
Uncommon EGFR mutations in lung carcinoma: features and treatment outcomes in a retrospective French cohort
Introduction
In advanced non-small cell lung carcinoma (NSCLC), epidermal growth factor receptor (EGFR) mutations account for approximately 10% to 15% of NSCLC cases in the non-Asian population and around 40% in the Asian population (1,2). Most EGFR mutations are deletions in exon 19 and L858R, i.e., amino acid substitution Leu858Arg in exon 21. These are referred to as common EGFR mutations and represent 85% to 90% of EGFR mutants (3). Therefore, around 10% harbor an EGFR mutation that is considered uncommon or rare.
The increasing use of next generation sequencing (NGS) has led to more frequent diagnosis of uncommon EGFR mutations. This higher rate of diagnosed mutations has resulted in uncertainty regarding the best treatment option(s) for each mutation. Up until 2017, only a limited number of mutations were investigated, mainly through polymerase chain reaction and Sanger sequencing. Those mutations were most frequently E709X and G719S in exon 18, in phase deletion in exon 19, insertions, T790M and S768I in exon 20 and L858R and L861Q in exon 21. Afterwards, NGS led to more extensive sequencing and the detection of more types of mutations (4). The treatment of metastatic NSCLC has been well documented for common mutations, particularly treatment using tyrosine kinase inhibitors (TKIs). The gold standard treatment for advanced NSCLC with common mutations is third generation TKIs like osimertinib, which have proven to provide better progression free survival (PFS) and overall survival (OS), with a safety profile similar to first generation TKIs (5). Current debate still remains over simultaneous use of TKI and VEGF antibodies, simultaneous use of chemotherapy and TKI with associated toxicity, and the role of immunotherapy (6-8) FLAURA 2 study.
The best management for rare mutations in NSCLC remains uncertain and is based on post hoc and/or case study analysis. For instance, FDA approval of 2nd generation TKI afatinib to treat some rare EGFR mutations (S768Q, L861Q, G719X) used a post hoc analysis of LUX Lung 2, 3 & 6 studies, which had a total of 32 pooled patients (9).
Literature on rare EGFR mutations in lung carcinomas underlines the need to split rare EGFR mutations into subgroups that may respond differently to treatment. These subgroups include: (I) exon 20 insertion/duplication (E20ID), (II) EGFR complex mutations (ECM), which are either a combination of two rare EGFR mutations or mixed rare EGFR mutation with one common EGFR mutation, and (III) other single uncommon mutations (OSUM).
Recent literature shows that OS for OSUM patients may be better with chemotherapy than TKIs, with exceptions including ECM (10). There are also developments of new TKIs for E20ID, including poziotinib, mobocertinib, which are still under phase 2 or 3 study in 2021 (11). Additionally, E20ID could benefit from the synergistic EGFR-MET antibody amivantamab, and TKI is still under trial (12). In the USA, the FDA has approved a license for amivantamab and mobocertinib for E20ID, but only as 2nd line treatment for now.
Our cohort takes another step towards better understanding rare EGFR mutations in lung carcinomas. Here, we provide one of the largest uncommon EGFR mutant multicentric cohorts with both exon 20 and other uncommon mutants, and we report on comprehensive patient characteristics and treatment outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1924/rc).
Methods
In this retrospective, regional study, patients were selected from the anatomic pathology databases of the Regional Oncology Center (CGFL, Dijon, France) and the Dijon-Burgundy University Hospital (CHU Dijon-Bourgogne, France). We collected available data on patient characteristics and tumor management from whichever French facility was in charge of patient treatment. This implied reviewing patient files from 12 different hospitals.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Guidelines of the International Conference on Harmonization. It was approved by the institutional ethics committee of the Cancer Center GF Leclerc and living patients were informed and had the opportunity to decline study participation.
Inclusion criteria were: patients over 18 years with lung cancer diagnosis of any stage, with a rare EGFR mutation identified at the CGFL or CHU Dijon from 1 January 2007 to 15 January 2021. We excluded patients with common EGFR mutations (exon 19 deletion and L858R) as well as secondary T790M mutations associated with an original common EGFR mutation for which the treatment strategy is well documented.
