
Household mold exposure in association with childhood asthma and allergic rhinitis in a northwestern city and a southern city of China
Introduction
Asthma prevalence has been increasing globally with the most pronounced increase in low and middle-income countries (1-3). Rhinitis is marked by irritation and inflammation of the nasal mucosa. According to the International Study of Asthma and Allergies in Childhood (ISAAC) study, about 17% of the children globally suffer from asthma or allergic rhinitis (4).
There is abundant epidemiologic evidence on the association of dampness and mold exposure with various respiratory problems, including upper respiratory tract symptoms, chronic wheezing and cough, and development of asthma in children (5-9). Epidemiologic studies conducted in different climates have suggested that home dampness and mold may also increase the risk of allergic rhinitis in children (5,10,11). More specifically, exposure to mold has been associated with the development or exacerbation of common respiratory diseases such as asthma, allergic rhinitis, and respiratory infections, with odds ratios (ORs) reaching 1.50 [95% confidence interval (CI): 1.25–1.80], 1.83 (95% CI: 1.75–1.91), and 1.49 (95% CI: 1.14–1.95), respectively (12). According to a review and meta-analysis, indoor dampness and mold can increase the risk of rhinitis (OR =1.12, 95% CI: 1.02–1.23) (11). Most of the studies included in this review were conducted in the humid climate. There have been few studies available on dampness or mold and children’s rhinitis in cold and dry areas where dampness-related mold problems about the sources of water damage and window condensation are indeed common in the winter (13,14).
Indoor dampness and mold are widespread in China. One Chinese study including seven northeast cities found that about 10% reported mold/mildew at home (13). Another Chinese study from Taiyuan, a northern city in Shanxi Province, showed that 20% of homes had dampness/mold problems recently (15). Among different dampness indicators, mold odor and visible mold have been reported to have the strongest association with asthma (7) and rhinitis (16). The ISAAC phase two studies, involving 21 countries, found that residential mold exposure was associated with increased rhinitis among school children (8–12 years) (17). Mold odor at home in Finland was associated with asthma incidence among children (18). The visible presence of mold was significantly associated with current symptoms of rhinitis (OR 1.55, 95% CI: 1.16–2.07) and rhinoconjunctivitis (OR 2.38, 95% CI: 1.51–3.75) in young children in Singapore (19). The CCHH (China, Children, Homes, Health) study reported that mold odor was associated with asthma (OR =1.90) and allergic rhinitis (OR =1.25–1.44) (8). In addition, self-reported dampness at home was also associated with asthma and rhinitis (8,20). However, data are still limited in China concerning household dampness and mold in relation to allergic diseases, especially in northwestern China.
The main aim of this study is to evaluate prevalence rates of asthma and allergic rhinitis in schoolchildren across two cities of distinct climate and the relationship between these diseases and indoor dampness-related mold exposure. We also aim to explore potential effect modifiers such as age, sex, and breastfeeding status. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1380/rc).
Methods
Study population
This survey was conducted in Lanzhou and Wuhan, which was part of the follow-up Four Chinese Cities (4CC2) study. This was a cross-sectional study carried out in 2017–2018 among elementary school children. The details of the survey design and sampling approach were described in prior publications (21,22).
Lanzhou, located in the northwest of China, has dry and cold winters and relatively cool summers, whereas Wuhan, located in central-southern China and at the middle reaches of the Yangtze River, has hot and humid summers and mild winter temperatures. Two primary schools, one from an urban and the other from a suburban area, were selected from each city. Informed consent forms were obtained. Parents of participating children completed the questionnaire at home and had their children returning it to their teachers in a sealed envelope. A total of 4,691 children (6–13 years old) completed the questionnaire survey, including 2,019 in Lanzhou and 2,672 in Wuhan.
Questionnaire data
Information on residential history, lifestyle, household characteristics, and children’s and parents’ health history were obtained through the questionnaire. The questionnaire was adapted from the American Thoracic Society (ATS) Epidemiologic Standardization Project questionnaire (23) and has proven to be an effective tool for assessing children’s respiratory health status and history (24,25). The questionnaire was in Chinese (Mandarin) and included detailed questions on sociodemographic factors, characteristics of the dwelling, and home environment, all tailored to suit the Chinese urban populations.
Presence of mold and the duration of mold exposure were characterized by the following questions: “Have you perceived mold due to dampness (such as mold odor or ever had visible mold) in your dwelling during the past 12 months, and how long does this situation last?” (none; <1 month/year; 1–3 months/year; >3 months/year). We created a categorical variable indicating whether mold was present in home. Answers to “none” were assigned to the reference category.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Committee on Ethics of Biomedicine Research, Duke Kunshan University, Jiangsu, China (No. FWA00021580). All the parents or legal guardians of participants completed the informed consent form.
