Reoperations after tricuspid valve repair: re-repair versus replacement
Introduction
Although most patients with tricuspid valve (TV) disease undergo TV repair including tricuspid annuloplasty (TAP), recurrent tricuspid regurgitation (TR) after initial repair is not infrequent during the follow-up, even after TAP using prosthetic rings (1-4). Failure of TAP and the resultant redo-TV surgery can be associated with high surgical risks (5,6). However, data on the results of reoperative TV surgery are scarce. This study was designed to evaluate the outcomes of tricuspid reoperations after initial TAP, to compare the results of TV re-repair with those of tricuspid valve replacement (TVR), and to analyze the factors associated with recurrent TR after TV re-repair.
Patients and methods
Patient characteristics
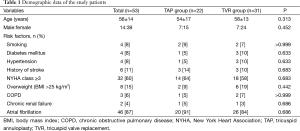
Our Institutional Review Board reviewed and approved the study protocol as a minimal risk retrospective study (no. H-1407-049-593) that did not require individual consent of the subjects. From January 1994 to December 2012, 53 patients (56±15 years, male:female =14:39) underwent TV reoperations after initial TAP. Previous tricuspid operations included De Vega TAP (n=35), Kay-type TAP (n=16), and prosthetic ring annuloplasty (n=2). Twenty-two patients underwent re-repair of TV (the TAP group) and the other 31 patients underwent TVR (the TVR group). There were no differences in preoperative characteristics and risk factors between the 2 groups (Table 1).
Full table
Surgical procedures and operative data
The surgical techniques for TAP and TVR have been described previously (7-9). In brief, those were performed under the aortic and bicaval cannulation, moderate hypothermic condition, and cold cardioplegic arrest through a median redo-sternotomy. In De Vega TAP, a 3-0 extended polytetrafluoroethylene (e-PTFE) suture was used. Annulus was reduced by tying down the annular plication suture of e-PTFE. To reduce the annulus reproducibly, it was performed while a cylinder-shaped sizer was inserted through the annulus. Three commercially available sizers (actual diameters of 29.5, 31.5, and 33.5 mm; labeled sizes of 27, 29 and 31 mm, respectively) were used. In the TAP group, 18 patients underwent De Vega annuloplasty, and 4 underwent TAP using prosthetic rings. In the TVR group, mechanical and bioprosthetic TVRs were performed in 16 and 15 patients, respectively. Tricuspid valve pathologies at reoperation were functional in 38 patients and organic in 15. Sixteen patients with functional TR underwent TVR without attempt of TAP at the discretion of the operating surgeon due to a concern of TAP failure. When performing TVR, mechanical valves were preferred in patients younger than 65 years.
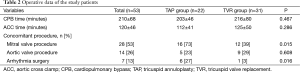
Average aortic cross-clamp and cardiopulmonary bypss times were 120±46 and 210±68 minutes, respectively, without any intergroup differences. Concomitant procedures were performed in 33 patients, such as mitral valve surgery (n=28) and aortic valve operation (n=14). In addition, 7 patients underwent arrhythmia surgery. Concomitant mitral valve operations (P=0.015) and arrhythmia surgeries (P=0.016) were more frequently performed in the TAP than TVR groups (Table 2). However, mean aortic cross clamp time was greater because TVR took more time than TAP.