For the EGFR analyses, DNA was isolated from tumor tissue samples using the Maxwell16 FFPE Plus LEV DNA Purification kit (Promega, Madison, WI, USA). The quantity of extracted genomic DNA was assessed by a fluorimetric method with a Qubit device. From 2007 to 2016, hotspot mutations in the EGFR gene were detected by allelic discrimination, fragment analysis and Sanger sequencing. From 2017, all coding exons of the EGFR gene (exons 18 to 21) were analyzed by NGS. Four hundred ng of genomic DNA were used for library preparation using the Agilent Sure Select XT reagent kit (AgilentTechnologies, Santa Clara, CA, USA). The entire enriched library was used in the hybridization and captured with Sure Select custom designed baits (Agilent Technologies). Following hybridization, the captured libraries were purified according to the manufacturer’s recommendations. Normalized libraries were pooled and DNA was sequenced on an Illumina MiSeq (Illumina) device using 2*111-bp paired-end reads.
Data was collected for patient characteristics, tumor characteristics and treatment. Patient characteristics included date of birth, sex, smoking status, body mass index (BMI), occupational exposure, cardiovascular and respiratory comorbidities, performance status at stage IV diagnosis, last follow-up or date of death. Tumor characteristics included date of lung cancer diagnosis, date of rare mutation diagnosis, date of stage IV diagnosis, histology, programmed cell death 1 ligand 1 (PD-L1) status, tumor localization, EGFR mutation(s), and allelic frequency if available. Finally, treatment data concerned prior treatment of stage IV and rare EGFR mutation diagnosis if any. Moreover, for each line of treatment after stage IV diagnosis, we collected date of treatment introduction, date of progression, date of ending and finally best objective response on computed tomography (CT) imaging when available.
Patient and tumor characteristics were described regardless of the disease stage at diagnosis. As for treatment options and outcomes, analyses were performed only in metastatic patients with available data for the 1st line of treatment. Data was presented overall and for the 3 subgroups: E20ID, ECM and OSUM.
We analyzed patient characteristics and treatment outcomes in terms of response rate, PFS and OS. OS was defined as time between the diagnosis of an uncommon mutation in EGFR during metastatic disease and death from any cause. PFS was defined as the time between treatment onset and progression or death. Objective response rate (ORR) was defined as the sum of complete and partial response rate on CT. Disease control rate (DCR) was defined as the sum of complete, partial and stable response.
Statistical analysis
Comparisons of tumor and patient characteristics according to the type of mutation were performed using Chi2, Fisher or Student tests, as appropriate. Median follow-up was obtained with a reverse Kaplan-Meier estimate. Survival analyses were performed using the Kaplan-Meier method, and comparisons between groups used the log rank test. As ECM is sometimes reported in previous literature (10,13) as having a better prognosis, the OS of this subgroup was also compared to pooled OSUM and E20ID. Prognostic factors for OS were determined with a Cox model and using backward stepwise selection of variables including gender, ever-smoker, obesity, professional exposure, cardiovascular comorbidity, performance status and tumor PD-L1 status.
Sub-analyses were carried out on two highly represented uncommon mutations: L861Q and G719X.
P value less than 5% was considered as significant. All tests were two-sided. Statistical analyses were performed using R v4.0.5 software.
Results
Patient and tumor characteristics
Of the estimated 9,520 NSCLC samples screened, we identified 164 samples with lung cancer and an uncommon EGFR mutation. After the withdrawal of duplicates (i.e., patients diagnosed twice) and screening errors, 119 patients with available medical records were reviewed. Out of those 119 patients, 97 had metastatic disease with at least one documented 1st line of treatment (see Figure 1).
