
The efficacy and safety of intrapleural hyperthermic perfusion in patients with malignant pleural effusion undergoing video-assisted thoracic surgery: a single-arm clinical trial
Introduction
Malignant pleural effusion (MPE) commonly occurs at the advanced stages of malignant tumors. It is estimated that there are more than 150,000 MPE cases in the United States each year (1). Patients with MPE have poor prognosis, with a median survival of 5 months (2). The treatment strategy for MPE can be categorized into conservative and surgical options. Thoracentesis is a conservative treatment used for patients with a poor Karnofsky performance status (KPS) score. The major purpose of that treatment is to relieve symptoms of dyspnea. Indwelling pleural catheter (IPC) is the most commonly used conservative treatment for MPE. IPC can cause spontaneous pleurodesis in 45.6% of patients with MPE (3). Pleurodesis is another conservative treatment that induces inflammation of the pleural cavity by administering, for example, talc, antibiotics, or silver nitrate. A meta-analysis reported that the success rate of talc pleurodesis for MPE was around 76% at 1 month, ranging from 47% to 89% across studies (4). C-reactive protein (CRP), which is a systemic inflammatory marker, is associated with the success rate of pleurodesis in MPE caused by mesothelioma (5). Surgery plays a limited role in the treatment of MPE. Rintoul et al. reported that video-assisted thoracoscopic partial pleurectomy for MPE caused by mesothelioma resulted in more surgical complications than talc pleurodesis, with a similar 1-year survival rate (6). However, there are differing opinions on the role of surgery for treating MPE. A recent meta-analysis found that hyperthermic intrathoracic chemotherapy (HITHOC) significantly prolonged the median survival of patients with MPE compared to those who did not receive HITHOC (7). The median survival of patients with MPE receiving cytoreductive surgery plus HITHOC reached as long as 20 months (8). Distilled water can cause the cytocidal effects of hypotonic shock and result in the lysis of cancer cells, which can be used in surgery to reduce the recurrence of cancer (9,10). Ba et al. reported that intrapleural hyperthermic perfusion (IHP) with distilled water at a temperature of 48 ℃ under ultrasound was a feasible and safe way to treat MPE, with a median survival of 13 months and a 100% control rate (11).
Although several studies have demonstrated the efficacy of intraperitoneal hyperthermic chemotherapy (IPHC), there were few studies focusing on the intrapleural hyperthemic perfusion for MPE with distilled water under video-assited thoracoscopic surgery. Therefore, this study explored the feasibility and safety of IHP under video-assisted thoracoscopic surgery (VATS) in patients with MPE. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-353/rc).
Methods
Patients and study design
This single-arm retrospective study analyzed the clinical efficacy and survival benefit of IHP using distilled water to treat MPE. The records of patients with MPE caused by non-small cell lung cancer (NSCLC) who were admitted to the Department of Cardiothoracic Surgery of Taizhou Hospital between January 2014 and December 2018 were reviewed. Personal ID numbers were deleted before delivery to one of the collaborators (MK). All patients received IHP with distilled water. Patients over 18 years with MPE confirmed by chest X-ray and/or computed tomography (CT) scan, pleural cytology, and/or biopsy were included. Evaluation of the patients’ cardiorespiratory function showed that they could tolerate general anesthesia. All patients underwent thorough examination before surgery, including medical history, physical examination, chest and abdominal CT scans, magnetic resonance imaging scan of the brain, and single-photon emission CT of the bone to evaluate the primary tumor and metastases sites. The clinical stage of the patient was evaluated according to the eighth edition of the American Joint Committee on Cancer TNM staging (12). All patients signed an informed consent form. Ethical approval was obtained from the Ethics Review Board of Taizhou Hospital (No. K20190739). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The efficacy of treatment was defined as follows: (I) complete remission (CR; no recurrence of pleural effusion after IHP for at least four weeks); (II) partial remission (PR; pleural effusion was decreased by 50% and the condition lasted for four weeks; or (III) no remission (NR; no decrease in the pleural effusion) (11,13,14). Patients were followed every two weeks for the first month, and every month thereafter. Chest radiographs or ultrasounds were performed during follow-up. All patients were followed until they died. Survival was calculated from the date of surgery. Information on pleural effusion and overall survival (OS) was retrieved from medical records.
Surgical technique
VATS was performed under general endotracheal anesthesia with double lumen endotracheal intubation ventilation. Vital signs were monitored with an electrocardiogram. Invasive pressure and end tidal CO2 were also monitored. Patients were placed at a 90-degree lateral position. A 1-cm port was placed in the seventh intercostal space along the middle axillary line and a 4-cm incision was made at the fourth intercostal space along the anterior axillary line. A trocar was inserted through the pleural cavity. The thoracoscope was placed at the port of the seventh intercostal space and used to explore the pleural cavity for the extent of adhesion and tumor dissemination. The adhesions, fibrinoid membrane, and pleural effusion were removed with a clamp and electrocautery. Sufficient disseminated tumor tissues were obtained for biopsy. The 32- and 28-F chest tubes were inserted into the two ports as inflow and outflow catheters. The tubes were fixed to the thoracic wall with sutures and connected to an extracorporeal circuit (roller pump, heat exchanger, and reservoir). A temperature sensor was placed into the thoracic cavity for real-time monitoring.
