
Effect of pulmonary vein cryoballoon ablation in dogs with coolant-nitrogen
Introduction
Atrial fibrillation (AF), which is the most common arrhythmia observed in clinical practice, affects approximately 1–2% of the entire population (1,2). Catheter ablation to isolate the pulmonary veins (PVs) provides the most effective treatment option. It is performed by either cryoballoon or radiofrequency ablation. A great number of studies have shown that the cryoballoon ablation of PVs is a safe and effective treatment for patients with AF (3-6). Compared to the radiofrequency ablation technique, the cryoballoon ablation technique has several advantages, including a shorter procedure duration, a higher degree of reproducibility, and a shorter learning curve (2,7). Due to its low risk of complications and proven efficacy, the Arctic Front Cardiac Cryoablation Catheter Family (Medtronic, Int.) is the leading system for cryoballoon ablation (4,8,9). Under this system, nitrous oxide (N2O) refrigerant is injected into the inner balloon via injection tubes to create a cryothermal lesion.
Liquid nitrogen is also widely used in surgery, and can achieve a temperature as low as −196 ℃ (10). However, its utility in percutaneous cardiac procedures has been limited due to its complex design and potential risks. In recent years, some novel cryoballoon systems have been developed in China, one of which uses nitrogen (N2) as the refrigerant. Effective innovations have made it possible to achieve pulmonary vein isolation (PVI) safely. This is the first study to use N2 refrigerant for cryoballoon ablation in dogs, and we evaluated the effectiveness and safety of the new cryoablation system for PVI. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-418/rc).
Methods
Laboratory animal preparation
Experiments were performed under a project license (No. 201821) granted by the Institutional Ethical Committee of Shanghai Putuo District People’s Hospital, in compliance with the guidelines of Shanghai Putuo District People’s Hospital for the care and use of animals. A protocol was prepared without registration. The use of the dog as a model for cardiac surgical and electrophysiological research is popular. There are subtle differences between hearts of dogs and human, and the model was used in most of the cryoballoon ablation research. In this study, 16 healthy adult Labrador dogs (weighing 25±5 kg), male or female, were enrolled in the study. Of these dogs, 13 underwent PVI procedures with cryoballoons delivering the refrigerant (N2O or N2), and the other 3 dogs served as baseline controls (see Table 1). For the study group, 26-mm cryoballoons (Cryofocus, Int., MN, USA) with refrigerant (N2) were used in 8 dogs, and for the control group, 28-mm second-generation cryoballoons with N2O (Arctic Front Advance; Medtronic, Inc.) were used in 5 dogs. For each experimental group, data of all the animals were included in the analysis. Each animal was fed, monitored and sacrificed in the same way to minimise potential confounders. All the procedures were performed under conscious sedation using intravenous injection of pentobarbital sodium, followed by diazepam and ketamine intramuscular injection. The dogs’ surface electrocardiogram and blood pressure was continuously monitored.
Table 1
| Follow-up time | Study group (n) | Control group (n) | Baseline control (n) |
|---|---|---|---|
| Before procedure | 8 | 5 | 3 |
| Immediately post-ablation | 8 | 5 | 0 |
| On the same day post-ablation (sacrificed) | 3 | 2 | 0 |
| 1-month post-ablation (sacrificed) | 5 | 3 | 0 |
The novel cryoballoon catheter system (Cryofocus, Inc.)
The system consists of a steerable catheter with a balloon, a 16-F deflectable delivery sheath, and external equipment that houses the N2 refrigerant (see Figure 1). The cryoablation equipment provides constant pressure and refrigerant based on multi-stage cooling and gradient pressurization technology. The temperature sensor is located at the proximal internal-end of the balloon, and real-time temperature data are shown on the screen of the device. The freezing flow rate is adjusted to maintain the proper temperature. If the temperature drops to the low limit, the equipment will automatically reduce the flow rate to stop any further drop, and vice versa. For security, the cooling output is controlled via a multivariable coupling system (pressure, temperature and flow). Another good design is the cryoballoon of the Cryofocus system has 18 jets (8 jets in Artic Front AdvanceTM), which results in an increased uniformity of cooling.

