The effectiveness of SGLT2 inhibitor in the incidence of atrial fibrillation/atrial flutter in patients with type 2 diabetes mellitus/heart failure: a systematic review and meta-analysis
Introduction
Atrial fibrillation/atrial flutter (AF/AFL) is the most common persistent arrhythmia. It currently affects 33 million people worldwide, and its incidence is expected to double in the next 40 years. For AF/AFL patients, premature mortality is increased by a factor of 2, and the risk of serious cardiovascular adverse events increases significantly (1). Type 2 diabetes mellitus (T2D) is an independent risk factor of AF/AFL, and the existence of T2D in AF/AFL is closely related to the aggravation of symptoms, the risk of stroke and embolism, and the increase of cardiovascular and cerebrovascular mortality (2,3). Therefore, the prevention and treatment of diabetes is of great significance to the control of AF. Sodium glucose cotransporter 2 inhibitors (SGLT2i) are a new type of oral hypoglycemic drug, which have been clearly highlighted in several large placebo-controlled randomized controlled trials (RCT) and meta-analyses: SGLT2i can potentially reduce heart failure (HF) and all-cause mortality, whether it is combined with T2D or not (4-7). However, some controversy remains regarding the difference of AF/AFL in T2D or HF patients, and the potential mechanism of SGLT2i may be the potential diuretic effect on myocardium and its influence on myocardial metabolism (8). A large-scale RCT, DECLARE-TIMI58, showed that dapagliflozin can reduce the incidence of AF/AFL adverse events in T2D patients with high risk, and this effect is consistent regardless of the patients' previous history of AF, atherosclerotic cardiovascular disease, or HF (9). For example, large randomized controlled studies such as DAPA-HF, CEREDENCE and EMPERIAL reported that the number of events in AF/AFL in SGLT2i intervention group decreased compared with the control group, but the research data of VERTIS CV and CAVAS showed that the number of events in AF/AFL in SGLT2i intervention group increased compared with the control group. This may be related to the sample size of the study, the follow-up time, and the number of patients lost to follow-up. The meta-analysis combined a large number of data to evaluate the consistency of the research results, so as to make systematic evaluation and summary, and proposed some new research questions, to point out the direction for further research. At the same time, the reliability of the results can be improved by measuring the accuracy of the efficacy and effect value of the clinical experimental studies of small samples. Therefore, we used meta analysis study the effect of SGLT2i on the occurrence and progression of AF/AFL in T2D/HF patients to play an important guiding role in the clinical diagnosis and treatment of AF complicated with T2D/HF. We located RCTs with available results to further evaluate the occurrence and progression of AF/AFL in T2D or HF patients and its differences according to age or gender. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-550/rc).
Methods
Inclusion and exclusion criteria
Research type
The research type was required to be RCT, open or double-blind trial, with a clear control group, the trial had been completed, and complete research results were available.
Participants
Patients aged 18 and over with T2D or HF were included, and patients with a short expected survival time such as those with type 1 diabetes, cardiogenic shock, severe liver and kidney damage, severe immune diseases, and malignant tumors were excluded.
Intervention measure
(I) Experimental group: a certain dose of SGLT2i was taken orally with or without other basic drugs.
(II) Control group: the dose of the experimental group was matched with the same dose of placebo or other drugs, with or without other basic drugs.
Outcome indicator
The main outcome measures were as follows: (I) incidence of AF/AFL; (II) cerebral infarction/infarction; (III) atrial/ventricular thrombosis; (IV) arterial thrombosis/embolism.
Secondary outcome indicators included the following: (I) arrhythmia or tachycardia; (II) ventricular tachycardia or supraventricular tachycardia; (III) palpitation; (IV) other arrhythmia.
Exclusion criteria
The exclusion criteria were as follows: repeated experimental research and overlapping data, duplicate published research and literature; documents with incomplete data or data which could not be extracted; comparison of hypoglycemic effect of non-RCT or SGLT2i combined with other drugs; research and literature in which the population does not meet the inclusion criteria; and research and literature on the failure of outcome indicators.
Information sources
The retrieval time was preset to 1 March, 2021. A computer search was conducted by 2 researchers in the Chinese Clinical Trial Registry website and ClinicalTrials.gov registration website, respectively, and they collected RCTs and articles involving patients who used SGLT2i to treat T2D and reported arrhythmia adverse events mainly including AF/AFL. The English-language keywords included the following: sodium glucose co transporter 2 inhibitors; type 2 diabetes; arrhythmology; atrial fibrillation/flutter; randomized controlled trial, and so on. The Chinese-language keywords included the following: sodium-glucose cotransporter 2 inhibitor; type 2 diabetes; arrhythmia; atrial fibrillation/atrial flutter; randomized controlled study, and so on. The search strategy was as follows: (Sodium-glucose cotransporter 2 inhibitors OR Dapagliflozin OR Empagliflozin OR Canagliflozin OR Ertugliflozin) AND Type 2 diabetes AND Randomized controlled trial AND (Arrhythmology OR Atrial fibrillation/flutter).