Definitions of asthma and allergic diseases
We assessed health outcomes according to the following definitions. Ever diagnosed asthma and allergic diseases by a doctor were defined as follows: (I) asthma: a “yes” answer to the question “Has a doctor ever diagnosed asthma in this child?”; (II) substance allergy: a “yes” answer to the question “Has a doctor ever diagnosed substance allergy in this child, i.e., allergy to food, drug, pollen, and chemical material?”; (III) allergic rhinitis: a “yes” answer to the question “Has a doctor ever diagnosed allergic rhinitis in this child?”. Having symptoms currently were defined as follows: (I) asthma: a “yes” answer to “has this child had an asthma attack in the last 12 months?”; (II) substance allergy: a “yes” answer to “has this child had an incident of substance allergy from food, drug, pollen, and chemical material, in the last 12 months?”; (III) allergic rhinitis: a “yes” answer to “has this child suffered from allergic rhinitis in the last 12 months?”.
Covariates
We considered a series of possible explanatory variables related to economic level and urbanization. Factors that have been shown previously or suspected to be associated with allergic diseases or associated symptoms were considered as the covariates in our statistical model. Sociodemographic factors included gender, age, and city. Early childhood factors included born pre-term, mother smoking during pregnancy or first year of birth, and breastfeeding duration. Breast-feeding was defined based on the question “The duration of the child’s exclusive breast-feeding?” (never; <4 months; 4–6 months; >6 months). Family health history factors were assessed by reports of father with asthma and mother with asthma. Factors related to the family residence were district (urban/suburban) and characteristics including child sleeping in shared or own room, sleeping in shared or own bed, whether kitchen was open or enclosed from the rest of the home, use of home space heating, use of kitchen ventilation device, whether house interior was remodeled in the past year, and use of air purifiers. ETS exposure was defined as parental smoking (no smoker; either father or mother smoker), and then classified into three levels according to the daily consumption of cigarettes (level 1, none; level 2, 1–10 cigarettes/day; level 3, >10 cigarettes/day). We established binary variables for presence or absence of any pets (no; yes). Other covariates included use of air freshers, cooking fuel type, and use of mosquito coils. Factors related to the family’s socioeconomic status included parental occupation and education level. Details of all home environmental and lifestyle factors are summarized in Table 1.
Table 1
| Variables | N (% of total) |
|---|---|
| Total | 4,691 (100.0) |
| Lanzhou | 2,019 (43.0) |
| Wuhan | 2,672 (57.0) |
| Urban area | 1,894 (40.4) |
| Boy | 2,680 (57.1) |
| <9 years old | 2,436 (51.9) |
| Questionnaire filler (total) | 4,579 (100) |
| Father | 1,323 (28.9) |
| Mother | 3,166 (69.1) |
| Grandparents | 90 (2.0) |
| Sleep in shared room | 2,140 (46.1) |
| Sleep in shared bed | 1,570 (33.9) |
| Open kitchen | 1,711 (37.2) |
| Cooking fuel type | |
| Coal | 153 (3.3) |
| Gas | 4,298 (93.1) |
| Electric/solar | 167 (3.6) |
| Use of kitchen ventilation device | 4,353 (95.3) |
| Use of home space heating | 2,772 (59.9) |
| Use of air purifiers | 712 (15.5) |
| Home remodeled in past year | 672 (14.5) |
| Use of mosquito coils | 3,162 (67.8) |
| Use of air freshers | 755 (16.2) |
| Child born pre-term | 336 (7.3) |
| Father’s occupation | |
| Blue collar | 1,892 (41.3) |
| White collar | 1,609 (35.1) |
| Military/other | 1,080 (23.6) |
| Mother’s occupation | |
| Blue collar | 1,549 (33.6) |
| White collar | 1,834 (40.9) |
| Military/other | 1,097 (24.5) |
| Father’s education | |
| Below or with senior high school | 3,229 (70.1) |
| Senior high school or above | 1,376 (29.9) |
| Mother’s education | |
| Below or with senior high school | 3,347 (72.9) |
| Senior high school or above | 1,247 (27.1) |
| Mother smoking during pregnancy | 33 (0.7) |
| Mother smoking in the first year of birth | 27 (0.6) |
| Breastfeeding duration | |
| 0 month | 846 (18.6) |
| <4 months | 672 (14.8) |
| 4–6 months | 747 (16.5) |
| >6 months | 2,277 (50.1) |
| Father with asthma | 40 (0.9) |
| Mother with asthma | 29 (0.6) |
Statistical methods
The six outcome variables were defined as ever diagnosed asthma, substance allergy, or allergic rhinitis by a doctor and having symptoms of asthma, substance allergy and allergic rhinitis currently. The key exposure variable was focused on household presence of mold. ORs and 95% CI were calculated using logistic regression for each of the six outcome measures. Chi-square test was used to compare the prevalence of asthma and allergic diseases and the differences in home mold, pet-keeping, and ETS exposure between cities. Multiple logistic regressions were used to assess the association between an outcome variable and presence of mold adjusting for gender, age, smoking status, presence of pets, factors related to the family residence, family’s socio-economic status and family history of asthma.
Logistic regression models were repeated for subgroups stratified by city, gender, age, urban or suburban residence, parental occupation type and education level. Potential effect modification of breast-feeding was examined in the overall children and separated by city, respectively. Significance was set at P value <0.05. All statistical analysis was performed using SPSS 22.0 (SPSS Ltd., USA).
Results
Characteristics of the study population
Of 5,098 eligible and invited participants, 4,691 returned the completed questionnaire (response rate of 92%). The mean age of the children was 9.1 years (SD =0.02; range, 6–13) and 2,680 (57.1%) were boys. A total of 2,019 (43%) children were from Lanzhou and 2,672 (57%) were from Wuhan. Most children (81.4%) were breastfed. The characteristics of the participants are shown in Table 1.