Perfusion technique
After the procedure inside the thorax, the tubes were connected to the perfusion system, and extracorporeal circulation was commenced. The heating circuit system contained preheated distilled water to 43 ℃. The pleural cavity was perfused with distilled water and the ipsilateral lung was collapsed. The rate of flow was set to 1,000–1,500 mL/min. The temperature of the pleural cavity was maintained at around 43 ℃. The perfusion procedure continued for 60 minutes. After perfusion, a thoracoscope was used to detect any active bleeding or air leaks. A 26F chest tube was placed through the seventh intercostal port in the thoracic cavity for drainage.
Statistical analysis
The data are presented as the median and range or the mean ± standard deviation. OS was analyzed using the Kaplan-Meier method with log-rank tests. The volume of pleural effusion was estimated through three-dimensional reconstruction of the CT scan of the chest with the Mimics Medical v.19.0 software (Materialise, Belgium). IBM SPSS v.22.0 (IBM SPSS, Inc, Chicago, IL, USA) was used for statistical calculations. The Kaplan-Meier method with a log-rank test was used for survival analysis. Cox proportional hazards regression models were applied to perform univariate analyses of the overall survival. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant.
Results
Patient characteristics
All 30 patients with MPE caused by NSCLC who received hyperthermic perfusion were retrospectively studied. The patient characteristics are summarized in Table 1. Of the 30 patients, 16 were males and 14 were females, with a mean age of 62.5 years (median: 63 years; range, 39–81 years). The mean volume of pleural effusion estimated by CT was 1,373±897 mL. The KPS scores of these patients before IHP ranged from 30 to 90, with a median value of 56. Of the 30 patients, 28 were diagnosed with lung adenocarcinoma and 2 with squamous cell cancer. Two patients had undergone lung cancer surgery. Thirteen patients only had pleural dissemination. Twelve patients had ipsilateral, contralateral, or bilateral lung metastases. Four patients had multiple extra-thoracic metastases including in the brain, bone, or liver. Twenty-four patients underwent genetic testing for epidermal growth factor receptor gene (EGFR) mutations with the pleural biopsy specimen, and 18 patients were diagnosed with EGFR-sensitive mutations. Six patients received tyrosine kinase inhibitor (TKI) treatment with icotinib, gefitinib, or osimertinib at least one month after IHP. No patient received systemic chemotherapy after surgery.
Table 1
| Characteristic | No. (%) |
|---|---|
| Age, years (mean ± SD) | 62.5±10.2 |
| Sex | |
| Male | 16 (53.3) |
| Female | 14 (46.7) |
| KPS, (range) | 56 [30–90] |
| Smoking history | 8 (26.7) |
| MPE volume, mL (mean ± SD) | 1,373±897 |
| Primary cancer | |
| Adenocarcinoma | 28 (93.3) |
| Squamous cell cancer | 2 (6.7) |
| T stage | |
| cTX | 6 (20.0) |
| cT1 | 3 (10.0) |
| cT2 | 4 (13.3) |
| cT3 | 2 (6.7) |
| cT4 | 15 (50.0) |
| N stage | |
| cN0 | 12 (41.9) |
| cN1 | 3 (7.3) |
| cN2 | 13 (41.9) |
| cN3 | 2 (6.5) |
| M stage | |
| cM1a | 19 (63.3) |
| cM1b | 1 (3.3) |
| cM1c | 10 (33.3) |
| EGFR mutation | |
| 19DEL | 7 (23.3) |
| L858R | 9 (30.0) |
| 19INS | 1 (3.3) |
| L861Q | 1 (3.3) |
| EGFR wild type | 6 (20.0) |
| No genetic testing | 6 (20.0) |
| TKI treatment | 6 (20.0) |
T: primary tumor; N: regional lymph nodes; M: distant metastases. EGFR, epidermal growth factor receptor gene; KPS, Karnofsky Performance Status Scale; MPE, malignant pleural effusion; SD, standard deviation; TKI, tyrosine kinase inhibitor.