Selection process and data extraction
The tasks of independent of screening research, literature, assessment and inclusion of research, literature, and extraction of required data were completed independently by 2 researchers in turn, who then made relevant tables, and finally cross-checked between themselves repeatedly. When screening documents, the types and titles of documents were read first, followed by the abstracts and full texts to judge whether the outcome indicators were included or not. The contents of selection data extraction included the following: (I) basic information features of included research and literature were as follows: research topic, national clinical trial (NCT) registration number, years, sample size, average age, basic diseases of included population, gender, intervention drugs, control group drugs, and follow-up time. (II) The key factors needed to evaluate the risk of literature bias; (III) required outcome index data.
Bias risk assessment included in the study
When evaluating the bias risk, we referred to the bias risk evaluation tool of RCTs recommended by Cochrane Handbook for Systematic Reviews of Intervention 5.1.0 (The Cochrane Collaboration, Copenhagen, Denmark, 2011). Two researchers worked independently and assessed the quality of included RCTs using Cochrane RoB 2.0. for assessing the risk of bias. This tool assesses randomization, allocation concealment, blinding, incomplete outcome data and selective reporting. Each of domains is rated as having a high-, moderate- or low-risk of bias. Publication bias was also qualitatively detected by drawing funnel plots. The asymmetry on both sides may have a publication bias. Further quantitative detection using harbord’s and begg’s test through STATA 17.0. If P>0.05 indicates no obvious publication bias, otherwise there is a publication bias.
Statistical analysis
All the above-mentioned data obtained are classified variables, without continuous variables or grade variables. After consultation between 2 researchers, OR was used as the effect analysis statistic. The effects were estimated by interval, and 95% CI was provided by default. Hypothesis testing was conducted to judge whether an effect was statistically significant according to the P value. The significance level of meta-analysis was set at α=0.05. The acquired data were statistically analyzed by RevMan 5.3 software (Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark, 2014), and a forest map was drawn. The heterogeneity analysis included in the research results adopting the Q test, and the test standard was set at α=0.1. At the same time, the heterogeneity was quantitatively judged by I2 value. If there was no statistical heterogeneity among the research results (assuming I2<50%), the fixed effects model was used for meta-analysis. If there was statistical heterogeneity among the research results (assuming I2≥50%), it was necessary to analyze the source of heterogeneity, exclude obvious clinical heterogeneity and methodological heterogeneity, and then use the random effects model for meta-analysis. If obvious clinical heterogeneity was found, subgroup analysis or sensitivity analysis could be used for further evaluation, or the merger was cancelled and descriptive analysis was conducted. In subgroup analysis, the data were analyzed with the same statistical effects model. Finally, painting the Galbraith plot by STATA 17.0 to further evaluate the heterogeneity of the primary outcomes. The sensitivity analysis of the main outcome was performed by eliminating it one by one to evaluate the reliability of the results.
Results
Study selection
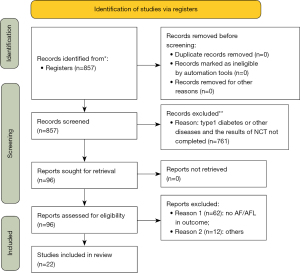
Through the above process, a computer network search was conducted for related research, followed by discussion and sorting of the retrieved articles. A total of 857 related studies were obtained in the initial examination. After screening layer by layer, 22 studies were finally included in the quantitative analysis, with a total of 52,951 participants. The specific screening process and results included in the literature are displayed in Figure 1.
Study characteristics
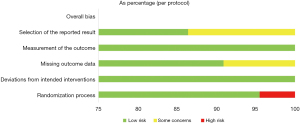
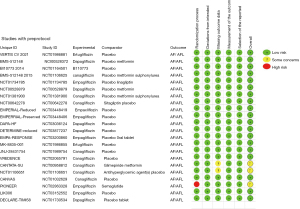
After discussion between 2 researchers, the general characteristic data (Table 1) and the bias risk evaluation results (Figures 2,3) included in the analysis were summarized. It shows an overall low risk in the individual studies.