Ablation strategy
The ablation strategy for the two systems was the same. Specifically, introducer sheaths were placed in both the right and left femoral veins. A 6-F decapolar catheter was inserted into the coronary sinus, and a 6-F quadripolar catheter was positioned at the right ventricle. A Brockenbrough transseptal needle and sheath were inserted through the right femoral venous entrance route. After successful transseptal puncture under fluoroscopy guidance and PV venography, the SL0 sheath was changed over-the-wire for a deflectable delivery sheath, and the cryoballoon catheter was inserted such that the left- and right-superior PVs were targeted for cryoablation. Before the balloon inflation, the circular mapping catheter was placed in the PV to record the potentials. The cryoballoon was then inflated and advanced toward the PV ostium. The vein occlusion was checked with contrast dye injection. After complete occlusion was verified, the freezing began. The duration of each freezing cycle was 180 s for the targeted PV. If time to isolation (TTI) was >60 s, the second cryoablation started. Before the right PVs were ablated, the right phrenic nerve (PN) was paced, and the diaphragmatic movements were closely monitored. In the case of the weakening of the diaphragmatic movements, a freezing stop was immediately triggered. After the ablation, the entrance block was confirmed by the absence of PV potentials, and pace-capture testing was used to assess the exit block with maximum output.
Histologic examination
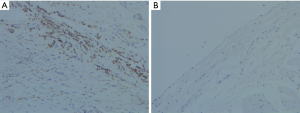
All the procedures were performed in Tenth People’s Hospital (co-construction hospital with the animal laboratory shared), Shanghai, China, bred in single housing under monitoring and fed with ad-libitum food. Three dogs of the study group and 2 dogs of the control group were euthanized on the same day post-ablation. The other 8 dogs of the two groups were euthanized 1 month post-ablation. The hearts, lungs, trachea, PVs, PNs, and esophagi were removed and examined. For the histological tissue preparation, each sample was fixed in 10% neutral buffer formalin, dehydrated, embedded in paraffin, sliced into 5-µm serial sections, and mounted onto glass slides. The sections were stained with hematoxylin and eosin (H&E) for general examination. Immunohistochemistry (IHC) was used to check the injury of the PVs, and apoptosis was detected using the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) method.
Statistical methods
The data were expressed as the mean ± standard deviation, or as the percentage with count data. The continuous variables were analyzed using the 2-sample t-test. The non-parametric variables were analyzed using the Mann-Whitney test. The categorical variables were analyzed using the chi-square test. For all the analyses, the P values were 2-sided, and a P value <0.05 was considered statistically significant. The analyses were conducted with the use of SPSS 20.0 software.
Results
Of the 8 dogs in the study group, 16 PVs were targeted, of which 2 left superior PVs were too small to be ablated. Of the 5 dogs in the control group, 10 PVs were targeted. No interventions were performed in the inferior PVs. Outcome evaluations were based on three criteria, including (I) the acute success of PVI, (II) an assessment of operation complications, and (III) a histopathology review of the lesion area.
The acute success of the PVIs was 100% in the study group, and 90% in the control group. The average ablation times of each PV in the study group were less than those in the control group (1.1±0.3 vs. 2.0±0.8; P=0.006). Compared to the Medtronic system, the novel Cryofocus system was associated with a shorter procedure duration (379±46 vs. 592±162 s; P=0.013) and a similar TTI (48.1±29.1 vs. 52.3±51.2 s). The PVI rate of a single ablation was higher in the study group than that in the control group (92.9% vs. 60.0%; P=0.05; see Table 2 and Figure 2).
Table 2
| Group | Mean ablation frequencies of each PV | Mean total ablation time (s) | Mean TTI (s) | Isolation rate of single ablation |
|---|---|---|---|---|
| Study group | 1.1±0.3* | 379±46* | 48.1±29.1 | 92.9% |
| Control group | 2.0±0.8 | 592±162 | 52.3±51.2 | 60.0% |
All the mean values are presented as the mean ± standard deviation. *, P<0.05, compared to the control group. PV, pulmonary vein; TTI, time to isolation.