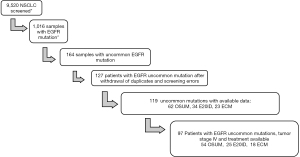
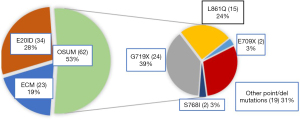
The annual number of diagnosed uncommon EGFR mutations more than doubled from 2016 to 2017, and then dropped back considerably in 2020 (Figure S1). Among the 119 rare mutations analyzed, 34 were single E20ID, 62 were OSUM, and 23 were ECM. The main mutations among OSUM were G179X and L861Q (Figure 2). Among 23 complex mutations, 14 were composed only of rare EGFR mutations and 9 of mixed uncommon/common mutations (5 L858R and 4 T790M). Among the 14 complex mutations harboring only rare mutations, S768I was found in 7 cases; G719X in 7 cases; and L861Q in 6 cases.
In this cohort, 93.3% were adenocarcinoma cases, 4 of which had a bronchioloalveolar presentation, and 3.3% were squamous cell carcinoma. When known, PD-L1 status was null in 44.6%, (1–50%) in 36.4%, and >50% in 19%. There were no significant differences in tumor characteristics in E20ID vs. OSUM and ECM (Table 1). In this cohort, 78.2% had a metastatic location at diagnosis: 26.8% cerebral, 61.3% pulmonary or pleural, 44.1% bone, 14% adrenal and 16.1% liver.
Table 1
| Characteristics | Overall (n=119) | E20ID (n=34) | OSUM (n=62) | ECM (n=23) | P value |
|---|---|---|---|---|---|
| Main patient characteristics | |||||
| Sexa | 0.86 | ||||
| Female | 68 (57.0%) | 20 (58.8%) | 36 (58.1%) | 12 (52.2%) | |
| Age, years, mean [SD] | 67 [10] | 70 [10] | 66 [11] | 66 [9] | |
| Smoking status (3 missing values)a | 0.004 | ||||
| Non-smoker | 46 (39.7%) | 20 (58.8%) | 18 (30.0%) | 8 (36.4%) | |
| Current smoker | 27 (23.3%) | 2 (5.8%) | 19 (30.7%) | 6 (26.1%) | |
| Former smoker | 43 (37.0%) | 12 (35.3%) | 23 (37.1%) | 8 (34.8%) | |
| BMI at diagnosis (20 missing values)b | 0.48 | ||||
| Mean | 25.1 | 26.6 | 24.6 | 24.3 | |
| Professional exposure (26 missing values)a | 0.40 | ||||
| Yes | 12 (13.0%) | 5 (14.7%) | 6 (16.2%) | 1 (4.0%) | |
| Performance status if known (13 missing data)a | 0.41 | ||||
| 0–1 | 77 (72.6%) | 24 (70.6%) | 34 (54.8%) | 19 (82.7%) | |
| 2 | 21 (19.8%) | 7 (20.6%) | 9 (14.5%) | 5 (21.7%) | |
| 3 | 8 (7.6%) | 1 (2.9%) | 7 (11.3%) | 0 | |
| Main tumor characteristics | |||||
| Histologya | 0.92 | ||||
| Adenocarcinoma | 111 (93.3%) | 33 (97.1%) | 58 (93.6%) | 20 (87.0%) | |
| Other | 8 (6.7%) | 1 (2.9%) | 6 (9.7%) | 1 (4.3%) | |
| PD-L1 status (% on data available) [45 (38%) missing values]c | 0.86 | ||||
| PD-L1 negative | 33 (44.6%) | 11 (45.8%) | 15 (35.0%) | 7 (41.0%) | |
| PD-L1 positive | 41 (55.4%) | 13 (54.2%) | 21 (49.0%) | 7 (41.0%) | |
| PD-L1 >50% | 14 (19.0%) | 4 (16.0%) | 7 (16.0%) | 3 (18.0%) | |
| Known initial metastasis [2 (1.6%) missing values]a | |||||
| Yes | 93 (78.2%) | 27 (79.4%) | 51 (82.0%) | 15 (71.0%) | 0.66 |
| Cerebral location | 25 (26.8%) | 7 (25.9%) | 17 (27.0%) | 1 (4.0%) | 0.07 |
a, Ki2 Pearson; b, F test; c, Ki2 Pearson on positivity. E20ID, exon 20 insertion/duplication; OSUM, other single uncommon mutation; ECM, EGFR complex mutation; BMI, body mass index; PD-L1, programmed cell death 1 ligand 1.