Table 1
| Research | Research design | NCT number | Sample size | Average age (years) | Condition | Gender | Intervention drugs | Control group | Follow-up time | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Female (%) | |||||||||
| VERTIS CV (10) | RCT | NCT01986881 | 8,246 | 64.4±8.1 | T2D | 5,769 | 2,477 | 30 | Ertugliflozin | Placebo | 6 y |
| BMS-512148 (11) | RCT | NCT00528372 | 407 | <65 | T2D | 50.5 | Dapagliflozin | Placebo | 102 w | ||
| BI10773 (12) | RCT | NCT01164501 | 741 | 63.9±8.8 | T2D Renal insufficiency | 430 | 308 | 41.7 | Empagliflozin | Placebo | 52 w |
| CANTATA-SU (13) | RCT | NCT00968812 | 1,452 | 56.2±9.22 | T2D | 756 | 694 | 47.9 | Canagliflozin | Glimepiride metformin | 104 w |
| NCT01106651 (2014) (14) | RCT | NCT01106651 | 716 | 63.6±6.24 | T2D | 396 | 318 | 44.5 | Canagliflozin | Placebo | 104 w |
| Sulfonylureas | |||||||||||
| DPP-4 inhibitor | |||||||||||
| Metformin | |||||||||||
| Insulin and its combination | |||||||||||
| CANVAS (15) | RCT | NCT01032629 | 4,330 | 62.4±8.02 | T2D High risk factors of cardiovascular disease | 2,861 | 1,469 | 33.9 | Canagliflozin | Placebo | 338 w |
| MK-8835-001 (16) | RCT | NCT01986855 | 468 | 67.3±8.6 | T2D | 231 | 236 | 50.5 | Ertugliflozin | Placebo | 52 w |
| JNJ-28431754 (17) | RCT | NCT01989754 | 5,813 | 64±8.35 | T2D Nephrotic syndrome | 3,648 | 2,164 | 37.2 | Canagliflozin | Placebo | 156 w |
| CREDENCE (18) | RCT | NCT02065791 | 4,401 | 63±9.2 | T2D Diabetic nephropathy | 2,907 | 1,494 | 66.1 | Canagliflozin | Placebo | 66 m |
| PIONEER 2 (19) | RCT | NCT02863328 | 822 | 58±10 | T2D | 415 | 406 | 49.5 | Empagliflozin | Semaglutide | 52 w |
| LIK006 (20) | RCT | NCT03152552 | 125 | 67.8±9.17 | T2D Cardiac failure | 89 | 35 | 28.2 | Lisiglitazone/Empagliflozin | Placebo | 36 w |
| DECLARE-TIMI58 (21) | RCT | NCT01730534 | 17,190 | 63.9±6.8 | T2D | 10,738 | 6,422 | 62.6 | Dapagliflozin | Placebo Metformin sulfonylureas |
5.2 y |
| High risk factors of cardiovascular disease | |||||||||||
| CANTATA-MSU (22) | RCT | NCT01106625 | 469 | 56.7±9.3 | T2D | 239 | 230 | 49.0 | Canagliflozin | Placebo | 52 w |
| Metformin sulfonylureas | |||||||||||
| NCT01734785 (2016) (23) | RCT | NCT01734785 | 332 | 55.2±9.7 | T2D | 198 | 134 | 40.4 | Empagliflozin | Placebo | 24 w |
| Metformin linagliptin | |||||||||||
| NCT00528879 (2015) (24) | RCT | NCT00528879 | 546 | 53.9 | T2D | 292 | 254 | 46.5 | Dapagliflozin | Placebo | 102 w |
| Metformin | |||||||||||
| NCT01381900 (2014) (25) | RCT | NCT01381900 | 678 | 56.3±8.94 | T2D | 362 | 314 | 46.4 | Canagliflozin | Placebo | 18 w |
| Metformin sulfonylureas | |||||||||||
| NCT00642278 (2013) (26) | RCT | NCT00642278 | 451 | 52.9±8.06 | T2D | 236 | 215 | 47.7 | Canagliflozin | Placebo | 12 w |
| Sitagliptin | |||||||||||
| EMPERIAL-Reduced (27) | RCT | NCT03448419 | 312 | 69.0±10.2 | HFrEF | 232 | 80 | 25.6 | Empagliflozin | Placebo | 12 w |
| EMPERIAL-Preserved (28) | RCT | NCT03448406 | 315 | 73.5±8.8 | HFmEF | 179 | 136 | 43.2 | Empagliflozin | Placebo | 12 w |
| DAPA-HF (29) | RCT | NCT03036124 | 4,744 | 66.3±10.9 | HFrEF Chronic heart failure | 3,635 | 1,109 | 23.4 | Dapagliflozin | Placebo | 27.8 m |
| DETERMINE-reduced (30) | RCT | NCT03877237 | 313 | 67.8±10.41 | HFrEF | 233 | 80 | 25.6 | Dapagliflozin | Placebo | 16 w |
| EMPA-RESPONSE (31) | RCT | NCT03200860 | 80 | 76 | Acute/congestive decompensated heart failure | 53 | 26 | 67.1 | Empagliflozin | Placebo | 30 d |
NCT, national clinical trial; RCT, randomized controlled trial; T2D, type 2 diabetes; HFrEF, heart failure with reduced ejection fraction; HFmEF, heart failure with mid-range ejection fraction.