As shown in Table 2, 477 (10.3%) homes reported the presence of mold, of which 293 (6.3%) had mold lasting less than a month, 73 (1.6%) had mold lasting for >3 months per year. The majority of households (85.0%) had pets. Nealy half (47.5%) of the children had ETS exposure at home. Compared with the children from Lanzhou, more children from Wuhan were exposed to residential mold (14.8% vs. 4.3%, χ2=137.480, P<0.001). Across the two cities, the overall prevalence of ever diagnosed asthma, substance allergy, and allergic rhinitis by a doctor was 2.0%, 11.0%, and 13.4%, respectively; and 0.9%, 5.9%, and 10.0% of children had current symptoms of asthma, substance allergy, and allergic rhinitis, respectively. When stratified by city, results showed that the prevalence rates of doctor-diagnosed asthma, substance allergy, allergic rhinitis, having current symptoms of allergic rhinitis symptom were all significantly higher in the Wuhan children (P<0.001). The greatest city-difference was observed for ever diagnosed allergic rhinitis (20.2% vs. 4.5%).
Table 2
| Personal characteristics | Total N (%) | Lanzhou N (%) | Wuhan N (%) | P for city difference |
|---|---|---|---|---|
| Ever diagnosed by a doctor | ||||
| Asthma | 94 (2.0) | 30 (1.5) | 64 (2.4) | 0.026 |
| Substance allergy | 514 (11.0) | 158 (7.8) | 356 (13.4) | <0.001 |
| Allergic rhinitis | 630 (13.4) | 91 (4.5) | 539 (20.2) | <0.001 |
| Having symptoms currently | ||||
| Asthma | 41 (0.9) | 16 (0.8) | 25 (0.9) | 0.573 |
| Substance allergy | 277 (5.9) | 106 (5.3) | 171 (6.5) | 0.068 |
| Allergic rhinitis | 467 (10.0) | 87 (4.3) | 380 (14.4) | <0.001 |
| Parental smoking | 0.067 | |||
| No smoker | 2,289 (52.5) | 918 (50.8) | 1,371 (53.6) | |
| Either smoker | 2,073 (47.5) | 888 (49.2) | 1,185 (46.4) | |
| Father smoking intensity | 0.229 | |||
| None | 2,378 (53.5) | 971 (52.2) | 1,407 (54.4) | |
| 1–10 cigarettes/day | 1,587 (35.7) | 689 (37.1) | 898 (34.7) | |
| >10 cigarettes/day | 479 (10.8) | 199 (10.7) | 280 (10.8) | |
| Mother smoking intensity | 0.080 | |||
| None | 4,229 (99.4) | 1,747 (99.7) | 2,482 (99.2) | |
| 1–10 cigarettes/day | 22 (0.5) | 4 (0.2) | 18 (0.7) | |
| >10 cigarettes/day | 3 (0.1) | 2 (0.1) | 1 (0.0) | |
| Presence of any pets | 3,959 (85.0) | 1,733 (85.8) | 2,226 (84.3) | 0.143 |
| Cat | 124 (2.7) | 32 (1.6) | 92 (3.5) | <0.001 |
| Dog | 344 (7.4) | 145 (7.2) | 199 (7.5) | 0.663 |
| Presence of mold | 477 (10.3) | 87 (4.3) | 390 (14.8) | <0.001 |
| Mold presence duration | <0.001 | |||
| None | 4,170 (89.7) | 1,932 (95.7) | 2,238 (85.2) | |
| <1 month/year | 293 (6.3) | 54 (2.7) | 239 (9.1) | |
| 1–3 months/year | 111 (2.4) | 13 (0.6) | 98 (3.7) | |
| >3 months/year | 73 (1.6) | 20 (1.0) | 53 (2.0) |
Association between indoor exposure factors and health outcomes
Univariate logistic regression models were first conducted to analyze the associations. Crude ORs, without adjustment for covariates, for associations between each indoor exposure variable (mold, ETS and pet keeping) and each health outcome are given in Table 3. Exposure to mold was positively associated with doctor-diagnosed asthma, doctor-diagnosed substance allergy, doctor-diagnosed allergic rhinitis, having symptoms of substance allergy, and having symptoms of allergic rhinitis, respectively. There were strong associations between mold presence of any duration and allergic rhinitis. However, no significant associations were found of any intensity of paternal smoking and pet keeping with any asthma and allergy outcomes.