Full table
Evaluation of early and long-term clinical outcomes
Patients underwent regular postoperative follow-up in the outpatient clinic at 3 to 4 months intervals. If the last clinic visit was not conducted at the scheduled time, they were contacted by telephone to confirm their condition. The anticoagulant dose was adjusted to obtain international normalized ratio (INR) of 2.5 in the patients with left-sided mechanical heart valves and/or atrial fibrillation, an INR of 3.0 for 6 months in patients with bioprosthetic tricuspid valves or prosthetic tricuspid rings, and an INR of 3.0 for a life-long period in patients with mechanical tricuspid valves. A specialized anticoagulation management team comprised of experienced pharmacists regularly followed up all patients. The clinical follow-up was closed on 30 June 2014. Follow-up was complete in all patients, with a follow-up duration of 81 months (range, 6−243 months). The operative mortality was defined as death within 30 days after surgery or during the same admission. Cardiac death included death related to cardiac events and sudden death during follow-up. The tricuspid valve-related events (TVRE) included the following according to the guidelines (10): (I) cardiac death; (II) structural valve deterioration (SVD) of the replaced valve or recurrent TR of the repaired valve; (III) TV reoperations; (IV) congestive heart failure requiring readmission; (V) a composite of thromboembolism and bleeding that caused death, hospitalization, or permanent injury, or that necessitated a transfusion; (VI) TV endocarditis; and (VII) permanent pacemaker implantation within 1 month post-surgery.
Echocardiographic evaluation
An initial postoperative echocardiographic evaluation was performed before discharge. Additional echocardiograms were performed during the follow-up based on the clinical symptoms and signs at the discretion of surgeons or the referring physicians. At least 1 echocardiogram was performed in 96% (45 of 47) of the survivors. The last echocardiogram was done at median 72 months (range, 1−233 months) after the surgery. The TR was graded from 0 to 4: 0= none; 1= mild; 2= moderate; 3= moderately severe; 4= severe. Recurrence of TR was defined as TR of grade 3 or 4 post-surgery.
Statistical analysis
The statistical analysis was performed using the SPSS software package (ver. 12.0, SPSS Inc., Chicago, IL, USA). The data are expressed as mean ± standard deviation, median (ranges), or proportions. Comparisons between the 2 groups were performed with the χ2 test or Fisher’s exact test for the categorical variables and Student’s t-test for the continuous variables. The survival rates were drawn by the Kaplan-Meier method, and analyses of factors associated with time-related events were performed with Cox regression analysis. Hazard ratios for categorical variables were obtained from the Cox regression model using Firth’s penalized maximum likelihood estimation due to separation. Multivariable analysis was also performed using the Cox regression model with Firth’s penalized maximum likelihood estimation. Variables with a P value <0.1 were entered into the multivariable analysis. A P value <0.05 was treated as statistically significant.
Results
Early results
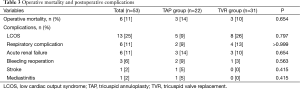
Operative mortality rate was 11% (6 of 53 patients). Postoperative morbidity included low cardiac output syndrome (n=13), acute renal failure (n=6), respiratory complications (n=6), and reoperations for bleeding (n=3). Incidence of operative mortality and postoperative morbidity were similar between the 2 groups (Table 3). Early postoperative echocardiography demonstrated grade 1 TR in 4 TAP group patients and grade 2 in 2.
Full table
Long-term survival
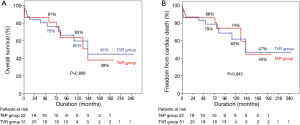
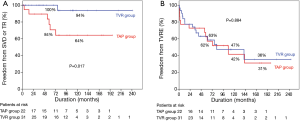
Among the 47 survivors, late death occurred in 14 patients (9 cardiac deaths). Overall survival at 5, 10, and 15 years were 78%, 61%, and 41%, respectively. Freedom rates from cardiac death at 5, 10, and 15 years were 82%, 67%, and 45%, respectively. There were no statistically significant differences in overall survival and freedom rates from cardiac death between the 2 groups (Figure 1).
Freedom from TVRE
In the TAP group, recurrent TR (≥ grade 3) occurred in 6 patients (TR grade 3 in 3 patients and grade 4 in 3) during the follow-up. One of 4 patients who underwent ring annuloplasty had recurrent TR just 3 months after surgery, although the patient had no TR at early postoperative echocardiography. Among these 6 patients, 2 patients underwent TV reoperations. Bleeding events occurred in 2 patients and readmission due to congestive heart failure was necessary in 7. In the TVR group, SVD occurred in 1 patient and the patient underwent TV reoperation. Bleeding events occurred in 7 patients. Readmission for congestive heart failure was necessary in 8 patients. Prosthetic valve endocarditis occurred in 1 patient.