The patients in this cohort were 57% women, and mean age at diagnosis was 67 years. Overall 39.7% were never-smokers, but the rate was much higher in the E20ID group (58.8%) compared to ECM & OSUM (31.7%). Smoking status was the only significantly different characteristic between the two subgroups (Table 1). Other patient characteristics included a mean BMI of 25.1 and professional exposure in 13%, mainly asbestos. Finally, performance status at diagnosis was mainly 0–1 (72.6% vs. 27.4% for 2 or 3).
Almost 37% of the 119 patients were still alive at last follow-up <6 months, 52% were deceased, and 11% were lost to follow-up. The mean follow-up time was 21 months (Table S1).
Out of the 97 metastatic patients with a 1st line of treatment, 25 were single E20ID, 54 were single OSUM, and 18 were ECM, among which 9 were common/uncommon combination and 9 were only composed of uncommon mutations (of which only one had E20ID mutation) (Figure S2).
Treatment options and respective outcomes for metastatic patients
The two main 1st line treatments in our 97 patients were chemotherapy (29%) and TKI (54%). Other treatments included palliative care for 9 (9.2%), immunotherapy for 5 (5.2%) and 1 radiotherapy as well as 1 surgical treatment for an isolated secondary metastasis.
The 1st line treatment administered to E20ID was mainly chemotherapy, whereas most OSUM and ECM patients received TKI. TKI were mostly 2nd or 3rd generation (afatinib 51%, gefitinib 21%, erlotinib 15%, osimertinib 6%). Chemotherapy was mainly platinum doublets (>85%), with some cases of monotherapy (Table S2).
A 2nd line treatment was given in around half of the 1st line treatment population, and about a quarter received a 3rd line treatment; 2nd line treatment was 48% (24 patients) chemotherapy; 19 of them had previously received TKI. TKI was the 2nd line treatment in 32% (16 patients), of whom 8 had already had TKI as 1st line; 14% of 2nd line therapy was immunotherapy.
The ORR was 45.4% in the overall population, 46.3% in OSUM, and 36% in E20ID. Regarding 1st line treatment with TKI, the OSUM group had an ORR of 63.6% compared to 20% for the E20ID group. The trend was the contrary for chemotherapy, with 25% ORR vs. 53.8%, respectively (Table S3). The difference in ORR was significant (P<0.002). There was a similar trend for DCR (Table S4). The ORR and DCR were better in the ECM group than for both E20ID and OSUM; 15 out of 18 were treated with TKI at first; two had complete response and six had partial response. Three patients were treated with chemotherapy and all three had partial response.
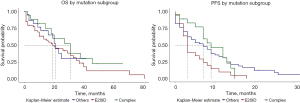
Overall median PFS was 7 months (95% CI: 4–9 months) (Figure S3). By mutation subgroup, median PFS was 3 months (95% CI: 3–9 months) for E20ID; 7 months (95% CI: 4–12) for OSUM; and 9 months for ECM [95% CI: 7–not available (NA)]; P=0.06 (Figure 3). By treatment group, PFS for all uncommon mutations showed a median PFS of 9 months for patients treated with TKI (95% CI: 7–13); 5 months for chemotherapy (95% CI: 3–9); and 1.5 months for palliative care (95% CI: 0–NA); P=0.002 (Figure S4).