Table 3
| Exposure factors | Ever diagnosed by a doctor | Having symptoms currently | ||||||
|---|---|---|---|---|---|---|---|---|
| Asthma OR (95% CI) |
Substance allergy OR (95% CI) |
Allergic rhinitis OR (95% CI) |
Asthma OR (95% CI) |
Substance allergy OR (95% CI) |
Allergic rhinitis OR (95% CI) |
|||
| Parental smoking (ref: no smoker) | 0.862 (0.568–1.310) |
1.041 (0.861–1.258) |
0.968 (0.814–1.151) |
0.639 (0.330–1.240) |
0.956 (0.743–1.230) |
0.932 (0.765–1.135) | ||
| Father smoking intensity (ref: none) | ||||||||
| 1–10 cigarettes/day | 0.950 (0.611–1.476) |
1.029 (0.838–1.262) |
1.019 (0.847–1.225) |
0.683 (0.334–1.398) |
0.947 (0.721–1.243) |
1.023 (0.831–1.261) |
||
| >10 cigarettes/day | 0.661 (0.298–1.464) |
1.195 (0.885–1.613) |
0.911 (0.678–1.224) |
0.615 (0.184–2.051) |
1.045 (0.696–1.570) |
0.752 (0.526–1.075) |
||
| Presence of any pet (ref: no) | 0.986 (0.555–1.752) |
0.867 (0.677–1.110) |
1.002 (0.792–1.2269) |
0.862 (0.381–1.952) |
0.813 (0.590–1.119) |
1.329 (0.993–1.779) |
||
| Mold (ref: no) | 2.459 (1.485–4.071)* |
1.822 (1.407–2.359)* |
2.682 (2.144–3.354)* |
1.510 (0.632–3.609) |
1.557 (1.098–2.210)* |
2.693 (2.104–3.448)* |
||
| Mold duration (ref: no) | ||||||||
| 1 month/year | 2.406 (1.291–4.484)* |
2.030 (1.489–2.768)* |
2.635 (1.998–3.474)* |
1.646 (0.581–4.664) |
1.524 (0.983–2.363) |
2.358 (1.722–3.230)* |
||
| 1–3 months/year | 3.197 (1.360–7.514)* |
1.592 (0.940–2.696) |
2.493 (1.608–3.865)* |
1.066 (0.145–7.853) |
1.310 (0.630–2.723) |
3.477 (2.236–5.407)* |
||
| >3 months/year | 1.576 (0.379–6.547) |
1.383 (0.704–2.714) |
3.188 (1.917–5.302)* |
1.652 (0.223–12.225) |
2.088 (0.989–4.406) |
2.945 (1.672–5.185)* |
||
*, indicates with statistical significance. OR, odds ratio; 95% CI, 95% confidence interval.
The covariate-adjusted ORs and 95% CI are shown in Table 4. Controlling for age, gender, city, parental asthma, factors related to family residence and home environment, and family’s socioeconomic status, consistent associations were found between presence of mold and doctor-diagnosed asthma (OR =2.399, 95% CI: 1.309–4.398), doctor-diagnosed substance allergy (OR =1.729, 95% CI: 1.282–2.332), doctor-diagnosed allergic rhinitis (OR =1.969, 95% CI: 1.491–2.600), having current symptoms of substance allergy (OR =1.537, 95% CI: 1.026–2.301) and allergic rhinitis (OR =2.242, 95% CI: 1.664–3.022), respectively. When analyzed by the durations of mold, there were also statistically significant associations between different mold durations and respiratory outcomes, notably for mold that lasted over 3 months per year showed a mostly strong association with doctor-diagnosed allergic rhinitis (OR =2.075, 95% CI: 1.106–3.895), mold lasted 1–3 months was mostly associated with doctor-diagnosed asthma (OR =3.392; 95% CI: 1.257–9.151) and then with current allergic rhinitis (OR =2.677; 95% CI: 1.586–4.519).
Table 4
| Mold | Ever diagnosed by a doctor | Having symptoms currently | |||||
|---|---|---|---|---|---|---|---|
| Asthma Adjust OR |
Substance allergy Adjust OR |
Allergic rhinitis Adjust OR |
Asthma Adjust OR |
Substance allergy Adjust OR | Allergic rhinitis Adjust OR |
||
| None | 1 | 1 | 1 | 1 | 1 | 1 | |
| Presence of mold | 2.399 (1.309–4.398)* |
1.729 (1.282–2.332)* |
1.969 (1.491–2.600)* |
1.583 (0.507–4.946) |
1.537 (1.026–2.301)* |
2.242 (1.664–3.022)* |
|
| Mold <1 month | 2.316 (1.084–4.949)* |
1.958 (1.364–2.811)* |
2.154 (1.528–3.037)* |
1.411 (0.307–6.480) |
1.519 (0.917–2.513) |
2.216 (1.527–3.217)* |
|
| Mold 1–3 months | 3.392 (1.257–9.151)* |
1.66 (0.951–2.899) |
1.572 (0.928–2.660) |
1.662 (0.203–13.607) |
1.337 (0.597–2.991) |
2.677 (1.586–4.519)* |
|
| Mold >3 months | 1.556 (0.355–6.823) |
1.096 (0.506–2.378) |
2.075 (1.106–3.895)* |
2.018 (0.248–16.409) |
1.880 (0.818–4.321) |
1.875 (0.941–3.738) |
|
*, indicates with statistical significance. a, ORs were adjusted for covariates: city, area, sex, age, parental smoking, sleep in shared or own room/bed, kitchen style, cook fuel, ventilation use, air purifier, decorated last year, keep pet, mosquito coils, air fresher, pre-term birth, parents’ occupation and education, breastfeeding duration and parental asthma history. OR, odds ratio.