Freedom from SVD or TR rates at 5, 10, 15 years were 88%, 79%, and 69%, respectively, and freedom from TVRE rates at 5, 10, 15 years were 62%, 45%, and 34%, respectively. Log-rank test demonstrated that freedom from SVD or TR rates were lower in the TAP than TVR groups (P=0.017). However, overall TVRE rates were not different in the 2 groups (P=0.884, Figure 2).
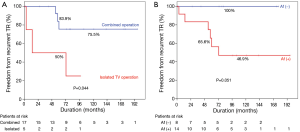
Subgroup analysis: risk factors for recurrent TR after re-repair
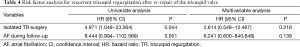
Univariate analyses demonstrated that isolated TV surgery (P=0.044) and presence of atrial fibrillation (P=0.051) were associated with recurrent TR after redo-TAP (Figure 3). There were no differences in recurrent TR between De Vega TAP and TAP using prosthetic ring (P=0.898). In the multivariable analysis, however, no variables showed statistically significant association with recurrent TR after re-repair (Table 4).
Full table
Discussion
This study demonstrated 3 main findings. First, operative mortality and complications of redo TV surgery after TAP were high. Second, the TVRE-free rates were similar regardless of type of surgery, although recurrent TR was frequently found after redo TAP. Third, atrial fibrillation and isolated TV reoperation were associated with recurrent TR after TV re-repair.
Tricuspid valve surgery accounts for less than 5% of cardiac surgeries (6), and most of the patients with TV disease undergo TAP rather than TVR. However, early and long-term results of TAP might be suboptimal. A previous study demonstrated that 15% of 789 patients experienced recurrent TR of equal or more than grade 3 at 1 month after TAP, even in the patients who underwent TAP using prosthetic rings (2). Failure of TAP and the subsequent TV reoperation can be a high-risk procedure. However, data demonstrating results of reoperative TV surgery after initial TAP are scarce (5,6). A previous study demonstrated that patients who underwent TV reoperation after TV repair had poor prognosis with high in-hospital (35%) and long-term (10-year survival rate of 40%) mortality rates (5). Another study demonstrated an in-hospital mortality rate of 13% and a 10-year survival rate of 56%, which were better than those in the former study but still high when considering the results of other heart valve surgeries (6). Results of the present study were in agreement with those of the latter study with an early mortality rate of 11% and a 10-year survival rate of 61%. Higher mortality rates in the former study might be due to the fact that patients enrolled in that study underwent operations a decade earlier and were in worse functional class and had more co-morbid conditions compared to the patients enrolled in the present study.
When selecting TV surgery, a theoretical disadvantage of TVR compared with TAP is that a large rigid prosthesis being inserted into a deformable, low-pressure right ventricular cavity could cause progressive right ventricular dysfunction (11,12). In our previous study, we demonstrated that freedom rates from cardiac death were lower after TVR compared to those after TAP in patients with functional TR (13). Other studies also demonstrated higher long-term mortality after TVR compared with that after TAP (11,12). In the reoperative cases such as patients enrolled in the present study, selecting type of TV surgery might be more complicated. Our hypothesis was that TAP is beneficial compared to TVR even in reoperation after initial TV repair. In the present study, however, we failed to demonstrate advantages of TAP compared with TVR in patients who underwent TV reoperation after previous TAP. This might be due to the high rate of recurrent TR after redo-TAP compared to reported TR recurrence rate after first time TAP (2,8,13). There might be an argue that recurrent TR after redo-TAP could be lowered if we performed prosthetic ring TAP. Although prosthetic rings have been widely used in many centers, we preferred to perform De Vega suture annuloplasty with some modifications. In our previous study, we reported 5- and 10-year TR free rates of 96.5% and 93.1%, respectively, after TAP using De Vega annuloplasty (8). This is comparable with TR free rates after prosthetic ring TAP demonstrated in the literature, although head-to-head comparison