In relation to the safety, there was no evidence of thrombus, esophageal injury, or pericardial tamponade in any of the dogs. Only 1 self-limited incidence of phrenic nerve paralysis (PNP) occurred in the control group, which was attributed to a right-superior PV ablation.
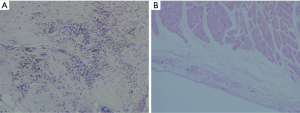
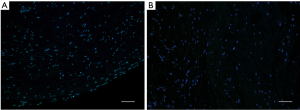
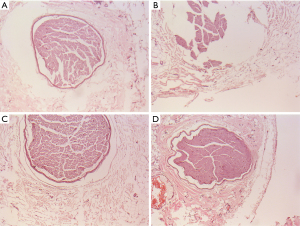
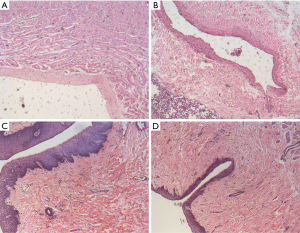
A histopathology review was conducted to assess the PV lesions. The pathological manifestations were similar in the two groups. Coagulation necrosis with inflammation was evident at the central part of the lesion (see Figures 3,4). At the peripheral zone, apoptosis became apparent 8 hours post-ablation (see Figure 5). The endothelial layers remained intact, and thrombus formation was not observed. No immediate PN injuries were observed microscopically in the study group; Edema and partial degeneration of PN was observed in 1 dog of the control group (see Figure 6). Esophageal injury was not observed in either group (see Figure 7).
Discussion
The use of a 3-dimensional mapping system, contact force-sensing technology, and irrigated radiofrequency catheter ablation have all been suggested to improve the safety and success of circumferential PVI; however, point-by-point radiofrequency ablation is still challenging due to the need for permanent and continuous transmural lesions (11-13). Various ablation technologies are being developed, among which cryoballoon ablation has unique characteristics that differs from the other types of energy ablation (14). Just a single delivery of cryoenergy has the ability to accomplish PVI, and thus it has been referred to as “one-shot” ablation. Cryoballoon ablation has been demonstrated to have the following 3 major advantages: (I) catheter stability; (II) a lower rate of acute procedural complications; and (III) a shorter procedure duration (15,16).
The cryoballoon freezes the surrounding tissue via the formation of intracellular ice crystals, which causes cell and tissue damage both during the freezing process and afterwards (17,18). Freezing results in immediate damage, including hypothermic stress and direct cell injury, while vascular-mediated injury and apoptotic cell death result in delayed damage. Factors affecting cryoablation efficacy include the tissue temperature, cooling rate, freezing duration, thawing rate, and blood flow (19). The cooling rate per second is a primary determinant of ablation outcomes. The optimal freeze-thaw cycle requires fast cooling and slow thawing. When rewarming, cell damage becomes more serious due to solution-effect injury and water recrystallization. Cryoablation depends on the elimination of energy and the absorption of heat from the tissue, and the efficacy is determined by the cooling rate.
The currently widely used second-generation cryoballoon (Arctic Front Advance, Medtronic, Minneapolis, MN, USA) was designed to use N2O as the refrigerant. While N2 is used in the novel cryoballoon catheter system (Cryofocus, Inc.), whose faster cooling rate may improve cryoablation efficacy. The boiling point of N2 (–196 ℃) and the critical temperature (–147 ℃) has a stronger freezing effect than that of N2O (which has a boiling point of –88.5 ℃ and a critical temperature of 36.5 ℃). The inner-cryoballoon of Cryofocus is equipped with a temperature sensor. Real-time temperature measurement data are fed back to the cryoablation equipment, enabling the freezing flow to be adjusted to ensure that the proper temperature can be maintained.
