Prediction and prognostic factors of post-recurrence survival in recurred patients with early-stage NSCLC who underwent complete resection
Introduction
Lung cancer has been understood as one of the major causes of cancer-related death worldwide. Despite recent development of chemotherapy and radiotherapy, the first choice for the treatment of early-stage non-small cell lung cancer (NSCLC) is surgery (1-4). Due to the developments in the early diagnosis and treatment, the survival rate of lung cancer patients has been improving. Nevertheless, tumor recurrence is still a major obstacle in the cure and long-term survival of patients, and has become the most common cause of death (1,5-7). This means that postoperative recurrence plays an important role in the long-term survival and cure of early-stage NSCLC patients (5). The recurrence rate has been reported between 20% and 85% in previous studies, depending on stage and follow-up period (8), with the local recurrence rate ranging from 22–50%; the distant recurrence rate, 48% to 78%; and the simultaneous local and distant recurrence rate, 3% to 20% (7-9). Despite the aggressive and multimodality treatments, most recurrent patients have little possibility of cure (1). As the majority of recurrence are distant metastasis, most patients have received chemotherapy, radiotherapy or combination therapy, but selected patients have underwent surgery (7,10-12). Relatively few studies on the predictive factors of the post-recurrence survival (PRS) rate have been reported and remain controversial (1,5,7,10). Documented prognostic factors that influence PRS rate are variable and include the disease free interval(DFI), the number of foci of metastatic disease, organ site of metastasis and use of post-recurrence therapy (1,4,13). And based on these predictive factors, few studies have been conducted on risk-stratify patients with respect to PRS (8). In this study, the clinicopathological records related with the PRS rate were retrospectively analyzed using the 7th edition of the TNM classification. Also by stratifying the recurred patients according to their survival rates, we hoped advancements in making predictions for their prognosis and the improvement of PRS can be reached.
Methods
Of the 357 patients who underwent a complete resection for stage I and II NSCLC at Dong-A University Hospital between January 1995 and December 2012, 141 patients who experienced a postoperative recurrence were selected for this retrospective study. Data of the patient characteristics, surgical resection type, histopathological findings, lymph node metastasis, post-recurrence therapy, death and so on was confirmed through their medical records or telephone call, and their association with PRS was analyzed. This study was approved by the Institutional Review Board of Dong-A University Hospital. Lung resection by lobectomy or larger with mediastinal lymph node dissection was performed in all patients and the surgical margin was no microscopically residual tumor. Patients were staged according to the 7th TNM staging proposed by International Staging Committee (ISC) of the International Association for the Study of Lung Cancer (IASLC). Histologic subtypes of lung cancer were determined according to the World Health Organization classification. Adjuvant therapy was given to patients diagnosed as pathologic stage II. The exceptions included those who refused therapy, those with poor performance status (PS) prior adjuvant therapy, those who couldn’t undergo complete adjuvant therapy due to deteriorating PS during the treatment or due to therapy-related toxicity, and those with stage changed from I to II by the 7th revision of TNM staging. The recurrence was categorized as local, distant, and simultaneous local and distant. Local recurrence was defined as the recurrence in the ipsilateral hemithorax including the mediastinal lymph nodes, and distant metastasis was defined as the recurrence in the contralateral hemithorax and extrathoracic organs. To assess the recurrence, blood examination (including serum tumor markers), plain chest X-ray, chest CT scan, abdominal ultrasonography and bone scintigraphy were conducted on an our outpatient department with every three months for the first 2 years after resection, and every 6 months thereafter. When feasible, histological confirmation was performed to diagnose the recurrence. Positron emission tomography/computed tomography (PET/CT) have been used to assess recurrence since they were introduced in 2004. Multiple recurrence was defined as having two or more noncontiguous recurrences within a single or multiple organs and the length of PRS was defined as the interval in months from the date of initial recurrence identified to death or the date of the last follow-up. The factors associated with the PRS of recurred patients were analyzed, and based on the hazard ratios of each factors, the risk scores were yielded. Risk scores were calculated by supposing the risk score of the factor having the lowest hazard ratio as 1.0, and by giving each factors the risk scores proportionate with their hazard ratios. The sum of risk scores were calculated in each recurred patients, and by analyzing the PRS, we could categorize the PRS of the recurred patients into three groups (based on the sum of each patient’s risk scores). The difference of PRS between each three groups was analyzed based on this categorization. All 141 patients were followed through March 2013 and complete follow-up was available in all patients. The median follow-up time for all 141 patients was 34.3 months. PRS was calculated by the Kaplan-Meier method, and univariate and multivariate analyses were performed by means of the Cox proportional hazard model using IBM SPSS Statistics 20 (SPSS Inc., IBM, Chicago, IL, USA). Only the factors that had a probability value less than 0.1 after the univariate analysis were entered into the multivariate analysis. Statistically significant differences were accepted when the probability value was less than 0.05.