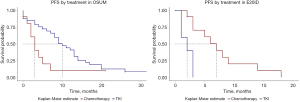
When comparing PFS for the two main treatments (chemotherapy vs. TKI) and by mutation subgroup, the trend is similar to those for ORR and DCR, with better and significant results for TKI in OSUM and better results for chemotherapy in E20ID (Figure 4). Indeed, median PFS in OSUM was 10 months for patients receiving TKI (95% CI: 8–14) and 3 months for those receiving chemotherapy (95% CI: 2–NA) with P=0.04, TKI hazard ratio (HR) =0.46 (0.21–0.99; P=0.05). In E20ID, median PFS was 7 months for chemotherapy (95% CI: 3–NA) and 2 months for TKI (95% CI: 1–NA), P=0.002, HR =6.03 (1.4–25.6; P=0.01). In ECM, median PFS was 12 months for TKI (95% CI: 7–NA). In this subgroup, statistical comparison or estimates were not relevant since there were only 3 complex mutations treated with chemotherapy as a 1st line treatment.
OS of metastatic patients
Median OS was 21 months (95% CI: 18–31) in the overall population (Figure S5).
By mutation subgroup, there were non-significant trends when the 3 groups were analyzed separately, with median OS of 31 months for the ECM group, 22 months for E20ID, and 19 months for OSUM (Figure 4). Despite very suggestive survival curves, median OS for the ECM group was also non-significantly greater than pooled E20ID-OSUM (P=0.3; HR =0.7; 95% CI: 0.34–1.34) (Figure S6).
Appendix 1 shows non-significant differences in OS by 1st line treatment in the whole population and in each uncommon mutation subgroup.
OS analysis therefore showed non-significant differences among subgroups of 1st line treatment in all rare EGFR mutations as well as by mutation subgroup. The results remained non-significant for all lines of treatment.
Other analyses
Prognostic factors for OS. Adjusted analysis showed two significant positive prognostic factors: female gender (HR =1.3; 95% CI: 1.1–3.4; P=0.026), and performance status 0, 1 or 2 (median OS: 31 months, 95% CI: 26–42 vs. 7 months, 95% CI: 2–18). Other explanatory variables were finally not significant (see Appendix 2). Above average allelic frequency was not associated with better OS or PFS response to TKI in our data.
L861Q and G719X mutations. Even though the result was not significant, L861Q-positive status in our cohort tended to be associated with better PFS and OS than other uncommon EGFR mutations. The opposite was true for G719X status (see Appendix 3).
In patients treated with immunotherapy, our limited results are presented in Appendix 4.
Finally in our data there was no significant differences in OS, FPS or ORR according to the type of TKI used as a 1st line treatment.
Discussion
On the one hand, the patient and tumor characteristics in our cohort are consistent with previous literature.
Considering the year of diagnosis, the large increase that began in 2017 can be linked to the increase in the use of NGS in France as abroad (4). As for 2020, the remarkable decrease is probably due to an increase in deferred diagnoses during the COVID-19 pandemic (14).
The distribution of rare mutations is roughly consistent with those described in previous literature, but with slightly higher proportions of E20ID and G719X (15). This is maybe due to the modest size of our cohort even though it can still be considered substantial for uncommon EGFR mutations. Histology in our cohort is in line with French nationwide EGFR profiling which indicates that 84% of all EGFR mutant NSCLC are adenocarcinomas (16). It should be noted that there was one case of sarcomatoid carcinoma, which is one of the very few cases reported so far for uncommon EGFR mutations (17).
In our cohort of rare EGFR mutants, the non-smoker proportion is higher than the 20% reported in EGFR non-mutant NSCLC. It is similar to the reported 60% of non-smokers in EGFR common mutants in our E20ID subgroup, whereas our OSUM & ECM subgroups had intermediate non-smoker proportions (16). Previous literature showed heterogeneous data, some reporting that E20ID was more frequent in smokers, and other cohorts like ours reporting a majority of never-smokers (3,13). Other characteristics are similar to previous reports showing a majority of women with rare EGFR mutants, with a mean age in the mid-sixties (10,18). Occupational exposure has rarely been studied and reported in uncommon EGFR mutants, but our results were very similar to the 12% to 14% exposure reported in NSCLC worldwide and in France (19,20).
On the other hand, treatment options and respective outcomes are somewhat different than previously reported.
Similar to prior work, our cohort illustrates the absence of strong consensus for the 1st line treatment of choice for these rare mutations (10).