Incidence of AF/AFL and supraventricular tachycardia or ventricular tachycardia
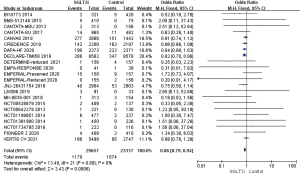
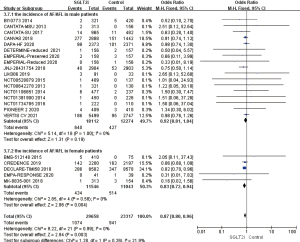
A total of 22 RCTs that mentioned AF/AFL occurring in patients with T2D or HF were included in heterogeneity analysis. Using the fixed effects model, OR =0.82, 95% CI: 0.73 to 0.93, and P=0.002, and the difference was statistically significant. It was suggested that the incidence of AF/AFL in the SGLT2i group was 18% lower than that in the placebo group, which indicated an obvious protective effect. A total of 10 RCTs mentioned that supraventricular tachycardia or ventricular tachycardia occurred during medication. The heterogeneity of the results was analyzed by I2 as 23%. Using the fixed effects model, the results showed that OR =0.83, 95% CI: 0.61 to 1.12, and P=0.21, the difference was not statistically significant (Figure 4). Galbraith plot suggests the symmetrical distribution of the studies on both sides of the regression line and suggests no significant heterogeneity (Figure S1).
Incidence of arrhythmia
A total of 22 RCT mentioned the adverse events of arrhythmia in patients with T2D or HF during medication. The heterogeneity analysis of the results showed that the I2 was 0% and the Q test showed P>0.05. Using the fixed effects model, the OR =0.86, 95% CI: 0.79 to 0.94, and P=0.0006, and the difference was statistically significant. The results showed that the incidence of arrhythmia in the SGLT2i group was 14% lower than that in the control group (Figure 5).
Incidence of cerebral infarction, intracardiac thrombosis, and arterial thrombosis
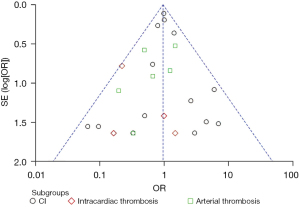
A total of 14 RCTs mentioned that patients had cerebral infarction during medication, and the results were analyzed by heterogeneity, whereby I2 was 10%. Using the fixed effects model, the OR =1.02, 95% CI: 0.80 to 1.28, and P=0.89, the difference was not statistically significant. A total of 5 RCTs mentioned the occurrence of intracardiac thrombosis during the course of medication, and the heterogeneity of the results was analyzed. Using the fixed effects model, the results showed that OR =0.31, 95% CI: 0.10 to 0.91, and P=0.03, which was statistically significant, suggesting that the incidence of intracardiac thrombosis in the SGLT2i group was significantly lower than that in the placebo group. A total of 6 RCTs mentioned the occurrence of arterial thrombosis in the course of medication, and the heterogeneity of the results was analyzed. Using the fixed effects model, OR =0.78 ,95% CI: 0.43 to 1.44, and P=0.43, the difference was not statistically significant (Figure 6). Galbraith plot suggests the symmetrical distribution of the studies on both sides of the regression line and suggests no significant heterogeneity (Figure S2).

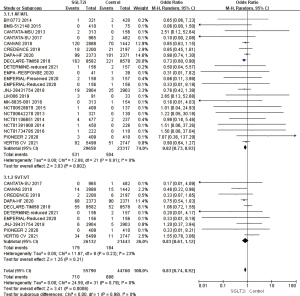
Comparison of the incidence of AF/AFL among different kinds of SGLT2i drugs
A total of 2 RCTs used ertugliflozin as they type of SGLT2i intervention drug. The heterogeneity analysis of the results showed that I2=58% (>50%). Using the random effects model, OR =0.57, 95% CI: 0.11 to 2.88, and P=0.50, and the difference was not statistically significant. A total of 8 RCTs used canagliflozin as they type of SGLT2i intervention drug. The heterogeneity of the results was analyzed, which showed that OR =0.87, 95% CI: 0.75 to 1.00, and P=0.05were not statistically significant. A total of 5 RCTs used dapagliflozin as they type of SGLT2i intervention drug. The results revealed OR =0.85, 95% CI: 0.74 to 0.98, and P=0.03, and the difference was statistically significant. It was suggested that the incidence of AF/AFL in the SGLT2i group was 15% lower than that in the placebo group, and there was a significant difference. A total of 7 RCTs used the SGLT2i intervention drug of empagliflozin. The results showed that OR=0.81, 95% CI: 0.36 to 1.83, and P=0.62, which was not statistically significant. In summary, it was suggested that the use of SGLT2i intervention drug dapagliflozin can reduce the incidence of AF/AFL by 15%, which has a significant protective effect, while ertugliflozin, canagliflozin, and empagliflozin have no significant effect on reducing the incidence of AF/AFL (Figure 7).
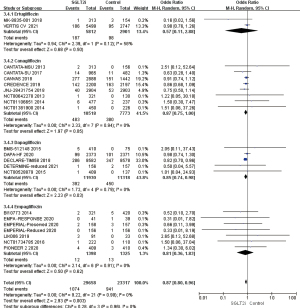
Disease differences in the incidence of AF/AFL
A total of 17 RCTs mentioned the AF/AFL rate of patients with T2D. The analysis of the results showed that OR =0.80, 95% CI: 0.69 to 0.92, and P=0.002 were measured by using the fixed effects model, and the difference was statistically significant. It was suggested that in T2D, the incidence of AF/AFL in the SGLT2i group was reduced by 20% compared with the control group, which was an obvious protective effect. A total of 5 RCTs which mentioned the incidence of AF/AFL in patients with HF were analyzed for heterogeneity. Using the fixed effects model, the results showed that OR =0.94, 95% CI: 0.72 to 1.24, P=0.67, the difference was not statistically significant, further analysis in patients with heart failure with reduced ejection fraction (HFrEF), the difference was not statistically significant (Figure 8).