Results
Patient characteristics
Of the 357 patients who underwent lung resection by lobectomy or larger for stage I and II NSCLC, 141 patients experienced recurrence. The mean follow-up time for these 141 patients was 34.3 months (range, 1.9–186.8 months), and the median time to initial recurrence was 14.2 months (range, 1.2–119.8 months). The mean age of the 141 patients at recurrence was 65.4±8.7 years and 110 patients (78%) were male. 108 patients had no or only one underlying disease, and the remaining 33 patients had two or more. 48 patients were non-smokers, and 82 patients were current smokers or ex-smokers. For the location and laterality of the tumor, 78 patients (55.3%) had it in the right lung; 63 (44.7%), in the left lung; 89 (63.1%), in the upper lobe (including the middle lobe); and 41 (29.1%), in the lower lobe. 106 patients (75.2%) underwent lobectomy and the remaining 35 patients (24.8%) underwent bilobectomy or pneumonectomy. The mean tumor size was 3.8 cm. At initial surgical resection, 26 patients (18.4%) were stage IA, 51 (36.2%) were IB, 18 (12.8%) were IIA and 46 (32.6%) were IIB. The most common pathological type of the resected specimens was adenocarcinoma (74 cases, 52.5%), followed by squamous cell carcinoma (53 cases, 37.6%) and other types of NSCLC (14 cases, 9.9%). In the histological grade, 37 cases (26.2%) were poor and 87 cases (61.7%) were moderate or well. Pathological examination of the dissected hilar or intrapulmonary lymph node confirmed metastasis in 50 patients (35.5%) (Table 1). Of the 141 patients, 110 received treatments after initial recurrence, and 31 received only supportive care. Of the 110 patients who received treatments, 19 underwent re-operation, and the remaining 91 received chemotherapy, radiation therapy or a combination of chemotherapy and radiotherapy.
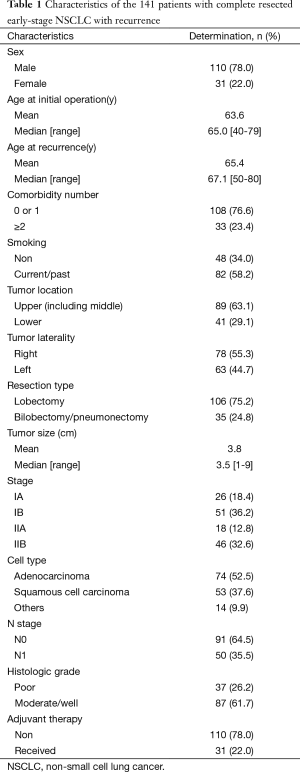
Full table
Post-recurrence survival
The 1- and 3-year survival rates of all patients after recurrence were 50.7% and 28.4%, respectively, and median survival time was 10.8 months (Figure 1). The patterns of recurrence included local only in 40 (28.4%), distant only in 86 (61%) and both local and distant in 15 patients (10.6%). Consequentially, distant recurrence accounted for 71.6% of all recurrent cases. Ninety-two patients (65.2%) had a single organ metastasis and 49 (34.8%) had multiple organ metastases. The lung was the most common recurrence site (63 cases), followed by the lymph node (47 cases), bone (29 cases), pleura (21 cases), liver (10 cases), brain (9 cases) and kidney (6 cases) (Table 2).