Our results suggest that there is no significant difference in OS when comparing OSUM, E20ID and ECM. However ECM had non-significantly better OS, as previously reported, particularly when patients were treated with 2nd generation TKI. However, there is still a high level of heterogeneity among complex mutations and their respective outcomes (10,21,22).
We found no significant differences in OS in 1st line treatment subgroups among OSUM, E20ID and ECM. However our data strongly suggests better PFS, ORR and DCR with 1st line TKI in OSUM, and with 1st line chemotherapy in E20ID. These results are consistent with results from a Chinese cohort showing similar trends in OS and PFS (23). Our data also reinforce and complete the existing results for ORR and DCR. Slight differences in the results might be linked to sampling bias as well as numerous differences in study design, including the inclusion of T790M mutation, only 1st generation TKI treatment, no distinction between OSUM and E20ID in the chemotherapy subgroup, and an Asian population with different characteristics.
Concerning OSUM, our results for OS are consistent with a recent similar French study (10). However, their results suggest significantly better OS after a 1st line of chemotherapy compared to TKI, but TKI treatment in this study were almost exclusively 1st generation (10). Our better result with a majority of 2nd and 3rd generation TKI for OSUM is also consistent with a recent international meta-analysis and another recent Japanese analysis suggesting better efficacy of 2nd generation TKI over 1st generation TKI (24,25). In addition, the previous French study did not mention other treatment outcomes such as PFS, ORR or DCR. Our results for PFS in OSUM patients follow the same trends reported in another study, though they reported a greater difference in PFS in favor of TKI (26). Considering our observation that OSUM had better PFS-ORR-DCR for TKI but non-significantly better OS for chemotherapy, we could hypothesize that there might be a mechanism of resistance to TKI that is not yet elucidated. This could lead to initial favorable outcomes followed by more aggressive tumor behavior, or late diagnosis and management of tumor progression.
For E20ID patients in our cohort, the median OS of 22 months is in line with a recent French cohort in which OS was 24 months; PFS was also similar at 7 months, and 70% of the E20ID group was treated with chemotherapy (27). This reinforces preclinical and clinical data in favor of the resistance of E20ID to most available TKI (15,28,29). Exceptions have however been reported for insertions which are not in the so-called loop but within the alpha-C-helix, which could explain some reports on TKI efficacy (15,30). It has also been recently suggested that E20ID could have better OS and PFS with chemotherapy than non-mutant adenocarcinoma (31).
For ECM, we were unable to perform statistical tests due to our small sample size. However, our results suggest no major difference in ORR between 1st line TKI and chemotherapy for complex mutations.
Immunotherapy in uncommon EGFR mutant NSCLC is an active field of research and its place is not univoc (15). Some reports suggests that the tumor micro-environment, which is not responsive to immunotherapy in patients with mutant EGFR, could be modified for instance with anti-angiogenic therapy (32). Still, immunotherapy outcomes could be better in uncommon EGFR mutations than in common ones (33). Our data do not reveal a significant trend due to the small number of patients and the almost exclusive use of immunotherapy, either compassionate use or simultaneous to radiotherapy.
Compared to other studies reporting on common EGFR mutations, the 21 months of OS for all uncommon mutations is close to the 22.6 months reported in patients with a common EGFR mutation, but other studies report OS as high as 35 months (3,27). Our PFS results also suggest that E20ID treated with chemotherapy and OSUM treated with TKI have outcomes similar to common EGFR mutations (3). More specifically, PFS of 10 months for OSUM treated with TKI can be compared to 11.1 months reported in common EGFR mutations, and 7 months for E20ID treated with chemotherapy is similar to 6.5 months in common EGFR mutations.
Finally, observations from the focus on L861Q and G719X patients. While not significant, L861Q positive status in our cohort tended to be associated with better PFS and OS than other uncommon EGFR mutations. These results are consistent with two previous reports but differ from another French cohort in which this variant had a rather poor prognosis when treated with 1st generation TKI (9,10,24). These data tend to confirm the resistance of L861Q to 1st generation TKI, and suggest the use of 2nd or 3rd generation TKI treatment rather than chemotherapy (15,34). On the contrary, patients with the G719X mutant tended to be associated with lower PFS and OS than other OSUM and ECM, even though the results were not significant. To sum up, the better than average OS and PFS in L861Q and lower than average in G719X is similar to what has been observed previously even though the literature remains heterogeneous (1,35).