In this study, there was a slight difference in the immediate success rate of PVI between the two groups. Additionally, the Cryofocus (N2) system had a higher PVI rate for single ablation (92.9%), less ablation times of each PV (1.1±0.3) and a shorter procedure duration (379±46 s) than the Medtronic system. This may be related to the rapid cooling rate of the cryoballoon with N2 refrigerant (3.07 ℃/s), which is faster than that of the cryoballoon with N2O refrigerant (1.59 ℃/s), and thus more likely to cause irreversible cell death.
TTI is an important parameter and is a strong predictor of longer-term PVI durability if it <60 s (20-24). The TTI was similar between the two groups, but it was a little bit shorter in the study group than in the control group. If the sample size had been larger, it was likely that the difference would have been statistically significant.
In this study, cryoablation caused cellular damage, the necrosis of tissues, and the infiltration of neutrophils. Lesions had well-demarcated boundaries and intact endothelial layers at the PVs in both groups. No thrombus was observed in the two groups. Apoptosis was observed 8 hours after ablation at the peripheral zone of ablation, where the temperatures and cooling rates achieved were less likely to be immediately lethal to the cells. Cellular injuries might activate caspases, which cleave proteins and cause membrane blebbing, chromatin condensation, genomic fragmentation, and programmed cell death (25).
Except for 1 PNP in the control group, there were no other complications in the two groups. Thus, the Cryofocus system appears to be safe, and the freezing efficacy of the N2 coolant is strong.
Compared to N2O refrigerant, the advantage of N2 refrigerant in PV cryoballoon ablation is stronger freezing effect so that it cost shorter time to complete the procedure. In a word, the novel cryoablation system with N2 refrigerant might have more efficacy than and a similar safety to that of the Medtronic system.
This study had several limitations. First, as this study was performed on dogs with a different PV anatomy to humans, the conclusions drawn in this study might not necessarily be applicable to humans. Second, an evaluation of 26 PVs in 13 dogs represents a small cohort. Third, we only measured the acute PVI rate due to the absence of any electrophysiological follow-up. Animal studies may provide useful information for clinical studies, but reliable estimates of human risk require further research.
Conclusions
In summary, the novel cryoablation system with N2 refrigerant had better efficacy than and a similar safety to that of the Medtronic system with N2O refrigerant. As this is the first study to evaluate the novel system, future studies need to be conducted to confirm our findings, especially those on safety.
Acknowledgments
Funding: This study was funded by Science and Technology Innovation Project of Health System in Putuo District, Shanghai (No. ptkwws202218).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-418/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-418/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-418/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 201821) granted by the Institutional Ethical Committee of Shanghai Putuo District People’s Hospital, in compliance with the guidelines of Shanghai Putuo District People’s Hospital for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie HY, Guo XG, Yang JD, et al. Safety and Efficacy Using the Second-Generation Cryoballoon in Patients With Atrial Fibrillation and a Common Ostium of Inferior Pulmonary Veins. Front Cardiovasc Med 2021;8:683315. [Crossref] [PubMed]
- Creta A, Kanthasamy V, Schilling RJ, et al. First experience of POLARx™ versus Arctic Front Advance™: An early technology comparison. J Cardiovasc Electrophysiol 2021;32:925-30. [Crossref] [PubMed]
- Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23. [Crossref] [PubMed]
- Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2016;374:2235-45. [Crossref] [PubMed]