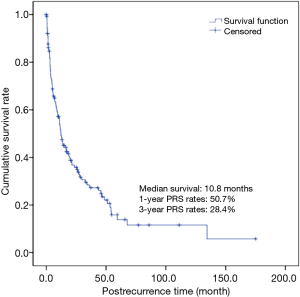
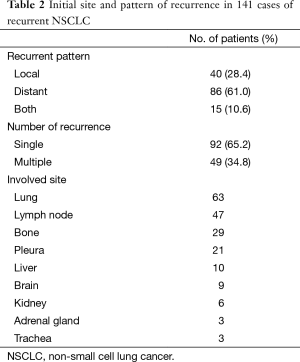
Full table
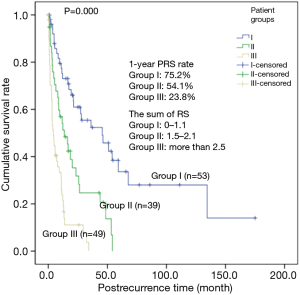
In this study, the difference in the PRS according to the patient characteristics, surgical resection type, histopathological findings, pathologic stage, post-recurrence therapy, recurrent pattern and so on were analyzed. The univariate analysis showed that resection type, histologic grade, symptom at initial recurrence, pulmonary metastasis and post-recurrence therapy had a significant influence on PRS (Table 3). A disease-free interval (DFI) >1 year was also a favorable predictor of PRS, although the difference was statistically insignificant. On multivariate analysis, extensive pulmonary resection, no post-recurrence therapy, symptom at initial recurrence, and poor histologic grade were found to be associated with a risk factor of PRS (Table 4). The risk scores of these four factors, which influence the PRS of recurred patients, were calculated in proportion with the hazard ratios of each factor. The sum of risk scores were calculated in each recurred patients, and by analyzing the PRS, we could categorize the PRS of the recurred patients into three groups. The patients having risk score sums of 1.1 or less had 1-year PRS rate of 75.2% (53 patients), those between 1.5 and 2.1, 54.1% (39 patients) and those having risk score sums of more than 2.5- had 1-year PRS rate of 23.8% (49 patients), which is suggestive of significant statistical difference of PRS rate between each groups (P=0.000) (Figure 2).
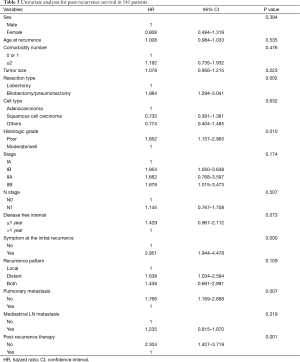
Full table

Full table
Discussion
In the present study, the risk factors that affected the PRS rates of the early-stage NSCLC (stage I or II) patients who underwent lung resection by lobectomy or larger were analyzed. Most of the operation was performed by only one surgeon, and the majority of the pathologic findings were read by one pathologist. Accordingly, the differences in the extensions of lymph node dissection and in the pathological opinions may be insignificant, so the pathologic staging is considered consistent. Under these circumstances, the differences in the PRS rates according to the patient characteristics, extent of surgery, histopathological findings, stage, post-recurrence therapy and recurrence patterns were analyzed.
In this study, the PRS rate of the cases without the presence of symptoms at recurrence was confirmed to have been three times higher than those of the cases with the presence of symptoms, regardless of the recurrent pattern. Moreover, most of the latter cases showed findings of extrathoracic metastasis. The presence of somatic symptoms at recurrence represent the more advanced recurrent disease and the consequent deterioration in the health status, which eventually result in premature mortality (8,14). Williams and co-workers also reported a more than twofold increase in post-recurrence mortality risk associated with the presence of symptoms at recurrence (8). Hung and co-workers reported that the majority of recurrence cases were distant and about 84% of them occurred within the first 2 years after operation (1). Therefore, although there could be a controversy, an intensive follow-up such as a routine PET/CT on all patients may be needed in the first 2 years after operation (15).
Furthermore, to improve PRS rates aggressive postoperative follow-up should be given in order to detect recurrence before symptoms occur.