Our multicenter study is one of the largest to focus on uncommon EGFR mutations. It is also one of few studies which have considered all rare mutations and then specifically analyzed ECM, E20ID and OSUM categories with pooled and separate analyses. It is also one of the very few of this size in which the TKI treatment were mainly 2nd and 3rd generation, which are now the most commonly prescribed TKI. However, our study has several limitations which are mainly due to its retrospective nature. It implies lack of randomization and numerous information as well as selection biases. In addition, most of our analysis was performed for the ECM, E20ID and OSUM subgroups, which contributes to general orientation on 1st line treatment, but those subgroups are still heterogeneous. Thus, a systematic literature review should be undertaken before making individual treatment decisions when available for the specific mutation diagnosed. Finally, our quantitative outcomes do not include assessment of quality of life under treatment, which has to be taken into account.
Conclusions
Our cohort illustrates the heterogeneity in patient characteristics, prognosis and treatment outcomes among NSCLC patients with uncommon EGFR mutants. Overall treatment outcomes in our cohort were better than reported for wild type patients, but not as good as in patients with common EGFR mutations.
Our data emphasizes differences between subgroups of patients with uncommon EGFR mutations. Those with exon 20 insertions had better ORR and PFS with chemotherapy, which, aside from ongoing trials, seems to be the best current treatment option for most. Other single uncommon EGFR mutation including complex mutation show better results with TKI, which should thus be considered as the 1st line treatment option.
There was no significant difference in OS among 1st line treatment or rare mutation subgroups. This adds valuable complementary information about uncommon EGFR mutations treated with TKIs—mostly 2nd and 3rd generation—to recent work suggesting that OS is better with chemotherapy than 1st generation TKI in uncommon EGFR mutations (E20ID excluded). This investigation provides data which could contribute to decisions about 1st line treatments in patients with uncommon EGFR mutants. However, it does not replace the need for a systematic literature review for specific mutations, which remains heterogeneous even among the described subgroups.
Acknowledgments
We thank Mrs Suzanne Rankin for reviewing the Medical English. We also sincerely thank our colleagues for their respective help in the process of data collection in the following hospitals: Auxerre (Dr. Chikouche, Dr. Neddaf), Chalon sur Saone (Dr. Grouet), Macon (Dr. Michaux), Nevers (Dr. De Faverges), Troyes (Dr. Delclaux et Kraoua), CHU de Dijon (Dr. Millière, Mme Chapusot et Pr Martin), Paray le Monial (Dr. Genety), Montceau les Mines (Dr. Simons).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1924/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1924/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1924/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Guidelines of the International Conference on Harmonization. It was approved by the institutional ethics committee of the Cancer Center GF Leclerc and living patients were informed and had the opportunity to decline study participation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Passaro A, Mok T, Peters S, et al. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J Thorac Oncol 2021;16:764-73. [Crossref] [PubMed]
- Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015;4:36-54. [PubMed]
- Leduc C, Merlio JP, Besse B, et al. Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: results of the nationwide French Cooperative Thoracic Intergroup (IFCT) program. Ann Oncol 2017;28:2715-24. [Crossref] [PubMed]