Mold effect modifiers
The associations between household presence of mold and childhood asthma and allergic diseases or symptoms were further analyzed in strata with city, gender, area, age group, paternal education and occupation, and maternal education and occupation (Figure 1). Due to the low prevalence of current asthma, no further group analysis was performed. The associations showed significant interaction with age and living area. There was a significant association between mold and doctor-diagnosed asthma among children living in suburban areas (adjusted OR =5.816, 95% CI: 2.390–14.149). Similarly, presence of mold was associated with a higher risk of doctor-diagnosed allergic rhinitis among children living in suburban areas (adjusted OR =2.473, 95% CI: 1.712–3.572). Younger children were more susceptible to mold-associated allergic rhinitis, both ever diagnosed by a doctor (OR =2.726, 95% CI: 1.894–3.924) and having symptoms currently (OR =2.813, 95% CI: 1.905–4.155). The ORs of allergic rhinitis were all significant under the grouping of city, gender, and paternal occupation. However, no significant interaction effects were identified between them.
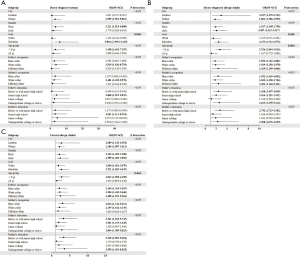
We also examined how mold effects were modified by breast-feeding (Table 5). Significant effects of presence of mold for asthma and allergic rhinitis were observed among breastfed children, with significant adjusted OR of 2.989 (95% CI: 1.497–5.967) for doctor-diagnosed asthma, 2.444 (95% CI: 1.801–3.317) for doctor-diagnosed allergic rhinitis, and 2.786 (95% CI: 2.004–3.874) for having allergic rhinitis symptoms currently. However, a higher risk of mold exposure for substance allergy was found among children who were not breastfed (OR =3.355, 95% CI: 1.674–6.725). We observed a similar pattern when stratified by city, Lanzhou and Wuhan.
Table 5
| Groups | Ever diagnosed by a doctor | Having symptoms currently | ||||
|---|---|---|---|---|---|---|
| Asthma | Substance allergy | Doctor-diagnosed AR | Current substance allergy | Current AR | ||
| All children | ||||||
| No breastfeeding | 1.508 (0.343–6.633) |
3.355 (1.674–6.725)* |
1.821 (0.916–3.620) |
2.034 (0.795–5.203) |
2.038 (0.973–4.270) |
|
| Breastfeeding | 2.989 (1.497–5.967)* |
1.595 (1.134–2.242)* |
2.444 (1.801–3.317)* |
1.46 (0.922–2.312) |
2.786 (2.004–3.874)* |
|
| Lanzhou only | ||||||
| No breastfeeding | 1.022 (0.247–8.025) |
1.066 (0.157–7.243) |
1.216 (0.105–14.116) |
1.199 (0.082–17.498) |
2.005 (0.143–28.066) |
|
| Breastfeeding | 1.550 (0.159–15.154) |
1.458 (0.626–3.399) |
4.743 (1.854–12.130)* |
0.512 (0.117–2.232) |
3.248 (1.203–8.767)* |
|
| Wuhan only | ||||||
| No breastfeeding | 7.711 (0.403–147.431) |
4.171 (1.762–9.876)* |
2.019 (0.867–4.701) |
1.606 (0.430–5.994) |
2.139 (0.885–5.172) |
|
| Breastfeeding | 3.160 (1.484–6.729)* |
1.469 (0.996–2.165) |
1.89 (1.364–2.619)* |
1.591 (0.954–2.653) |
2.322 (1.628–3.313)* |
|
*, numbers indicate with statistical significance. a, odds ratios were adjusted for covariates: city, area, sex, age, parental smoking, sleep in shared or own room/bed, kitchen style, cook fuel, ventilation use, air purifier, decorated last year, presence of pet, mosquito coils, air fresher, pre-term birth, parents’ occupation and education and parental asthma history. 95% CI, 95% confidence interval; AR, allergic rhinitis.
Discussion
In this cross-sectional study, we found that exposure to household dampness-related mold increased the risk of asthma, substance allergy and allergic rhinitis in children. We also identified more susceptible subgroups, e.g., children of younger age and those living in suburban areas had higher mold-associated asthma and allergic rhinitis risks. The results showed somewhat inconsistent associations among breastfed and non-breastfed children. Furthermore, the effects of household mold on allergic rhinitis were stronger in Lanzhou while the effect on asthma was stronger in Wuhan, while the prevalence rates of mold, asthma, and allergic rhinitis were all significantly higher in Wuhan children.
Our results are supported by those from previous studies that measured microbials associated with dampness or mold (26-28). An early study evaluated the measurement by enzyme immunoassay of extracellular polysaccharides of Aspergillus in 31 homes of children and reported that Penicillium species in dust of the living room had significant associations with childhood respiratory symptoms (27). A cross-sectional survey conducted in 44 schools across Taiwan for 6–15 years old schoolchildren found that the Aspergillus or Penicillium and spores of airborne fungi were significantly associated with the prevalence of asthma and asthmatic symptoms (26). A prospective cohort study with 475 premature and atopy risk newborns found significant associations between respiratory tract infections and exposures to Penicillium spores as well as between rhinitis and its related symptoms and Aspergillus exposures (29). However, previous studies of dampness and mold and respiratory and allergic diseases showed conflicting results (30-32) partially due to the classification of dampness in studies. Our data support the conclusion of a review that there is sufficient evidence on the association between dampness or mold and asthma symptoms (6). We further provide evidence that residential mold exposure is associated with the prevalence of asthma and allergic rhinitis in children.