Some studies on the impact of specific distant metastatic organ sites on PRS in resected NSCLC have been reported, but they were conducted with small cohorts or without discriminating the local recurrence from the distant metastasis. Accordingly, no definite results could be drawn (1). Sugimura et al. reported a better PRS in cases of recurrence confined to the lung (7). Yoshino et al. demonstrated a favorable PRS in cases of intrapulmonary metastasis than in cases of other organ metastases, and confirmed that bone metastasis was a marginally unfavorable factor (16). According to Hung et al., distant metastases confined within the contralateral lung showed significantly better PRS than those with distant metastases outside the contralateral lung, and bone metastases showed significantly worse PRS (1). However, Williams et al. and Nakagawa et al. reported that liver metastasis was associated with the worse prognostic factor for PRS (5,8). In this study, the lung metastases showed significantly better PRS than the other organ metastases. This was because more than half of the lung metastases in this study were localized single metastases, and most of these patients received post-recurrence therapy.
DFI has been shown to be a significant prognostic factor of PRS in NSCLC. According to Walsh et al., DFI than 12 months was the favorable prognostic factor of the overall postoperative survival and PRS (17). Recently, several studies have reported that a longer DFI is associated with prolonged PRS (1,3). In this study, no statistically significant association between the DFI and the PRS was found, but a marginally significant association was observed. Therefore, an analysis with larger cohort may show a statistically significant association between the DFI and the PRS.
Surgical resection by lobectomy or larger and extensive lymphadenectomy are the treatment of choice for early-stage NSCLC patients, but pneumonectomy and bilobectomy may increase the postoperative mortality and morbidity (18). Kozower et al. also reported that pneumonectomy and bilobectomy were predictors of increased risk of mortality and morbidity after surgery (19). According to Puri et al. and Ludwig et al., patients undergoing pneumonectomy for early-stage NSCLC decreased their long-term survival compared with those treated by lesser resections (20,21). This means the postoperative complication rate in early-stage NSCLC patients who had undergone bilobectomy or pneumonectomy was high and the recurrence in these patients resulted in a high mortality rate. Accordingly, the extent of the surgery and PRS were thought to be associated with each other and this study showed similar results. In other words, to improve PRS rates in recurrences (to lower the sum of risk scores in each patients), the extent of lung resection should be minimized by every possible measures.
It is difficult to understand that the histologic grade of initial lung cancer is the prognostic factor of PRS. According to our previous study, however, the histologic grade was poorer the higher the recurrence rate was and the shorter the DFI was. This implies that histologic grade can be used as a marker of tumor aggressiveness or occult disease at resection. Therefore, histologic grade and PRS are considered to be associated with each other, and a statistically significant difference between them was confirmed in this study. Clinically, for those patients with poor histologic grades, efforts should be made to reduce other risk scores in order to improve PRS rates in recurrences. That is, for those patients intensive follow-up should be taken in order to detect recurrences before the symptom develops, and in recurrences, intensive post-recurrence therapy should be given.
The recent developments of various treatment methods and enhancement of their effects have significantly improved post-recurrence prognoses. Moreover, the treatment of recurrent NSCLC has also significantly prolonged the overall survival and PRS (1,7,17). Systemic chemotherapy combined with radiotherapy has often been used in most cases to treat recurrence of lung cancer (8,16). However, recently, surgical resection has also been used in localized local or distant recurrence (7,8,12). Yoshino et al. reported that patients who underwent metastasectomy for recurrence in distant organs had significantly longer survival while those with chemotherapy had marginally prolonged survival (16). Nakagawa et al. reported a prolonged survival in stage I NSCLC patients with post-recurrence therapy (5). In a large cohort study conducted by Sugimura et al. that targeted stages I to III NSCLC patients, treatment, whether it was surgery or combination chemotherapy with radiation, significantly improved PRS over no treatment in resected NSCLC after local recurrence and in the case of initial recurrence localized to the lung, surgical resection were recommended. In addition, they emphasized that the treatment of the recurrent patients should be focused on local control of recurrent disease such as surgical resection or radiotherapy (7). In this study, an improved PRS rate was also observed in the recurrent patients with post-recurrence therapy such as chemotherapy, radiotherapy and surgical resection. No statistically significant difference in the PRS rates was observed between the group that underwent re-operation and the group that received other treatments but a marginally significant difference was found (P=0.058). Nakagawa et al. also reported similar results (5). However, if the one patient who died of respiratory failure after re-operation was excluded, the difference in the PRS rates of the group that underwent re-operation and the group that received chemotherapy and/or radiotherapy was statistically significant (P=0.032). The overall 5-year survival of the patients who underwent re-operation after recurrence was 44.9%, much better than that of the patients (11.5%) who received other treatments and the 2-year PRS of the patients who underwent re-operation was 69.1%, which was excellent. Because only the patients who were in a good enough condition to undergo surgery and those who had localized recurrence were selected, the PRS outcomes in the patients who underwent re-operation were favorable. This explanation coincides with that of the study of Song et al. (11). Therefore, if a recurrent patient is suitable for surgery, re-operation should be actively considered. Furthermore, an intensive postoperative follow-up should be conducted to provide patients with active treatments such as surgery when they are in better health with less symptoms. Further comparative studies regarding the prognosis of post-recurrence therapy with larger cohort may be needed in the future.