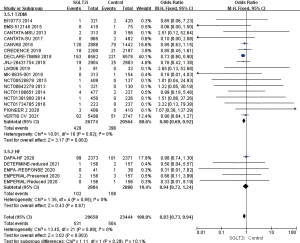
Age difference in the incidence of AF/AFL
A total of 14 RCTs included patients with AF/AFL who were younger than 65 years old. Of these, 13 studies were excluded due to large bias. The heterogeneity of the results was analyzed using a fixed effects model. The results showed that OR =0.91, 95% CI: 0.79 to 1.05, and P=0.19 were measured, and the difference was not statistically significant. A total of 8 RCTs included AF/AFL patients aged over 65 years. The results of heterogeneity analysis yielded I2%=0%, so the fixed effects model was used, which revealed OR =0.89, 95% CI: 0.74 to 1.06, P=0.18. The difference was not statistically significant, suggesting that SGLT2i has no significant age difference in the incidence of AF/AFL in patients with T2D (Figure 9).
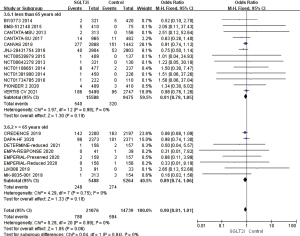
Gender differences in the incidence of AF/AFL
The AF/AFL patients were predominantly male in 17 studies, the results of heterogeneity analysis, I2%=0%, using a fixed effects model, measured OR =0.92, 95% CI: 0.81 to 1.04, P=0.19, the difference was not statistically significant. The AF/AFL patients were predominantly female in 5 studies, the heterogeneity analysis of the results, I2%=0%, using the fixed effect model, measured OR =0.83, 95% CI: 0.72 to 0.94, P=0.004, the difference was statistically significant, suggesting that the incidence of AF/AFL in female patients in the SGLT2i group was 17% lower than that in the placebo group, which had obvious protective effect. To sum up, it was suggested that the protective effect of SGLT2i on the incidence of AF/AFL in female patients was better than that in male patients, and there was a significant gender difference (Figure 10).
Sensitivity analysis
Sensitivity analysis was carried out according to the efficacy indexes of drugs (incidence of arrhythmia, AF/AFL, supraventricular tachycardia and ventricular tachycardia, cerebral infarction, intracardiac thrombus and arterial thrombosis, drug type, disease, gender, and age difference). The effectiveness index results did not change significantly, suggesting that the results were stable.
Reporting biases
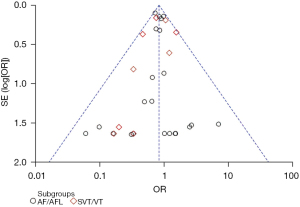
According to the outcome of AF/AFL and its complications, a funnel chart (Figure 8) was drawn using RevMan 5.3 software to qualitatively test the publication bias. The results showed that the left and right distribution of each study point was basically symmetrical, and they were all in the area surrounded by 2 diagonal lines. It notes that the included studies were no publication bias, supporting the reliability of the results. Further analysis, AF/AFL as the outcome index is symmetrical distributed on both sides of the funnel, and more top studies, supporting the effectiveness of SGLT2i in patients with T2D/HF, while the treatment of intracardiac thrombosis/arterial thrombosis distribution on left sides of the funnel map, and a considerable number of studies focused on the bottom of the funnel, indicating that such studies maybe limited to small samples and tend to publish positive results, which requires large samples to further prove the reliability of the results (Figures 11,12). Quantitative test of publication bias for outcome of AF/AFL and its complications using STATA 17.0 software. For AF/AFL outcome, Harbord test suggested no small study: z=−0.15, P=0.8798>0.05, begg test suggested: z=−1.25, P=0.2239>0.05, all showed no publication bias. For the outcome of AF/AFL complications, Harbord test suggested no small study: z=−0.15, P=0.1323>0.05, begg test suggested: z=−0.91, P=0.3875>0.05, all indicating that there is no publication bias. It supports the reliability of the study findings.