Furthermore, we demonstrated that the associations between household mold and childhood allergic rhinitis were both significant in two cities with different climates. Generally, the southern cities had more rain and warmer than the northern cities in China. These climatic differences could lead to differences in the indoor air climate, thus could increase differences in the status of household dampness-related exposures. As our analysis (Table 2) showed, the proportions of families who reported household mold were substantially higher in Wuhan than in Lanzhou. However, the result showed that, although household mold exposure was lower in Lanzhou than in Wuhan, the positive relationships of mold and allergic rhinitis had no differences between children from Lanzhou and Wuhan. It was supported by findings in a previous study conducted in southern and northern cities of China (33). Similarly, a six-cities study showed that associations between mold signs and symptoms, including rhinitis, were stronger in Beijing as compared to southern China (9). This indicates that climate may modify health associations. It can be important to study health effects of mold in different subgroups concerning different climate zones. However, due to the data limitation, we were not able to analyze the modification effect of meteorological factors on these associations directly. Our research mainly found that using the same research methods in southern cities and northern cities, northern children suffering from allergic rhinitis are more susceptible to indoor humidity. This is lacking in previous studies.
Our findings for asthma and allergic rhinitis are also in line with recent studies conducted in urban and suburban areas, which found increased risk related to mold exposure in suburbs (5,9,34,35). The farm environment has been demonstrated to present a higher exposure to bio-contaminants, in particular to endotoxins but also molds (35). Flamant-Hulin et al. (5) observed more children with current asthma had experienced mold exposure in their homes (OR =3.38, 95% CI: 1.16–9.90), especially among children living in rural areas. Niculita-Hirzel et al. (10) found dwellings in suburban zones showed the most frequent fungal contamination in the owners’ bedroom and the highest diversity of fungal genera among dwellings. Likewise, a systematic review reported higher aerobiome diversity in rural locations (34). However, two studies experimentally found the rural environment to benefit health, examined that rural aerobiome shifted immune function away from allergic (Th2-type) responses (36,37). The available epidemiological evidence between urban and suburban allergens and allergic reactions is limited and does not allow firm conclusions to be drawn.
Numerous studies have investigated the health effects of breast-feeding on sensitization, but the results remain ambiguous. Several studies have suggested that prolonged breastfeeding increases the risk of developing allergic diseases (38-40). An Australian high-risk cohort study found an increased risk of sensitization with prolonged breastfeeding (41); and similar results were presented in a cohort study from New Zealand (39). However, the Osaka Maternal and Child Health Study did not find a statistically significant relationship between the duration of breastfeeding and the risk of wheezing or asthma in Japanese infants (42). These discrepancies between the various studies can possibly be attributed to differences in the study design, geographical location, ethnicity, and methodological factors (43,44). Compared with these studies, the present study intended to explore the modification of breastfeeding on the effects of household mold on childhood asthma and allergic diseases. Our findings suggest that breastfeeding may enhance asthma and allergic rhinitis risk of mold, but not substance allergy, in children.
Overcrowding or certain personal habits may increase the indoor dampness load (e.g., drying of clothes indoors, keeping an aquarium, frequent showering, or cooking). China is a large country with diverse climate zones and the causes of dampness and indoor mold could be different in different climate zones. The prevalence of mold can be higher in southern China, linked to a warmer and more humid climate. However, the health risk linked to water damage and mold odor can be higher in northern China, possibly due to less ventilation in the homes because of the cold weather (9). Early-life exposure to molds could cause recurrent irritation and immune activation in the respiratory tract, inducing prolonged inflammation, prompting the genesis of inflammatory-related diseases, like asthma and rhinitis (6). Certain fungal species are associated with inflammatory processes, and molds may contain substances that have inflammatory effects such as β-1-3-glucans in the cell wall structure, microbial volatile organic compounds (mVOCs), extracellular polysaccharides, and mycotoxins (7,11). A previous study found that higher loads of β-(1,3)-glucan and endotoxin were associated with poorer health outcomes, and this has also been observed with β-(1,3)-glucan and airway inflammation and airway obstruction in adults as well as respiratory symptoms and peak expiratory flow variability in children (45,46). By any means, the current evidence is insufficient to fully elucidate the causal relationship between the mold exposure and asthma and allergy exacerbations, and warrants further discussions.
This study has several limitations. Firstly, as in all cross-sectional epidemiological studies, no causal inference could be made about associations of mold with childhood asthma and allergic rhinitis. Secondly, exposures to outdoor pollutants (e.g., NO2) or exposures in school/daycare environment may be potential influencing factors (47) and were not considered in our study. We used questions to partly characterize outdoor exposure, such as home location. Thirdly, except for mold odor or visible mold, we had no related questions to deduce mold problems about the sources of water damage or moist spots on surfaces, which may contribute to household mold (8). Future studies may be benefited from considering these limitations to ascertain the role of dampness-related household mold in asthma and allergic diseases.
