A novel method for lymphadenectomy along the left laryngeal recurrent nerve during thoracoscopic esophagectomy for esophageal carcinoma
Introduction
Esophageal cancers are strongly invasive and malignant tumors, and lymph node (LN) dissection is crucial for staging and determining the prognosis associated with surgical treatment (1-3). In esophageal carcinoma, LN metastasis mostly likely occurs to the neck, mediastinum, and abdomen (4). LNs along the recurrent laryngeal nerves (RLNs) are located at the cervical base continuous to the upper mediastinum. As a major lymphatic chain into the neck, LNs along the RLNs are thought to be highly involved in metastasis (4,5). In addition, the left RLN can be easily injured during surgery because of its long course from the aortic arch to the neck.
Minimally invasive esophagectomy has been widely acceptable for the advantages of a magnified operative view and less invasiveness since the 1990s (6-9). Compared to the lateral decubitus position, better operative exposure has been demonstration in the prone position due to the working field formed by gravity and pneumothorax (10-12). Noshiro et al. (12) showed thoracoscopic esophagectomy in the prone position provides better surgeon ergonomics and better operative exposure around the left RLN during an aggressive esophagectomy (12). Oshikiri et al. (13) reported a method that draws the proximal portion of the divided esophagus and tissue that includes the left RLN and LNs through a gap between the vertebral body and the right scapula to improve lymphadenectomy along left RLN. However, lymphadenectomy along left RLN still thought to be a burdensome step because of limited operative exposure. To achieve a complete dissection of this portion, stable operative views and advanced surgical skill are required.
We herein present a novel method for en bloc dissection of the LNs along the left RLN during thoracoscopic esophagectomy, and retrospectively review the outcomes of this method in the semi-prone position.
Methods
We retrospectively reviewed the records of 110 consecutive patients with potentially curable thoracic esophageal cancer who received thoracoscopic esophagectomy in the semi-prone position between September 2014 and September 2015. All patients had squamous cell carcinoma located at the esophagus, histologically proven by esophagogastroduodenoscopy. Clinical stage was assessed by thoraco-abdominal enhanced computed tomography (CT), cervical ultrasonography, and endoscopic ultrasonography. Cancer was staged according to seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual (14). From September 2014 to April 2015, 60 patients received conventional surgery, and from April 2015 to September 2015, 50 patients received surgery with the novel method.
Under a combination of epidural and general anesthesia, a single-lumen endotracheal tube was inserted for ventilation. The patient was initially placed in the semi-prone position (Figure 1A). To introduce the thoracoscope (30°), an observation port was placed at the seventh intercostal space (ICS) along the mid-axillary line, and another 10-mm port was placed at the ninth ICS in the scapular line. Two 5-mm ports were placed at the third ICS along the mid-axillary line and just inferior to the tip of scapula, respectively (Figure 1B).
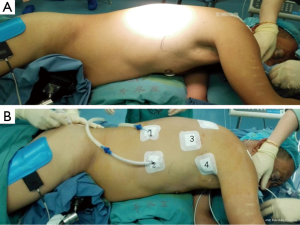
The chest cavity was inflated with a CO2 insufflation pressure of 8 to 10 mmHg. All procedures were performed by a surgeon who had performed more than 1,000 minimally invasive esophagectomies with patients in the semi-prone position with conventional extended lymphadenectomy.
Thoracic surgery procedures
In both conventional and novel method, the upper thoracic esophagus was mobilized from both sides of the mediastinal pleura. The LNs along the RLN were dissected, and the azygous vein was doubly ligated and transected. Circumferential mobilization of the thoracic middle and lower esophagus, with all surrounding mediastinal pleura, peri-esophageal tissues, and LNs was performed. The thoracic duct was carefully preserved. The tissues, including the left RLN and LNs, between the upper esophagus and trachea or bronchus were not released.
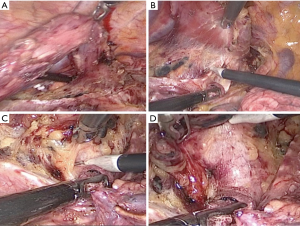
In the novel method, using a traction line to suspension the incompletely stripping esophagus (Figure 2A). Tissues including the left RLN and LNs were extended, which improved the operative exposure. The tissue is released in the area close to the trachea and left main bronchus to dissect the ventral and cranial borders. The trachea was pushed aside by an assistant to exposed surgical field (Figure 2B,C). The left RLN and LNs were easily recognizable (Figure 2D). First, the LNs below the aortic arch was dissected (Figure 3A). The left RLN to the thyroid gland and the LNs along the left RLN were separated using endoscopic scissors, keeping the remaining LNs attached to the esophagus (Figure 3B). The tracheoesophageal arteries were identified and ligated. Consequently, en bloc lymphadenectomy along the left RLN was performed (Figure 3C,D). Finally, circumferential mobilization of the esophagus was completely performed to cervical region.