Discussion
As a new type of hypoglycemic drug, SGLT2i acts through renal tubular sodium-glucose symporter and has shown great benefits in reducing blood glucose through osmotic diuresis, especially in T2D patients. However, mounting studies have found that SGLT2i has a huge beneficial effect on cardiovascular disease, especially HF (32,33), but there have been few studies on the impact of AF/AFL and its complications, and there have been no clinical trials on patients with AF/AFL. After retrieval of current studies of SGLT2i on T2D and HF patients, meta-analysis showed that the incidence of AF/AFL and intracardiac thrombosis in patients treated with SGLT2i were significantly lower than those in placebo groups; the incidence of AF/AFL decreased by 18%, and the rate of intracardiac thrombus formation decreased by 69%. Among them, the protective effect of dapagliflozin on T2D female patients was more obvious, and there was no significant age difference in its protective effect. The difference was statistically significant. Conversely, empagliflozin, canagliflozin, and ertugliflozin exerted no significant protective effects on the incidence of AF/AFL, stroke and arterial thrombosis in men with HF, and had no significant protective effects on supraventricular and ventricular tachycardia, but they had obvious protective effects on global arrhythmias including slow arrhythmias. Therefore, they had significant protective effects for other arrhythmias. In particular, the role of slow arrhythmia still requires further exploration. From the results of heterogeneity testing, the above analysis basically had no obvious heterogeneity, which shows that the current results are reliable.
Previously, a meta-analysis of the incidence of AF/AFL in T2D patients conducted by Li et al. found that regardless of age, body weight, glycosylated hemoglobin (HbA1c), and baseline systolic blood pressure, SGLT2i may give specific AF/AFL reduction benefits in T2D susceptible people, with a reduction rate of up to 24% (34). The benefits of this reduction in AF/AFL may be partly due to the pharmacological effects of reducing the incidence of HbA1c, weight, blood pressure, and HF. However, there was no further detailed discussion on the reduction rate of AF/AFL complications and its differences according to gender and age, it was only revealed that it has no obvious protective effect on cerebrovascular events, and the curative effect of dapagliflozin is significant, which is consistent with the results of this meta-analysis. However, the reason for the inconsistent incidence of AF/AFL may be that the meta-analysis included not only T2D patients but also HF patients, which yielded an AF/AFL rate of 18%. Further subgroup analysis showed that the combined incidence of AF/AFL in 17 studies (including the latest study and further evaluation of quality) could be reduced by 20%. There was a certain difference between the two. In addition, Li et al.’s research on the incidence of AF/AFL was limited to T2D patients, and its differential role in HF patients was not further explored. This study complements this limitation and finds that it does not show the same effect on the incidence of AF/AFL in patients with HF as in T2D, and its reduction is not statistically significant, but the current research on the effectiveness and safety of SGLT2i in patients with HF is limited, so its protective effect needs to be verified by further research.
As a hypoglycemic drug, the mechanism of SGLT2i reducing the incidence of AF/AFL may be related to atrial fibrosis and cardiac remodeling. On the one hand, increased glucose excretion will essentially lead to additional osmotic diuresis, which will lead to lower arterial blood pressure, delay myocardial structural remodeling, and then lead to atrial fibrosis (35). On the other hand, in the case of diuresis, the loss of glucose in urine may help to lose weight and maintain it for a prolonged period, thereby reducing the progress of cardiac remodeling (36). In addition, this study further analyzed the effect of SGLT2i on myocardial ischemia and found that SGLT2i can significantly improve myocardial ischemia and coronary artery ischemia. Long-term myocardial ischemia is closely related to the occurrence of arrhythmias and cardiac structural remodeling, which may further explain the mechanism of SGLT2i improving the incidence of AF/AFL, but further research is still needed.
The meta-analysis further revealed that SGLT2i can significantly reduce the incidence of intracardiac thrombosis, but has a poor effect on cerebral infarction and arterial thrombosis. The mechanism may be related to SGLT2i reducing the incidence of AF/AFL and arrhythmia in patients with T2D/HF. The study found that 99% of thromboses in patients with non-valvular AF came from the left atrial appendage. The mechanism can be explained by the fact that AF itself enhances the coagulation cascade, impairing platelet reactivity and fibrinolysis, and these processes are magnified along with pre-existing complications. Secondly, structural changes such as atrial fibrosis and endothelial dysfunction are related to the development of AF, which promotes further atrial remodeling, thus providing a suitable platform for clot formation and subsequent embolism (37). However, SGLT2i is not effective in the treatment of cerebral infarction or arterial thrombosis, which may be because the formation mechanism of cerebral infarction or arterial thrombosis is related to intracardiac thrombosis.
The meta-analysis found that the curative effect of female patients with SGLT2i was better than that of men. Medicare data show that 55% of cases of AF in 2007 were female (38). Westerman et al. reported that there are great gender differences in the occurrence of AF (39). The pathogenesis, etiology, and response to treatment of AF are different between women and men, and the risk of stroke is significantly higher for women than men. At the same time, as a risk factor, the congestive HF, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74, and gender category (female) (CHA2DS2-VASC) score of women was 1 point higher than that of men, suggesting that the incidence of thrombosis in women with AF was significantly higher than that in men. The use of SGLT2i can significantly improve the incidence of female AF/AFL and provide important reference value for future clinical research.
SGLT2i has been shown to reduce the risk of HF events for patients with T2D or at high risk of cardiovascular disease. The United States Food and Drug Administration has expanded the regulatory labels of empagliflozin to reduce cardiovascular risk in patients with T2D and cardiovascular disease. The inclusion of SGLT2i in people without diabetes is being actively studied in the context of the treatment of HF patients. Although there is cumulative data to support such treatments, cardiologists’ infrequent use of SGLT2i may be due to a lack of familiarity (40). At the same time, China also approved SGLT2i for T2D combined with HF, but the same problems exist with the use of SGLT2i. Continuing to strengthen the research on SGLT2i will benefit more clinical T2D/HF or AF/AFL patients.