Formerly, the effect of post-recurrence chemotherapy was controversial. Yano et al. reported that systemic chemotherapy containing cisplatin had no significant effect on PRS, but local treatments such as radiotherapy was effective (22). Shaw et al. reported similar results (23). In this study, no significant difference in the PRS between the patients who received chemotherapy only and the patients who received local treatments after recurrence was observed, but the cases of chemotherapy followed by local treatment showed significantly better PRS than the cases of local treatment only (P=0.005). Therefore, if a patient’s general condition is favorable, chemotherapy after local treatment (especially re-operation) may be the optimal treatment strategy. However, the many new chemotherapy regimens have recently been developed (e.g., epidermal growth factor receptor-tyrosine kinase inhibitors, which is used in targeted therapy). Shimada et al. reported that epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) can improve PRS in patients with recurrent NSCLC (24). In this study, the effect of EGFR-TKI on recurrent NSCLC patients was not included in the survey of risk factors on PRS, as EGFR-TKI wasn’t available in our institution until 2012. In conclusion, in our study, an improved PRS rate was confirmed in the patients who received post-recurrence therapy regardless of chemotherapy after recurrence, when compared with no treatment. This result coincides with that of the study of Sugimura et al. (7). Hereafter a prospective randomized trials are needed in the future to investigate the effect of many new chemotherapy regimens on PRS benefit.
The biases and limitations of this study are as follows. First, patient selection bias might be inevitable due to the single-institute retrospective study. Second, pathologic evaluation for decisive diagnosis of recurrence was not conducted on the majority of patients and diagnosis of recurrence was merely inferred based on imaging studies. Consequently, it was difficult to distinguish a second primary tumor from pulmonary metastases. Third, there was a selection bias for the treatment. This might have been due to the no evaluation of the PS at the time of recurrence, which is important in determining the patient’s post-recurrence therapy strategy. Fourth, the effects of the highlighted agent, EGFR-TKI and which organs are involved in the metastasis on the PRS rates wasn’t examined thoroughly. Lastly, there were limitations regarding the lack of cross-validation of risk scores described in this study. There were not enough recurrent patients since 2013 until now in a single-center to perform cross-validations. In the future, cross-validation using big data (i.e., multicenter studies) should be needed. Considering such limitations, biases and the difficulty in generalizing about multifactorial patient backgrounds, clinical application of risk scores proposed in this study to predict PRS may be controversial.