Conclusions
The prevalence of household mold was substantially higher in the southern Chinese city of Wuhan than the northwestern city of Lanzhou. Household mold exposures, especially with a longer duration, were risk factors for childhood asthma and allergic rhinitis in both cities. The higher effect of mold exposure was found in the suburban area and among younger children. Minimizing family mold exposure may be an effective way to reduce children’s risk of asthma and allergic rhinitis.
Acknowledgments
We are particularly indebted to the children, their parents and the schools for their time and enthusiastic participation. We also appreciate all those who helped us during the implementation of the project.
Funding: This work was supported by the Beijing Natural Science Foundation (7202106).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “Children’s Respiratory Health and Air Quality”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1380/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1380/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1380/coif). The series “Children’s Respiratory Health and Air Quality” was commissioned by the editorial office without any funding or sponsorship. JZ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Thoracic Disease. JG serves as an unpaid editorial board member of Journal of Thoracic Disease. XD reports funding from the Beijing Natural Science Foundation (7202106). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Committee on Ethics of Biomedicine Research, Duke Kunshan University, Jiangsu (No. FWA00021580). All the parents or legal guardians of participants completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chiesa Fuxench ZC. Atopic Dermatitis: Disease Background and Risk Factors. Adv Exp Med Biol 2017;1027:11-9. [Crossref] [PubMed]
- Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006;368:733-43. [Crossref] [PubMed]
- Lundbäck B, Backman H, Lötvall J, et al. Is asthma prevalence still increasing? Expert Rev Respir Med 2016;10:39-51. [Crossref] [PubMed]
- Behbehani NA, Abal A, Syabbalo NC, et al. Prevalence of asthma, allergic rhinitis, and eczema in 13- to 14-year-old children in Kuwait: an ISAAC study. International Study of Asthma and Allergies in Childhood. Ann Allergy Asthma Immunol 2000;85:58-63. [Crossref] [PubMed]
- Flamant-Hulin M, Annesi-Maesano I, Caillaud D. Relationships between molds and asthma suggesting non-allergic mechanisms. A rural-urban comparison. Pediatr Allergy Immunol 2013;24:345-51. [Crossref] [PubMed]
- Mendell MJ, Mirer AG, Cheung K, et al. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119:748-56. [Crossref] [PubMed]
- Quansah R, Jaakkola MS, Hugg TT, et al. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS One 2012;7:e47526. [Crossref] [PubMed]
- Wang J, Zhao Z, Zhang Y, et al. Asthma, allergic rhinitis and eczema among parents of preschool children in relation to climate, and dampness and mold in dwellings in China. Environ Int 2019;130:104910. [Crossref] [PubMed]
- Zhang X, Norbäck D, Fan Q, et al. Dampness and mold in homes across China: Associations with rhinitis, ocular, throat and dermal symptoms, headache and fatigue among adults. Indoor Air 2019;29:30-42. [Crossref] [PubMed]
- Niculita-Hirzel H, Yang S, Hager Jörin C, et al. Fungal Contaminants in Energy Efficient Dwellings: Impact of Ventilation Type and Level of Urbanization. Int J Environ Res Public Health 2020;17:4936. [Crossref] [PubMed]
- Tischer CG, Hohmann C, Thiering E, et al. Meta-analysis of mould and dampness exposure on asthma and allergy in eight European birth cohorts: an ENRIECO initiative. Allergy 2011;66:1570-9. [Crossref] [PubMed]
- Rantala A, Jaakkola JJ, Jaakkola MS. Respiratory infections in adults with atopic disease and IgE antibodies to common aeroallergens. PLoS One 2013;8:e68582. [Crossref] [PubMed]
- Dong GH, Qian ZM, Wang J, et al. Residential characteristics and household risk factors and respiratory diseases in Chinese women: the Seven Northeast Cities (SNEC) study. Sci Total Environ 2013;463-464:389-94. [Crossref] [PubMed]
- Lu C, Deng Q, Li Y, et al. Outdoor air pollution, meteorological conditions and indoor factors in dwellings in relation to sick building syndrome (SBS) among adults in China. Sci Total Environ 2016;560-561:186-96. [Crossref] [PubMed]
- Fan XJ, Yang C, Zhang L, et al. Asthma symptoms among Chinese children: the role of ventilation and PM10 exposure at school and home. Int J Tuberc Lung Dis 2017;21:1187-93. [Crossref] [PubMed]
- Jaakkola MS, Quansah R, Hugg TT, et al. Association of indoor dampness and molds with rhinitis risk: a systematic review and meta-analysis. J Allergy Clin Immunol 2013;132:1099-1110.e18. [Crossref] [PubMed]
- Weinmayr G, Gehring U, Genuneit J, et al. Dampness and moulds in relation to respiratory and allergic symptoms in children: results from Phase Two of the International Study of Asthma and Allergies in Childhood (ISAAC Phase Two). Clin Exp Allergy 2013;43:762-74. [Crossref] [PubMed]