Overall, SGLT2i can effectively reduce the risk of AF/AFL and intracardiac thrombosis in T2D patients, so the use of SGLT2i in T2D patients should be considered to prevent the occurrence of AF/AFL and intracardiac thrombosis, and there are significant gender differences. However, whether it can improve the risk of AF/AFL in patients with HF requires many large-scale placebo-controlled trials in the HF population to clarify whether SGLT2i shows beneficial effects in reducing arrhythmias such as AF/AFL and tachycardia.
The shortcomings and limitations of this study were as follows: there was a significant relationship between the occurrence of AF/AFL and age, but the meta-analysis did not reveal that there is a significant age difference in the reduction of AF/AFL incidence by SGLT2i. Whether this was related to the lack of inclusion in the study or the need for more detailed gender stratification needs to be verified by further analysis. At present, the number of studies on SGLT2i in patients with HF is limited, and further studies are needed to explore the role of SGLT2i in patients with HF. In the study on the efficacy of SGLT2i in patients with T2D, part of the control group was not a placebo control, which may have affected the results of the study to some extent. The safety of SGLT2i treatment was not further analyzed, which needs to be explored in future.
Conclusions
This meta-analysis found that SGLT2i, as a new oral hypoglycemic drug, has a good effect not only on HF patients, but also on the incidence of AF/AFL in T2D patients, which can be reduced by 20%. The incidence of intracardiac thrombosis decreased by 69%. Among the types of SGLT2i, the effect of dapagliflozin is better than that of empagliflozin, cagagliflozin, and ertugliflozin, and the effect is better for females than males. However, due to the limited statistical data, it was found that the effect of taking SGLT2i is similar in the elderly and young people. Conversely, SGLT2i has no significant effect on the incidence of AF/AFL in patients with HF, but a large number of studies have shown that SGLT2i has a significant effect on patients with HF, whether it is HFrEF or heart failure with preserved ejection fraction (HFpEF), the conclusion remains to be further verified. This study will provide some guidance for the clinical use of SGLT2i. With the gradual deepening of SGLT2i research and widespread clinical use, it will play a more extensive value in the cardiovascular field.
Acknowledgments
Funding: This study was supported by Shanxi Youth Top Talent Fund, Organization Department of Shanxi Provincial CPC Committee (No. 2016005).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-550/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-550/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wijesurendra RS, Casadei B. Mechanisms of atrial fibrillation. Heart 2019;105:1860-7. [Crossref] [PubMed]
- Wang A, Green JB, Halperin JL, et al. Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol 2019;74:1107-15. [Crossref] [PubMed]
- Plitt A, McGuire DK, Giugliano RP. Atrial Fibrillation, Type 2 Diabetes, and Non-Vitamin K Antagonist Oral Anticoagulants: A Review. JAMA Cardiol 2017;2:442-8. [Crossref] [PubMed]
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019;380:2295-306. [Crossref] [PubMed]
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31-9. [Crossref] [PubMed]
- Böhm M, Slawik J, Brueckmann M, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. Eur J Heart Fail 2020;22:126-35. [Crossref] [PubMed]
- Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018;61:2108-17. [Crossref] [PubMed]
- Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J 2015;36:3250-7. [Crossref] [PubMed]
- Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation 2020;141:1227-34. [Crossref] [PubMed]
- Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease, The VERTIS CV Study (MK-8835-004). 2021. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01986881?term=NCT01986881&draw=1&rank=1
- A Phase III Study of BMS-512148 (Dapagliflozin) in Patients With Type 2 Diabetes Who Are Not Well Controlled With Diet and Exercise. 2015. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT00528372?term=NCT00528372&draw=2&rank=1
- Efficacy and Safety of Empagliflozin (BI 10773) in Patients With Type 2 Diabetes and Renal Impairment. 2014. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01164501?term=NCT01164501&draw=2&rank=1
-
CANagliflozin Treatment And Trial Analysis-Sulfonylurea (CANTATA-SU) SGLT2 Add-on to Metformin Versus Glimepiride 2017 . Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT00968812?term=NCT00968812&draw=2&rank=1 - A Safety and Efficacy Study of Canagliflozin in Older Patients (55 to 80 Years of Age) With Type 2 Diabetes Mellitus. 2014. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01106651?term=NCT01106651&draw=2&rank=1
- CANVAS - CANagliflozin cardioVascular Assessment Study (CANVAS). 2018. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01032629?term=NCT01032629&draw=2&rank=1
