, nausea/vomiting/diarrhea; PICU, pediatric intensive care unit; SBOWC, Sophia Benzodiazepine and Opioid Withdrawal Checklist; WAT, withdrawal assessment tool.
Mechanisms
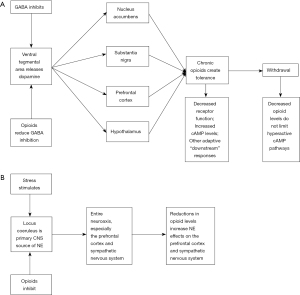
The model for opioid addiction in outpatients involves 3 stages (5,20-22). The first stage includes opiate-induced pleasure and reward sensations in the brain. This stage can also involve intoxication. The second stage includes opioid tolerance which requires increasing doses to experience pleasure sensation and withdrawal symptoms occur when the drug cannot be obtained. The third stage involves chronic relapses and drug use which may be associated with environmental or emotional factors or psychiatric factors. All 3 stages are associated with changes in neural cells and neural networks, especially ventral tegmental area, the nucleus accumbens, and the locus coeruleus. Opioid use disorders usually involve the episodic use of opioids by various routes in patients who may also use other drugs and have underlying psychiatric comorbidity. In contrast, opioid use in ICU patients has clear therapeutic goals, but it often includes the use of additional sedative medications in patients who have complex acute syndromes with complicated care strategies which can cause pain, anxiety, and fear. The same neural circuits are involved, but the CNS drug levels are likely much higher, and the timeframe for developing these adverse effects is quite compressed. Studies on opioid withdrawal and ICU patients are quite difficult. Figure 1 summarizes basic information on opioid withdrawal in adults (5,23,24).
Korak-Leiter et al. prospectively studied 29 patients who required postoperative ventilator support and management with a combination of narcotic and sedative (25). Fourteen patients received sufentanil and midazolam, and 15 patients received sufentanil and propofol. In both groups, the patients had an increase in withdrawal related symptoms at the beginning of narcotic weaning. Somatic sensory evoked potentials recovered more quickly in patients on sufentanil and propofol. The plasma beta-endorphin levels increased to higher levels in patients on sufentanil and midazolam. These investigators concluded that long-term administration of an opioid with a benzodiazepine result in tolerance to the opioid and the requirement for higher levels of infusion which in turn delayed recovery of endogenous opioid synthesis and this was associated with a longer duration of withdrawal syndrome. They suggested an alpha-2 agonist should be used whenever patients start to have withdrawal symptoms. This study uses relatively straightforward methods to characterize the intensity of withdrawal symptoms and signs, objective neurologic information using evoked potentials, and measurement of endogenous opioid compounds. Measurement of endogenous opioid to may provide a simple way to classify patients and predict the development of a withdrawal syndrome.
Possibilities for an adult assessment tool
For adult patients, opioid withdrawal can be assessed by using self-reported symptoms, by observing symptoms, such as increased lacrimation and rhinorrhea, or by measuring physiological parameters, such as blood pressure or level of opioids in urine analysis. However, there are also scales developed for assessing the severity of withdrawal or to differentiate between opioid toxicity and acute opioid withdrawal in outpatients (Tables 2,3). In the past, the Himmelsbach scale, The Opioid Withdrawal Scale, and the Subjective Opiate Withdrawal Scale were used (18). At this time, the most commonly used assessment tool is the Clinical Opiate Withdrawal Scale (COWS). It can be completed in two minutes, assesses the severity of withdrawal signs or symptoms based on a score of 5 “mild” and 36 “severe” (18). This tool was designed for adult patients able to verbally communicate their symptoms in an outpatient setting, but might be used in other settings, depending on the clinical status of the patient. Its use in ICU patients seems doubtful.