- Boveda S, Metzner A, Nguyen DQ, et al. Single-Procedure Outcomes and Quality-of-Life Improvement 12 Months Post-Cryoballoon Ablation in Persistent Atrial Fibrillation: Results From the Multicenter CRYO4PERSISTENT AF Trial. JACC Clin Electrophysiol 2018;4:1440-7. [Crossref] [PubMed]
- Su WW, Reddy VY, Bhasin K, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020;17:1841-7. [Crossref] [PubMed]
- Providencia R, Defaye P, Lambiase PD, et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48-57. [PubMed]
- Ciconte G, Sieira-Moret J, Hacioglu E, et al. Single 3-Minute versus Double 4-Minute Freeze Strategy for Second-Generation Cryoballoon Ablation: A Single-Center Experience. J Cardiovasc Electrophysiol 2016;27:796-803. [Crossref] [PubMed]
- Irfan G, de Asmundis C, Mugnai G, et al. One-year follow-up after second-generation cryoballoon ablation for atrial fibrillation in a large cohort of patients: a single-centre experience. Europace 2016;18:987-93. [Crossref] [PubMed]
- Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology 2009;59:229-43. [Crossref] [PubMed]
- Straube F, Pongratz J, Kosmalla A, et al. Cryoballoon Ablation Strategy in Persistent Atrial Fibrillation. Front Cardiovasc Med 2021;8:758408. [Crossref] [PubMed]
- Shigeta T, Yamauchi Y, Sagawa Y, et al. Cryoballoon ablation of the left atrial posterior wall reduces recurrence of persistent atrial fibrillation in patients with non-paroxysmal atrial fibrillation. J Arrhythm 2021;37:1477-87. [Crossref] [PubMed]
- Omran H, Gutleben KJ, Molatta S, et al. Second generation cryoballoon ablation for persistent atrial fibrillation: an updated meta-analysis. Clin Res Cardiol 2018;107:182-92. [Crossref] [PubMed]
- Andrade JG, Deyell MW, Verma A, et al. The Cryoballoon vs Irrigated Radiofrequency Catheter Ablation (CIRCA-DOSE) Study Results in Context. Arrhythm Electrophysiol Rev 2020;9:34-9. [Crossref] [PubMed]
- He X, Chen Y, Zhou Y, et al. One-Year Clinical Outcome of Pulmonary Vein Isolation Using the Second-Generation Cryoballoon: A Meta-Analysis. Pacing Clin Electrophysiol 2016;39:182-9. [Crossref] [PubMed]
- Koektuerk B, Yorgun H, Koch A, et al. Pulmonary vein isolation in patients with paroxysmal atrial fibrillation: Long-term clinical outcomes with first- and second-generation cryoballoons. Herz 2017;42:91-7. [Crossref] [PubMed]
- Snyder KK, Baust JG, Baust JM, et al. Cryoablation of cardiac arrhythmias. Philadelphia, PA: Elservier/Saunders, 2011.
- Barnett AS, Bahnson TD, Piccini JP. Recent Advances in Lesion Formation for Catheter Ablation of Atrial Fibrillation. Circ Arrhythm Electrophysiol 2016;9:e003299. [Crossref] [PubMed]
- Aryana A, Mugnai G, Singh SM, et al. Procedural and biophysical indicators of durable pulmonary vein isolation during cryoballoon ablation of atrial fibrillation. Heart Rhythm 2016;13:424-32. [Crossref] [PubMed]
- Su W, Aryana A, Passman R, et al. Cryoballoon Best Practices II: Practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm 2018;15:1348-55. [Crossref] [PubMed]
- Reissmann B, Wissner E, Deiss S, et al. First insights into cryoballoon-based pulmonary vein isolation taking the individual time-to-isolation into account. Europace 2017;19:1676-80. [Crossref] [PubMed]
- Chun KR, Stich M, Fürnkranz A, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm 2017;14:495-500. [Crossref] [PubMed]
- Ferrero-de-Loma-Osorio Á, García-Fernández A, Castillo-Castillo J, et al. Time-to-Effect-Based Dosing Strategy for Cryoballoon Ablation in Patients With Paroxysmal Atrial Fibrillation: Results of the plusONE Multicenter Randomized Controlled Noninferiority Trial. Circ Arrhythm Electrophysiol 2017;10:e005318. [Crossref] [PubMed]
- Aryana A, Kenigsberg DN, Kowalski M, et al. Verification of a novel atrial fibrillation cryoablation dosing algorithm guided by time-to-pulmonary vein isolation: Results from the Cryo-DOSING Study (Cryoballoon-ablation DOSING Based on the Assessment of Time-to-Effect and Pulmonary Vein Isolation Guidance). Heart Rhythm 2017;14:1319-25. [Crossref] [PubMed]
- Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int 2005;95:1187-91. [Crossref] [PubMed]