- Freedman AN, Klabunde CN, Wiant K, et al. Use of Next-Generation Sequencing Tests to Guide Cancer Treatment: Results From a Nationally Representative Survey of Oncologists in the United States. JCO Precis Oncol 2018;2:1-13. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Chen F, Chen N, Yu Y, et al. Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients With Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis. Front Oncol 2020;10:904. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Jin R, Zhao J, Xia L, et al. Application of immune checkpoint inhibitors in EGFR-mutant non-small-cell lung cancer: from bed to bench. Ther Adv Med Oncol 2020;12:1758835920930333. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Brindel A, Althakfi W, Barritault M, et al. Uncommon EGFR mutations in lung adenocarcinoma: features and response to tyrosine kinase inhibitors. J Thorac Dis 2020;12:4643-50. [Crossref] [PubMed]
- Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019;4:5. [Crossref] [PubMed]
- König D, Savic Prince S, Rothschild SI. Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations. Cancers (Basel) 2021;13:804. [Crossref] [PubMed]
- Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014;25:126-31. [Crossref] [PubMed]
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol 2020;21:1035-44. [Crossref] [PubMed]
- Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol 2020;61:167-79. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- He Q, Shi X, Zhu H, et al. A case treated with Crizotinib after secondary MET amplification of A double Rare L747S and G719S EGFR mutation Pulmonary Sarcomatoid Carcinoma. Ann Oncol 2020;31:544-6. [Crossref] [PubMed]
- Heigener DF, Schumann C, Sebastian M, et al. Afatinib in Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations Pretreated With Reversible EGFR Inhibitors. Oncologist 2015;20:1167-74. [Crossref] [PubMed]
- Boffetta P, Autier P, Boniol M, et al. An estimate of cancers attributable to occupational exposures in France. J Occup Environ Med 2010;52:399-406. [Crossref] [PubMed]
- Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889-902. [Crossref] [PubMed]
- Chen K, Yu X, Wang H, et al. Uncommon mutation types of epidermal growth factor receptor and response to EGFR tyrosine kinase inhibitors in Chinese non-small cell lung cancer patients. Cancer Chemother Pharmacol 2017;80:1179-87. [Crossref] [PubMed]
- Gristina V, Malapelle U, Galvano A, et al. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat Rev 2020;85:101994. [Crossref] [PubMed]
- Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer 2017;114:96-102. [Crossref] [PubMed]
- Yang JC, Schuler M, Popat S, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J Thorac Oncol 2020;15:803-15. [Crossref] [PubMed]
- Tanaka I, Morise M, Kodama Y, et al. Potential for afatinib as an optimal treatment for advanced non-small cell lung carcinoma in patients with uncommon EGFR mutations. Lung Cancer 2019;127:169-71. [Crossref] [PubMed]
- Li H, Wang C, Wang Z, et al. Efficacy and long-term survival of advanced lung adenocarcinoma patients with uncommon EGFR mutations treated with 1st generation EGFR-TKIs compared with chemotherapy as first-line therapy. Lung Cancer 2019;130:42-9. [Crossref] [PubMed]
- Chouaid C, Filleron T, Debieuvre D, et al. EGFR Exon 20 insertion: Prognostic and predictive values in advanced non-small cell lung cancer, a real-world study. J Clin Oncol 2021;39:9062. [Crossref]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Woo HS, Ahn HK, Lee HY, et al. Epidermal growth factor receptor (EGFR) exon 20 mutations in non-small-cell lung cancer and resistance to EGFR-tyrosine kinase inhibitors. Invest New Drugs 2014;32:1311-5. [Crossref] [PubMed]
- Chang JH, Han Y, Ryu YJ, et al. Uncommon EGFR mutations of lung adenocarcinoma in one tertiary institution, Korea: clinical characteristics and treatment response to tyrosine kinase inhibitors. Eur Respir J 2018;52:PA2853.
- Choudhury NJ, Schoenfeld AJ, Flynn J, et al. Response to Standard Therapies and Comprehensive Genomic Analysis for Patients with Lung Adenocarcinoma with EGFR Exon 20 Insertions. Clin Cancer Res 2021;27:2920-7. [Crossref] [PubMed]
- Santaniello A, Napolitano F, Servetto A, et al. Tumour Microenvironment and Immune Evasion in EGFR Addicted NSCLC: Hurdles and Possibilities. Cancers (Basel) 2019;11:1419. [Crossref] [PubMed]
- Yamada T, Hirai S, Katayama Y, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med 2019;8:1521-9. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016;107:1179-86. [Crossref] [PubMed]
- Xu J, Jin B, Chu T, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer 2016;96:87-92. [Crossref] [PubMed]