Table 3
| Assessment tool | Subjective Opiate Withdrawal Scale (1987) (7,17) | Diagnostic and Statistical Manual of Mental Disorders fifth edition (7,19) | COWS (1999) (7,18) |
|---|---|---|---|
| Intended patient population | Adult patients in with high opioid physical dependence in an outpatient environment | No specific patient population or setting listed | Adult patients in with high opioid physical dependence in an outpatient environment |
| Tool Description | A sixteen-item tool covering signs and symptoms the patient reports occurred in the last 24 h. Graded on a five-point scale, 0 being “not at all” and 4 being “extremely”. Severity can range from 0 to 64 | Not a tool but a list of diagnostic criteria for opioid withdrawal | An eleven-item tool covering signs and symptoms associated with pulse rate, gastrointestinal system, CNS, and musculoskeletal system. Can be completed in two minutes by evaluator (i.e., nurse or physician) during outpatient patient visits. Is scaled based on severity between mild, moderate, moderately severe, and severe. Score ranges from 0–36 |
| Symptoms recorded | |||
| Restlessness/agitation | Yes | No | Yes |
| Yawning | Yes | Yes | Yes |
| Tremor | Yes | No | Yes |
| Anxiety | Yes | No | Yes |
| Pupil size | No | Yes | Yes |
| Fever | No | Yes | No |
| Sleep changes | No | Yes | No |
| Startle to touch | No | No | No |
| Time to gain calm | No | No | No |
| Inconsolable crying | No | No | No |
| Grimacing | No | No | No |
| Hallucinations | No | No | No |
| Seizure | No | No | No |
| Tachycardia | No | No | Yes |
| Hypertension | No | No | No |
| Tachypnea | No | No | No |
| Lacrimation/rhinorrhea | Yes | Yes | Yes |
| Sneezing | No | No | No |
| Frequent suction | No | No | No |
| N/V/D | Yes | Yes | Yes |
| Feeding retention | No | No | No |
| Sweating | Yes | Yes | Yes |
| Piloerection | Yes | Yes | Yes |
| Hot/cold flushes | Yes | No | Yes |
| Mottling | No | No | No |
| Bone/joint/muscle aches | Yes | Yes | Yes |
| Uncoordinated movement | Yes | No | No |
| Muscle tone | No | No | No |
| Effectiveness with intended patient population | Intended to assess effectiveness of outpatient opiate withdrawal therapies used through the progression of treatment | ||
| Effectiveness with adult critically ill patients with IOWS | Not applied to critically ill hospitalized adult patients | Not applied to critically ill hospitalized adult patients | Not applied to critically ill hospitalized adult patients |
COWS, Clinical Opiate Withdrawal Scale; CNS, central nervous system; N/V/D, nausea/vomiting/diarrhea; IOWS, iatrogenic opioid withdrawal syndrome.
Since WAT-1 is a popular tool used by researchers to assess the severity of withdrawal symptoms for critically ill pediatric patients, there has been an attempt to use it in critically ill adult patients. Capilnean et al. conducted a prospective observational open cohort study of fifty-two critically ill adults on mechanical ventilation (16). They assessed withdrawal symptoms using the DSM-5 to establish the diagnosis and the WAT-1 for comparison. In contrast to its use in pediatric patients, when applied to critically ill adult patients, the WAT-1 was 50% sensitive and 65.9% specific, resulting in a positive likelihood ratio 1.47 and a negative likelihood ratio of 0.758. Agreement between these 2 tools had kappa value of 0.102 (P=0.390). Interrater reliability of the DSM-5 was 90.1%, and the interrater reliability of the WAT-1 was 89.1%. Therefore, the WAT-1 appears to be inadequate for use in adult patients in part because it relies on nurses’ interpreting nonverbal responses. For example, the WAT-1 includes “time to gain calm” and “startle to touch” among their factors. The SOS also includes factors, such as “inconsolable crying” and “grimacing”. While these factors are designed to assess central nervous system activity, they primarily occur in pediatric and neonate patients. Therefore, the results can be skewed by the lack of these responses in adults without substitution of other symptoms which reflect central nervous system activity during withdrawal.
Treatment and possible consequences of opioid withdrawal
The diagnosis of IOWS in critically ill adults with complicated medical care in ICUs represents a difficult problem. These patients often require opioid and sedative infusions. Other medical problems can lead to changes in the central nervous system and autonomic nervous system which might be confused with iatrogenic opioid withdrawal. Possible explanations for the development of these symptoms include direct drug toxicity, the development of pain and anxiety, the development of delirium, and the withdrawal syndrome. The development of IOWS can significantly complicate the care of these patients, prolong mechanical ventilation, and prolong length of stay in the hospital. In addition, it is possible that patients with this syndrome are more likely to leave the hospital on opioids and require chronic opioid medications (26). The approach to management should include both prevention with limitation of the amounts of infused opioids to the extent possible and avoiding combinations with sedatives when possible. Slow reductions in the rate of the infusion may reduce the frequency of this syndrome. When it develops, using drugs, such as clonidine and dexmedetomidine, appear to have sound pharmacologic rationale.
Conclusions
Iatrogenic opioid withdrawal syndromes have been well-documented in pediatric patients, but studies on IOWS in critically ill adults have been limited. The two main diagnostic tools for children, the WAT-1 and the SOS, do not work well in adults. Studies on IOWS in adults have used different diagnostic criteria. This syndrome needs more recognition and research in critically ill adults to develop a better diagnostic tool and, hopefully, better treatment guidelines.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-157/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-157/coif). KMN serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2021 to July 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herzig SJ, Rothberg MB, Cheung M, et al. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med 2014;9:73-81. [Crossref] [PubMed]
- Gupta M, Gokarakonda SB, Attia FN. Withdrawal Syndromes. Treasure Island (FL): StatPearls Publishing; 2022.