is impossible. Ten-year TR-free rate of 64% in the present study is markedly low when considering results of our previous study (8). To identify the risk factors for recurrent TR after redo-TAP, the subgroup analysis was performed in the 22 TAP group patients. Cox regression analysis revealed that recurrent TR after redo-TAP occurred frequently in patients who underwent reoperation due to isolated TV disease and patients with atrial fibrillation, although it was marginally significant. On the contrary, method of TAP was not a risk factor for recurrent TR, although only small number of patients underwent TAP using a prosthetic ring. Association between atrial fibrillation and progression of TR in native TV or repaired TV has been well demonstrated (14,15). The finding of this study is in agreement with that in previous studies. In addition, isolated TR was identified as another risk factor. When TR after initial repair was concomitantly occurred with other cardiac problems such as left sided-valvular dysfunction, these cardiac problems, rather than repaired TV itself could be a cause of TR. In this situation, TR would resolve after correction of left-sided heart problems even without re-repair of the TV. On the other hand, when TR recurred after initial TAP without any combined disease, there might be factors such as right ventricular enlargement and resultant severe leaflet tethering (16), which may not be corrected even after re-repair of the tricuspid annulus, although we did not demonstrate such data.
Study limitations
The present study had limitations that must be noted. First, this study was a retrospective study that was conducted at a single institution. Second, the indications for TAP and TVR were not precisely defined because of the retrospective nature of the present study. Finally, the number of patients enrolled in the present study was relatively small to draw statistically significant conclusions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Navia JL, Nowicki ER, Blackstone EH, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg 2010;139:1473-82. [PubMed]
- McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674-85. [PubMed]
- Sales VL, McCarthy PM. Durability of functional tricuspid valve repair. Semin Thorac Cardiovasc Surg 2010;22:97-103. [PubMed]
- De Vega NG. Selective, adjustable and permanent annuloplasty. An original technic for the treatment of tricuspid insufficiency. Rev Esp Cardiol 1972;25:555-6. [PubMed]
- Bernal JM, Morales D, Revuelta C, et al. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg 2005;130:498-503. [PubMed]
- Jeganathan R, Armstrong S, Al-Alao B, et al. The risk and outcomes of reoperative tricuspid valve surgery. Ann Thorac Surg 2013;95:119-24. [PubMed]
- Hwang HY, Kim KH, Kim KB, et al. Mechanical tricuspid valve replacement is not superior in patients younger than 65 years who need long-term anticoagulation. Ann Thorac Surg 2012;93:1154-60. [PubMed]
- Hwang HY, Chang HW, Jeong DS, et al. De Vega annuloplasty for functional tricupsid regurgitation: concept of tricuspid valve orifice index to optimize tricuspid valve annular reduction. J Korean Med Sci 2013;28:1756-61. [PubMed]
- Jeong DS, Kim KH. Tricuspid annuloplasty using the MC3 ring for functional tricuspid regurgitation. Circ J 2010;74:278-83. [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523-8. [PubMed]
- Singh SK, Tang GH, Maganti MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg 2006;82:1735-41; discussion 1741.
- Bajzer CT, Stewart WJ, Cosgrove DM, et al. Tricuspid valve surgery and intraoperative echocardiography: factors affecting survival, clinical outcome, and echocardiographic success. J Am Coll Cardiol 1998;32:1023-31. [PubMed]
- Hwang HY, Kim KH, Kim KB, et al. Treatment for severe functional tricuspid regurgitation: annuloplasty versus valve replacement. Eur J Cardiothorac Surg 2014;46:e21-7. [PubMed]
- Kwak JJ, Kim YJ, Kim MK, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J 2008;155:732-7. [PubMed]
- Calafiore AM, Iac AL, Romeo A, et al. Echocardiographic-based treatment of functional tricuspid regurgitation. J Thorac Cardiovasc Surg 2011;142:308-13. [PubMed]
- Fukuda S, Gillinov AM, McCarthy PM, et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation 2006;114:I582-7. [PubMed]