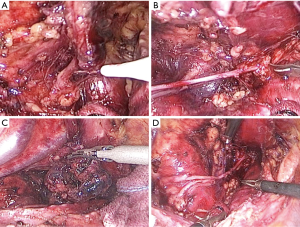
In conventional surgery, the tissue surrounding the left RLN and LNs was released from the undivided esophagus proximally, toward the neck. The esophagus needs to be continuously held by the surgeon’s left hand to expose the surgical field. Finally, the left RLN is sharply isolated from the explored tissue.
Lymphadenectomy of the entire subcarinal LNs was performed. All thoracic procedures were completed by placement of an intercostal drain and closure of the thoracic incisions.
Data collection and statistical analysis
Data collected included patient demographic information, the operative time for the lymphadenectomy along the left RLN and for the entire thoracic procedure, estimated blood loss, the number of harvested LNs during the entire thoracic procedure and along the left RLN, perioperative morbidity related to the left RLN, and the duration of postoperative hospital stay. Data were compared between the two groups.
Data collected were recorded in Microsoft Excel for further processing. All statistical analyses were performed using SPSS version 22.0. Student’s t-test and chi-square test were used to compare categorical variables, as appropriate The Mann-Whitney test was used to compare continuous variables. A two-sided P value <0.05 was considered to be statistically significant.
Results
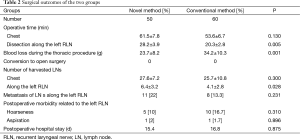
Patient and tumor characteristics are shown in Table 1. No significant differences were found between the two groups in age, sex, tumor location and depth, stage, and preoperative therapy (Table 1). Conversion to open surgery and intraoperative morbidity related to the left RLN did not occur in either group. During the thoracic procedure, there was no significant difference between the groups in the total operative time (chest) (61.5±7.8 vs. 53.5±6.7 min, P=0.13), while the operative time for lymphadenectomy along the left RLN was significantly longer in the novel method group than in the conventional method group (28.2±3.9 vs. 20.3±2.8 min, P=0.005). The number of harvested LNs along the left RLN in the novel method group was significantly less than in the conventional surgery group (6.4±3.2 vs. 4.1±2.8 min, P=0.028). Blood loss during thoracic procedures in the novel method group was significantly less than in the conventional surgery group (23.7±8.2 vs. 34.2±10.3 g, P=0.001). There was no significant difference of postoperative morbidity related to the left RLN and duration of the thoracic procedure between the groups. Hoarseness occurred in 10% of patients in the novel method group, and in 16.7% of patients in the conventional method group (P=0.31). None of the other operative results were significantly different between the two groups (Table 2).

Full table
Full table
Discussion
In this study, the novel method significantly increased the number of harvested LNs along the left RLN, and was associated with decreased blood loss compared to the conventional method. The survival rate has been shown to be significantly better in extensive three-field dissection than two-field dissection for patients with esophageal carcinoma (4,15,16). The rational for the aggressive procedure is based on high involvement of LNs along the RLN, and survival is significantly improved by radical dissection of these nodes (17,18). Completely lymphadenectomy of these nodes is recommended; however, significant postoperative morbidity and mortality are associated with this procedure (15,19). When the operative field is sufficiently exposed, lymphadenectomy in this area can be performed precisely and accurately through strictly defined and traced.
Minimally invasive esophagectomy via a thoracoscopic procedure is associated with less operative blood loss, fewer respiratory complications, and preserved respiratory vital capacity (11,20,21). However, complete dissection of the LNs along the left RLN during thoracoscopy is challenging and requires substantial technical skill. In the left upper mediastinum there are three important anatomical structures: the left RLN, which should be preserved; LNs, which should be dissected; and the left tracheoesophageal artery, which should be cut. These structures intermingle in limited working space, which complicates lymphadenectomy along the left RLN. The left RLN can easily injured during this procedure because of its long course from the aortic arch to the neck. Therefore, it is necessary to understand the interrelationship of these three anatomical structures, and their logical separation (13). In addition, the layer including the thoracic duct should be preserved.
In thoracoscopic procedures, the magnified operative view can enhance meticulous dissection of LNs, thereby preserving mediastinal structures. It is difficult to achieve satisfactory operative exposure with a patient in the left lateral decubitus position during thoracoscopic esophagectomy because mediastinal organs and structures are shifted to the left, the right lung is collapsed by single lung anesthesia and overlies the esophagus, and exudates collect in the posterior mediastinum of the operative field. The surgeon’s hand-eye coordination hard to obtain smoothly if the assistant exposes the operative views not quickly and correctly. In the prone position with an artificial pneumothorax, mediastinal structures are exposed spontaneously and exudates accumulate in the right anterior thorax, not in the operative field (10-12). Nevertheless, disadvantages of the prone position include the need of an assistant help to expose the operative field, emergent or elective conversion to open surgery, and endotracheal tube displacement. Some have suggested that the prone position is unsuitable for patients who received neoadjuvant chemoradiotherapy and for those with large tumors adjacent to neighboring organs (12,22,23). The semi-prone position not only maintains the advantages of the prone position, but also overcomes some of the disadvantages. The collapsed lung and structures are exposed spontaneously and exudates accumulate in the right anterior thorax. In addition, it is easier to manage displacement of the endotracheal tube.