Clinical efficacy and survival
All patients were reevaluated for pleural effusion after IHP. The response rate was 96.7%, with 63.3% achieving PR and 33.3% achieving CR. The median postoperative hospital stay was 7 days. The follow-up time after IHP ranged from 2 to 46 months. The median survival was 12 months (range: 2–46 months). The survival time of patients who received TKI treatment after IHP ranged from 13 to 45 months, with a median survival of 28 months. The OS curves of the patients are shown in Figure 1. The 1- and 2-year survival rates were 54.8% and 16%, respectively. Univariate analysis of predictors of OS showed that the male sex (P=0.010), no TKI treatment (P=0.030), and clinical efficacy of PR (P=0.030) were associated with poorer prognosis (Table 2).
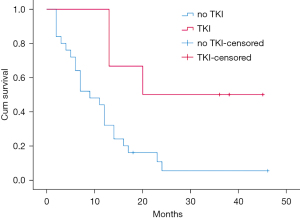
Table 2
| Variable | Univariate Cox analysis | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| Clinical efficacy | |||
| PR | Reference | ||
| CR | 0.349 | 0.135–0.903 | 0.030 |
| KPS score | |||
| ≤50 | Reference | ||
| >50 | 0.752 | 0.339–1.670 | 0.484 |
| TKI treatment | |||
| No | Reference | ||
| Yes | 0.257 | 0.075–0.874 | 0.030 |
| Serum CEA level, ng/mL | |||
| <5 | Reference | ||
| ≥5 | 0.627 | 0.252–1.562 | 0.316 |
| Sex | |||
| Female | Reference | ||
| Male | 2.955 | 1.296–6.737 | 0.010 |
| Age, years | |||
| ≤60 | Reference | ||
| >60 | 2.229 | 0.914–5.435 | 0.078 |
| Smoking history | |||
| No | Reference | ||
| Yes | 2.382 | 0.996–5.696 | 0.051 |
| Histologic type | |||
| Squamous cell cancer | Reference | ||
| Adenocarcinoma | 2.312 | 0.310–17.221 | 0.413 |
| EGFR mutation | |||
| Wild type | Reference | ||
| Mutation | 0.937 | 0.334–2.629 | 0.902 |
| T stage | |||
| TX, T1, or T2 | Reference | ||
| T3 or T4 | 0.929 | 0.414–2.085 | 0.858 |
| N stage | |||
| N0 or N1 | Reference | ||
| N2 or N3 | 1.663 | 0.754–3.669 | 0.208 |
| M stage | |||
| M1a | Reference | ||
| M1b or M1c | 1.185 | 0.533–2.635 | 0.677 |
T: primary tumor; N: regional lymph nodes; M: distant metastases. HR, hazard ratio; CI, confidence interval; PR, partial remission; CR, complete remission; KPS, Karnofsky Performance Status Scale; TKI, tyrosine kinase inhibitor; CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor gene.
Complications and adverse effects
No serious complications were observed after IPH treatment. No patient was admitted to the intensive care unit (ICU), and there were no perioperative deaths. A few patients experienced mild gastrointestinal reactions, which was attributed to the adverse effects of anesthesia.
Discussion
This study demonstrated that IHP with 43 ℃ distilled water under VATS provides a feasible and safe method for the treatment of MPE.
There remains no effective way to treat disseminated pleural cancer with MPE. Patients with MPE have poor prognosis, and the median survival has been reported to be only 5 months (2). Many studies have investigated the use of HITHOC to locally control pleural effusion (8,15-19). Matsuzaki et al. reported that the mean survival time of patients with MPE without combination therapy of lung resection and IPHC was only 6 months (8). However, patients in the combination therapy group were at the early stages of MPE, which may not truly reflect the efficacy of lung resection and IPHC.
Cisplatin was the most commonly used chemotherapeutic agent for IHP in several studies (8,19). However, there were serious adverse effects associated with cisplatin, such as renal toxicity, bone marrow suppression, and cytotoxicity. The poor condition of patients with MPE suggests that they may not be able to tolerate the adverse effects of chemotherapeutic agents. Distilled water is often used for pleural lavage and peritoneal lavage during surgery to eradicate free cancer cells (9,10). Furthermore, the cytocidal effects of hypotonic shock with distilled water on cancer cells have been demonstrated (10). Ba et al. reported that the patients undergoing IHP with distilled water reached a median survival of 13 months (11). However, in that study, patients received IHP under B-ultrasound, and patients with encapsulated pleural effusion or extensive pleural adhesions were excluded. VATS has the advantage of removing the pleural effusion and fibrinoid membrane, which could improve pulmonary function. Additionally, the biopsy taken during surgery provides sufficient tissue for genetic testing. Furthermore, VATS surgery is associated with less pain, enhanced recovery, and a shorter hospitalization time.
Zhang et al. reported that EGFR mutation-positive lung cancers were sensitive to intrapleural perfusion with hyperthermic chemotherapy complete treatment. Hyperthermia promoted the accumulation of cisplatin in lung cancer cells and downregulated the EGFR protein level, leading to quenching of the EGFR signal and induction of apoptosis (20). Further study is warranted to confirm the superior efficacy of IHP with distilled water on patients with EGFR mutations and clarify the underlying mechanisms.