- A Study of the Efficacy and Safety of Ertugliflozin in Participants With Type 2 Diabetes Mellitus With Stage 3 Chronic Kidney Disease Who Have Inadequate Glycemic Control on Antihyperglycemic Therapy (MK-8835-001). 2018. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01986855?term=NCT01986855&draw=2&rank=1
- A Study of the Effects of Canagliflozin (JNJ-28431754) on Renal Endpoints in Adult Participants With Type 2 Diabetes Mellitus (CANVAS-R). 2018. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01989754?term=NCT01989754&draw=2&rank=1
-
Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) 2019 . Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT02065791?term=NCT02065791&draw=2&rank=1 - Efficacy and Safety of Oral Semaglutide Versus Empagliflozin in Subjects With Type 2 Diabetes Mellitus (PIONEER2). 2020. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT02863328?term=NCT02863328&draw=2&rank=1
- A Dose Finding Study to Assess the Effect of LIK066 Compared to Placebo or Empagliflozin in Patients With Type 2 Diabetes Mellitus and Heart Failure. 2019. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT03152552?term=NCT03152552&draw=2&rank=1
- Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). 2019. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT 01730534?term=NCT01730534&draw=2&rank=1
-
The CANTATA-MSU Trial (CANagliflozin Treatment And Trial Analysis - Metformin and SUlphonylurea) 2013 . Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01106625?term=NCT01106625&draw=2&rank=1 - Safety and Efficacy of the Combination of Empagliflozin and Linagliptin Compared to Linagliptin Alone Over 24 Weeks in Patients With Type 2 Diabetes. 2016. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01734785?term=NCT01734785&draw=2&rank=1
- A Phase III Study of BMS-512148 (Dapagliflozin) in Patients With Type 2 Diabetes Who Are Not Well Controlled on Metformin Alone. 2015. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT00528879?term=NCT00528879&draw=2&rank=1
- A Efficacy, Safety, and Tolerability Study of Canagliflozin in Patients With Type 2 Diabetes Mellitus With Inadequate Glycemic Control on Metformin Alone or in Combination With a Sulphonylurea.2014. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01381900?term=NCT01381900&draw=2&rank=1
- An Efficacy, Safety, and Tolerability Study of Canagliflozin (JNJ-28431754) in Patients With Type 2 Diabetes. 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT00642278
- This Study Tests Empagliflozin in Patients With Chronic Heart Failure With Reduced Ejection Fraction (HFrEF). The Study Looks at How Far Patients Can Walk in 6 Minutes and at Their Heart Failure Symptoms. 2020. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03448419?term=NCT03448419&draw=2&rank=1
- This Study Tests Empagliflozin in Patients With Chronic Heart Failure With Preserved Ejection Fraction (HFpEF). The Study Looks at How Far Patients Can Walk in 6 Minutes and at Their Heart Failure Symptoms. 2020. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03448406?term=NCT03448406&draw=2&rank=1
-
Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure (DAPA-HF) 2020 . Available online: https://www.clinicaltrials.gov/ct2/show/NCT03036124?term=NCT03036124&draw=2&rank=1 - DETERMINE-reduced - Dapagliflozin Effect on Exercise Capacity Using a 6-minute Walk Test in Patients With Heart Failure With Reduced Ejection Fraction.2021. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03877237?term=NCT03877237&draw=2&rank=1
-
Effects of Empagliflozin on Clinical Outcomes in Patients With Acute Decompensated Heart Failure (EMPA-RESPONSE) 2020 . Available online: https://www.clinicaltrials.Gov/ct2/show/results/NCT03200860?term=NCT03200860&draw=2&rank=1 - Hernandez M, Sullivan RD, McCune ME, et al. Sodium-Glucose Cotransporter-2 Inhibitors Improve Heart Failure with Reduced Ejection Fraction Outcomes by Reducing Edema and Congestion. Diagnostics (Basel) 2022;12:989. [Crossref] [PubMed]
- Htoo PT, Buse J, Cavender M, et al. Cardiovascular Effectiveness of Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists in Older Patients in Routine Clinical Care With or Without History of Atherosclerotic Cardiovascular Diseases or Heart Failure. J Am Heart Assoc 2022;11:e022376. [Crossref] [PubMed]
- Li WJ, Chen XQ, Xu LL, et al. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol 2020;19:130. [Crossref] [PubMed]
- Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265-325. [Crossref] [PubMed]
- Kim YG, Han KD, Choi JI, et al. The impact of body weight and diabetes on new onset atrial fibrillation: a nationwide population based study. Cardiovasc Diabetol 2019;18:128. [Crossref] [PubMed]
- Ding WY, Gupta D, Lip GYH. Atrial fibrillation and the prothrombotic state: revisiting Virchow's triad in 2020. Heart 2020;106:1463-8. [Crossref] [PubMed]
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67-e492. [Crossref] [PubMed]
- Westerman S, Wenger N. Gender Differences in Atrial Fibrillation: A Review of Epidemiology, Management, and Outcomes. Curr Cardiol Rev 2019;15:136-44. [Crossref] [PubMed]
- Vardeny O, Vaduganathan M. Practical Guide to Prescribing Sodium-Glucose Cotransporter 2 Inhibitors for Cardiologists. JACC Heart Fail 2019;7:169-72. [Crossref] [PubMed]