The left RLN, LNs along the left RLN, and the tracheo-esophageal and primary esophageal arteries are integrated in a two-dimensional membrane in the left upper mediastinum (13). During conventional surgery, the esophagus needs to be continuously held by the surgeon’s left hand. The operative exposure is unsatisfactory, and distinguishing the left RLN and lymphadenectomy are difficult. However, with the noel method the esophagus is simply drawn away from trachea, the working space and operative field between esophagus and trachea is extended, the left RLN is easier to distinguish from the LNs, and the trachea-esophageal artery can be reliably ligated. In addition, the surgeon’s left hand is freed and lymphadenectomy along the left RLN easier and safer.
During lymphadenectomy along the left RLN, the operative time in the novel method group was significantly longer than in the conventional group. Better operative exposure was not associated with shortened operative time in this series. The initial learning curve does not appear to prolong the operative time. The chief surgeon in this study had performed over 1,000 cases of thoracoscopic esophagectomy in the semi-prone position (24). The main reason for the prolonged operative time was that it took more time to dissect the LNs completely and remove them en bloc. Furthermore, we should assess specific measurements of task performance or surgeon fatigue in future studies to advocate improved surgeon ergonomics.
Conclusions
In conclusion, the novel method for en bloc dissection of LNs along the left RLN with the patient in the semi-prone position is technically safe and feasible, and provides good surgeon ergonomics and excellent operative exposure. During lymphadenectomy along the left RLN, blood loss was less and the number of harvested LNs along the left RLN was greater in the novel method group. We believe complete en bloc dissection of the LNs along the RLN by thoracoscopy in the semi-prone position may possibly improve long-term outcomes.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 81400681).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [PubMed]
- Lin CS, Cheng CT, Liu CY, et al. Radical Lymph Node Dissection in Primary Esophagectomy for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg 2015;100:278-86. [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esoph- agus. Ann Surg 1994;220:364-72. [PubMed]
- Shiozaki H, Yano M, Tsujinaka T, et al. Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis Esophagus 2001;14:191-6. [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- McAnena OJ, Rogers J, Williams NS. Right thoracoscopically assisted oesophagectomy for cancer. Br J Surg 1994;81:236-8. [PubMed]
- Law S, Fok M, Chu KM, et al. Thoracoscopic esophagectomy for esophageal cancer. Surgery 1997;122:8-14. [PubMed]
- Kawahara K, Maekawa T, Okabayashi K, et al. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc 1999;13:218-23. [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203:7-16. [PubMed]
- Fabian T, Martin J, Katigbak M, et al. Thoracoscopic esophageal mobilization during minimally invasive esophagectomy: a head-to-head comparison of prone versus decubitus positions. Surg Endosc 2008;22:2485-91. [PubMed]
- Noshiro H, Iwasaki H, Kobayashi K, et al. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc 2010;24:2965-73. [PubMed]
- Oshikiri T, Yasuda T, Harada H, et al. A new method (the "Bascule method") for lymphadenectomy along the left recurrent laryngeal nerve during prone esophagectomy for esophageal cancer. Surg Endosc 2015;29:2442-50. [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-4. [PubMed]
- Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20. [PubMed]
- Igaki H, Tachimori Y, Kato H. Improved survival for patients with upper and/or middle mediastinal lymph node metastasis of squamous cell carcinoma of the lower thoracic esophagus treated with 3-field dissection. Ann Surg 2004;239:483-90. [PubMed]
- Watson A. Operable esophageal cancer: current results from the West. World J Surg 1994;18:361-6. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Li J, Shen Y, Tan L, et al. Is minimally invasive esophagectomy beneficial to elderly patients with esophageal cancer? Surg Endosc 2015;29:925-30. [PubMed]
- Fabian T, McKelvey AA, Kent MS, et al. Prone thoracoscopic esophageal mobilization for minimally invasive esophagectomy. Surg Endosc 2007;21:1667-70. [PubMed]
- Kuwabara K, Matsuda S, Fushimi K, et al. Quantitative comparison of the difficulty of performing laparoscopic colectomy at different tumor locations. World J Surg 2010;34:133-9. [PubMed]
- Wang H, Shen Y, Feng M, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg 2015;149:1006-14; discussion 1014-5.e4.