Treatment with TKIs with or without pleurodesis resulted in good control of MPE in patients with EGFR-mutant NSCLC. The progression-free survival without re-accumulation of MPE achieved 21.7 months and the OS was 31.1 months (21). In our study, the median OS of patients receiving TKI treatment was as long as 28 months, which was comparable with that of the study by Kashiwabara et al. (21).
There were some limitations to this study. First, this was a retrospective study with few patients. Second, it was a single-arm study without a comparison control group. Third, the patients included were heterogeneous.
Conclusions
IHP with 43 ℃ distilled water under VATS is a feasible and safe treatment for patients with MPE. It can effectively remove pleural effusion and prevent the re-accumulation of the effusion. Larger studies are warranted to confirm these results.
Acknowledgments
Funding: This work was supported by the Taizhou Municipal Science and Technology Bureau (Nos. 1801ky09 and 20ywb09) and the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Nos. 2019KY773 and 2020KY353). The funding had no role in the design of the study the collection, analysis, and interpretation of the data, nor the preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-353/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-353/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-353/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients signed an informed consent form. Ethical approval was obtained from the Ethics Review Board of Taizhou Hospital (No. K20190739).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 2015;20:654-9. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Xia H, Wang XJ, Zhou Q, et al. Efficacy and safety of talc pleurodesis for malignant pleural effusion: a meta-analysis. PLoS One 2014;9:e87060. [Crossref] [PubMed]
- Mercer RM, Macready J, Jeffries H, et al. Clinically important associations of pleurodesis success in malignant pleural effusion: Analysis of the TIME1 data set. Respirology 2020;25:750-5. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Zhou H, Wu W, Tang X, et al. Effect of hyperthermic intrathoracic chemotherapy (HITHOC) on the malignant pleural effusion: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e5532. [Crossref] [PubMed]
- Matsuzaki Y, Shibata K, Yoshioka M, et al. Intrapleural perfusion hyperthermo-chemotherapy for malignant pleural dissemination and effusion. Ann Thorac Surg 1995;59:127-31. [Crossref] [PubMed]
- Huguet EL, Keeling NJ. Distilled water peritoneal lavage after colorectal cancer surgery. Dis Colon Rectum 2004;47:2114-9. [Crossref] [PubMed]
- Kosuga T, Shiozaki A, Ichikawa D, et al. Pleural lavage with distilled water during surgery for esophageal squamous cell carcinoma. Oncol Rep 2011;26:577-86. [PubMed]
- Ba M, Long H, Wang Y, et al. Intrapleural hyperthermic perfusion using distilled water at 48 °C for malignant pleural effusion. J Cancer Res Clin Oncol 2013;139:2005-12. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Feng X, Zhu L, Xiong X, et al. Therapeutical effect of intrapleural perfusion with hyperthermic chemotherapy on malignant pleural effusion under video-assisted thoracoscopic surgery. Int J Hyperthermia 2018;34:479-85. [Crossref] [PubMed]
- Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer 2008;62:139-44. [Crossref] [PubMed]
- Matsuzaki Y, Edagawa M, Shimizu T, et al. Intrapleural hyperthermic perfusion with chemotherapy increases apoptosis in malignant pleuritis. Ann Thorac Surg 2004;78:1769-72. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer 1993;72:426-31. [Crossref] [PubMed]
- Shigemura N, Akashi A, Ohta M, et al. Combined surgery of intrapleural perfusion hyperthermic chemotherapy and panpleuropneumonectomy for lung cancer with advanced pleural spread: a pilot study. Interact Cardiovasc Thorac Surg 2003;2:671-5. [Crossref] [PubMed]
- Suzuki K, Funai K, Shundo Y, et al. Extrapleural pneumonectomy after hyperthermo-chemotherapy for the lung cancer patients with malignant pleural effusion. Kyobu Geka 2004;57:1023-7. [PubMed]
- Yellin A, Simansky DA, Paley M, et al. Hyperthermic pleural perfusion with cisplatin: early clinical experience. Cancer 2001;92:2197-203. [Crossref] [PubMed]
- Zhang H, Zhan C, Ke J, et al. EGFR kinase domain mutation positive lung cancers are sensitive to intrapleural perfusion with hyperthermic chemotherapy (IPHC) complete treatment. Oncotarget 2016;7:3367-78. [Crossref] [PubMed]
- Kashiwabara K, Fuji S, Tsumura S, et al. Prognosis of EGFR-mutant Lung Adenocarcinoma Patients With Malignant Pleural Effusion Receiving First-line EGFR-TKI Therapy Without Pleurodesis: A Single-institute Retrospective Study. Anticancer Res 2020;40:1117-21. [Crossref] [PubMed]