In summary, the risk factors that affected the PRS were extensive pulmonary resection, symptoms at initial recurrence, poor histologic differentiation, and no post-recurrence therapy. Based on the hazard ratios of each risk factor, the risk scores were yielded. Risk scores are calculated by supposing the risk score of the factor having the lowest hazard ratio as 1.0, and by giving each factors the risk scores proportionate with their hazard ratios. The sum of risk scores were calculated in each recurred patients, and by analyzing the PRS, we could categorize the PRS of the recurred patients into three groups (based on the sum of each patient’s risk scores). The patients whose risk score sums were 1.1 or less were assigned to Group I; between 1.5 and 2.1, to Group II; and more than 2.5, to Group III. Significant differences in their PRS rates were confirmed (Bilobectomy or pneumonectomy: HR, 2.039 and RS, 1.0; no post-recurrence therapy: HR, 2.330 and RS, 1.1; poor histological differentiation: HR, 3.125 and RS, 1.5; and symptoms at initial recurrence: HR, 3.154 and RS, 1.5). Furthermore, in the study of Williams et al., the risk factors that affected the PRS were analyzed to be converted to their risk scores, and the sum of each patient’s risk scores were calculated, as in this study. The study created empirical four groups according to the sum of each patient’s risk scores, and median survival across the four groups were calculated. Significant differences between the groups were confirmed (8). Therefore, each groups of patients may be used to predict the prognosis. And in order to improve the PRS rates, efforts to reduce these risk factors may be needed.
Acknowledgements
This work was supported by the Dong-A University research fund, Korea.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hung JJ, Jeng WJ, Hsu WH, et al. Prognostic factors of postrecurrence survival in completely resected stage I non-small cell lung cancer with distant metastasis. Thorax 2010;65:241-5. [PubMed]
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-42S.
- Yano T, Haro A, Yoshida T, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol 2010;102:852-5. [PubMed]
- Kim MY, Kim JS, Lee JS. Radiotherapy for Locoregional Recurrent Non-Small Cell Lung Cancer. J Lung Cancer 2011;10:37-43.
- Nakagawa T, Okumura N, Ohata K, et al. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:499-504. [PubMed]
- Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 2002;20:1989-95. [PubMed]
- Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 2007;83:409-17; discussioin 417-8.
- Williams BA, Sugimura H, Endo C, et al. Predicting postrecurrence survival among completely resected nonsmall-cell lung cancer patients. Ann Thorac Surg 2006;81:1021-7. [PubMed]
- Jang KM, Lee KS, Shim YM, et al. The rates and CT patterns of locoregional recurrence after resection surgery of lung cancer: correlation with histopathology and tumor staging. J Thorac Imaging 2003;18:225-30. [PubMed]
- Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64:192-6. [PubMed]
- Song IH, Yeom SW, Heo S, et al. Prognostic factors for post-recurrence survival in patients with completely resected Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:262-7. [PubMed]
- Voltolini L, Paladini P, Luzzi L, et al. Iterative surgical resections for local recurrent and second primary bronchogenic carcinoma. Eur J Cardiothorac Surg 2000;18:529-34. [PubMed]
- Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am 2001;10:243-55. vii. [PubMed]
- Vigano A, Donaldson N, Higginson IJ, et al. Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer 2004;101:1090-8. [PubMed]
- Cho S, Lee EB. A follow-up of integrated positron emission tomography/computed tomography after curative resection of non-small-cell lung cancer in asymptomatic patients. J Thorac Cardiovasc Surg 2010;139:1447-51. [PubMed]
- Yoshino I, Yohena T, Kitajima M, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg 2001;7:204-9. [PubMed]
- Walsh GL, O'Connor M, Willis KM, et al. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg 1995;60:1563-70; discussion 1570-2. [PubMed]
- Hung JJ, Jeng WJ, Hsu WH, et al. Time trends of overall survival and survival after recurrence in completely resected stage I non-small cell lung cancer. J Thorac Oncol 2012;7:397-405. [PubMed]
- Kozower BD, Sheng S, O'Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3. [PubMed]
- Puri V, Garg N, Engelhardt EE, et al. Tumor location is not an independent prognostic factor in early stage non-small cell lung cancer. Ann Thorac Surg 2010;89:1053-9. [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [PubMed]
- Yano T, Hara N, Ichinose Y, et al. Local recurrence after complete resection for non-small-cell carcinoma of the lung. Significance of local control by radiation treatment. J Thorac Cardiovasc Surg 1994;107:8-12. [PubMed]
- Shaw EG, Brindle JS, Creagan ET, et al. Locally recurrent non-small-cell lung cancer after complete surgical resection. Mayo Clin Proc 1992;67:1129-33. [PubMed]
- Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013;143:1626-34. [PubMed]