- Jaakkola JJ, Hwang BF, Jaakkola N. Home dampness and molds, parental atopy, and asthma in childhood: a six-year population-based cohort study. Environ Health Perspect 2005;113:357-61. [Crossref] [PubMed]
- Tham KW, Zuraimi MS, Koh D, et al. Associations between home dampness and presence of molds with asthma and allergic symptoms among young children in the tropics. Pediatr Allergy Immunol 2007;18:418-24. [Crossref] [PubMed]
- Wang J, Li B, Yu W, et al. Rhinitis symptoms and asthma among parents of preschool children in relation to the home environment in Chongqing, China. PLoS One 2014;9:e94731. [Crossref] [PubMed]
- Li S, Cao S, Duan X, et al. Long-term exposure to PM2.5 and Children's lung function: a dose-based association analysis. J Thorac Dis 2020;12:6379-95. [Crossref] [PubMed]
- Zhang JJ, Kan H, Kipen HM. Respiratory health, children's lung function, and air quality in four Chinese cities: two snapshots in 1993-1996 and 2017-2018. J Thorac Dis 2020;12:6311-4. [Crossref] [PubMed]
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978;118:1-120. [PubMed]
- Dzhambov AM, Lercher P, Rüdisser J, et al. Allergic symptoms in association with naturalness, greenness, and greyness: A cross-sectional study in schoolchildren in the Alps. Environ Res 2021;198:110456. [Crossref] [PubMed]
- Zhang JJ, Hu W, Wei F, et al. Children's respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environ Health Perspect 2002;110:961-7. [Crossref] [PubMed]
- Chen CH, Chao HJ, Chan CC, et al. Current asthma in schoolchildren is related to fungal spores in classrooms. Chest 2014;146:123-34. [Crossref] [PubMed]
- Douwes J, van der Sluis B, Doekes G, et al. Fungal extracellular polysaccharides in house dust as a marker for exposure to fungi: relations with culturable fungi, reported home dampness, and respiratory symptoms. J Allergy Clin Immunol 1999;103:494-500. [Crossref] [PubMed]
- North ML, Brook JR, Lee EY, et al. The Kingston Allergy Birth Cohort: Exploring parentally reported respiratory outcomes through the lens of the exposome. Ann Allergy Asthma Immunol 2017;118:465-73. [Crossref] [PubMed]
- Müller A, Lehmann I, Seiffart A, et al. Increased incidence of allergic sensitisation and respiratory diseases due to mould exposure: results of the Leipzig Allergy Risk children Study (LARS). Int J Hyg Environ Health 2002;204:363-5. [Crossref] [PubMed]
- Braun-Fahrländer C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002;347:869-77. [Crossref] [PubMed]
- Douwes J, van Strien R, Doekes G, et al. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol 2006;117:1067-73. [Crossref] [PubMed]
- Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011;364:701-9. [Crossref] [PubMed]
- Cai J, Li B, Yu W, et al. Household dampness-related exposures in relation to childhood asthma and rhinitis in China: A multicentre observational study. Environ Int 2019;126:735-46. [Crossref] [PubMed]
- Flies EJ, Clarke LJ, Brook BW, et al. Urbanisation reduces the abundance and diversity of airborne microbes - but what does that mean for our health? A systematic review. Sci Total Environ 2020;738:140337. [Crossref] [PubMed]
- Schram D, Doekes G, Boeve M, et al. Bacterial and fungal components in house dust of farm children, Rudolf Steiner school children and reference children--the PARSIFAL Study. Allergy 2005;60:611-8. [Crossref] [PubMed]
- Alenius H, Pakarinen J, Saris O, et al. Contrasting immunological effects of two disparate dusts - preliminary observations. Int Arch Allergy Immunol 2009;149:81-90. [Crossref] [PubMed]
- Roy SR, Schiltz AM, Marotta A, et al. Bacterial DNA in house and farm barn dust. J Allergy Clin Immunol 2003;112:571-8. [Crossref] [PubMed]
- Oddy WH, Peat JK. Breastfeeding, asthma, and atopic disease: an epidemiological review of the literature. J Hum Lact 2003;19:250-61; quiz 262-6. [Crossref] [PubMed]
- Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 2002;360:901-7. [Crossref] [PubMed]
- Wright AL, Holberg CJ, Taussig LM, et al. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax 2001;56:192-7. [Crossref] [PubMed]
- Mihrshahi S, Ampon R, Webb K, et al. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clin Exp Allergy 2007;37:671-9. [Crossref] [PubMed]
- Miyake Y, Tanaka K, Sasaki S, et al. Breastfeeding and the risk of wheeze and asthma in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol 2008;19:490-6. [Crossref] [PubMed]
- Litonjua AA, Celedón JC, Hausmann J, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol 2005;115:751-7. [Crossref] [PubMed]
- Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol 2001;108:S2-8. [Crossref] [PubMed]
- Sigsgaard T, Bonefeld-Jørgensen EC, Kjaergaard SK, et al. Cytokine release from the nasal mucosa and whole blood after experimental exposures to organic dusts. Eur Respir J 2000;16:140-5. [Crossref] [PubMed]
- Thorn J, Rylander R. Airways inflammation and glucan in a rowhouse area. Am J Respir Crit Care Med 1998;157:1798-803. [Crossref] [PubMed]
- Deng Q, Lu C, Yu Y, et al. Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Respir Med 2016;121:67-73. [Crossref] [PubMed]