- Shah M, Huecker MR. Opioid Withdrawal. Treasure Island (FL): StatPearls Publishing; 2021.
- Berger FK. Substance use disorder 2020. Available online: https://medlineplus.gov/ency/article/001522.htm
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 2002;1:13-20. [Crossref] [PubMed]
- Kreek MJ, Levran O, Reed B, et al. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest 2012;122:3387-93. [Crossref] [PubMed]
- Chiu AW, Contreras S, Mehta S, et al. Iatrogenic Opioid Withdrawal in Critically Ill Patients: A Review of Assessment Tools and Management. Ann Pharmacother 2017;51:1099-111. [Crossref] [PubMed]
- Duceppe MA, Perreault MM, Frenette AJ, et al. Frequency, risk factors and symptomatology of iatrogenic withdrawal from opioids and benzodiazepines in critically Ill neonates, children and adults: A systematic review of clinical studies. J Clin Pharm Ther 2019;44:148-56. [Crossref] [PubMed]
- Taesotikul S, Dilokpattanamongkol P, Tangsujaritvijit V, et al. Incidence and clinical manifestation of iatrogenic opioid withdrawal syndrome in mechanically ventilated patients. Curr Med Res Opin 2021;37:1213-9. [Crossref] [PubMed]
- Hyun DG, Huh JW, Hong SB, et al. Iatrogenic Opioid Withdrawal Syndrome in Critically Ill Patients: a Retrospective Cohort Study. J Korean Med Sci 2020;35:e106. [Crossref] [PubMed]
- Arroyo-Novoa CM, Figueroa-Ramos MI, Balas M, et al. Opioid and Benzodiazepine Withdrawal Syndromes in Trauma ICU Patients: A Prospective Exploratory Study. Crit Care Explor 2020;2:e0089. [Crossref] [PubMed]
- Zerrouki K, Li Q, Delucilla L, et al. Symptomatology of opioid-associated withdrawal syndrome in critically ill adults: a descriptive study. Crit Care 2019;23:403.
- Brown C, Albrecht R, Pettit H, et al. Opioid and benzodiazepine withdrawal syndrome in adult burn patients. Am Surg 2000;66:367-70; discussion 370-1. [PubMed]
- Cammarano WB, Pittet JF, Weitz S, et al. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med 1998;26:676-84. [Crossref] [PubMed]
- Wang PP, Huang E, Feng X, et al. Opioid-associated iatrogenic withdrawal in critically ill adult patients: a multicenter prospective observational study. Ann Intensive Care 2017;7:88. [Crossref] [PubMed]
- Capilnean A, Martone A, Rosu VA, et al. Validation of the Withdrawal Assessment Tool-1 in Adult Intensive Care Patients. Am J Crit Care 2019;28:361-9. [Crossref] [PubMed]
- Ista E, van Dijk M, de Hoog M, et al. Construction of the Sophia Observation withdrawal Symptoms-scale (SOS) for critically ill children. Intensive Care Med 2009;35:1075-81. [Crossref] [PubMed]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253-9. [Crossref] [PubMed]
- American Psychiatry Association. Opioid Withdrawal. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatry Publishing, 2013;547-9.
- Peltz G, Südhof TC. The Neurobiology of Opioid Addiction and the Potential for Prevention Strategies. JAMA 2018;319:2071-2. [Crossref] [PubMed]
- Koob GF. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry 2020;87:44-53. [Crossref] [PubMed]
- Kakko J, Alho H, Baldacchino A, et al. Craving in Opioid Use Disorder: From Neurobiology to Clinical Practice. Front Psychiatry 2019;10:592. [Crossref] [PubMed]
- Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res 2010;1314:162-74. [Crossref] [PubMed]
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 2008;154:384-96. [Crossref] [PubMed]
- Korak-Leiter M, Likar R, Oher M, et al. Withdrawal following sufentanil/propofol and sufentanil/midazolam. Sedation in surgical ICU patients: correlation with central nervous parameters and endogenous opioids. Intensive Care Med 2005;31:380-7. [Crossref] [PubMed]
- von Oelreich E, Eriksson M, Sjölund KF, et al. Opioid Use After Intensive Care: A Nationwide Cohort Study. Crit Care Med 2021;49:462-71. [Crossref] [PubMed]


