
Comparison of the mid-term clinical efficacy and short-term complications of Y-type coronary artery bypass grafting and sequential bypass grafting of the great saphenous vein: a retrospective cohort study
Introduction
Coronary artery bypass grafting (CABG), is internationally recognized as the most effective treatment for coronary heart disease (CHD) (1). In the operation, blocked coronary arteries are replaced to improve myocardial blood supply, relieve angina pectoris, improve quality of life, and reduce the risk of death from CHD (2). Under the approach, grafts or bridged blood vessels are used to create a pathway between the ascending aortic root and the blocked diseased coronary artery to allow the heart to pump blood through the bridge in the aorta (3-5). Unlike great saphenous veins (SVs), arterial bridges provide long-term patency, but questions remain as to the short-term effects of arterial bridges, which may result in complications, such as radial spasm (6). The traditional large SV has attracted attention because it has a high short-term patency and is easier to access than the arterial bridge. However, a relevant postoperative study has shown that the 10- and 15-year postoperative patency rates of large SVs are only 50% and 25%, respectively, and the long-term effect of large SVs is not as good as that of arterial bridge vessels (7). The 10-year patency rate of arterial bridge vessels has been reported to be as high as 85%, and the long-term benefit is higher than that of large SV. Thus, it is recommended that different approaches be adopted depending on the age of the patients. Generally, a total arterial coronary artery bypass transplantation is recommended for patients aged <50 years (8).
CHD refers to heart disease caused by myocardial ischemia and hypoxia caused by coronary atherosclerosis (9). CABG is internationally recognized as one of the most effective methods for treating CHD, especially for patients in whom multiple branches and multiple lesions are the main indications. The large SV is generally sufficient in terms of its large diameter and length, and it has a high patency rate in the short term, and little effect on lower limb activities after removal (10). It has been used as a standard bypass material, and other than the anterior descending branch, it is the most commonly used bridge vessel in coronary arteries (11). At present, the large SV bypass method is mainly orderly, point-to-point. However, there have been no reports on the application of Y-type compound bridge anastomosis of the great SV in CABG. The establishment of a Y-shaped bridge avoids the aortic manipulation of proximal aorto-coronary anastomosis, thereby preventing intra-aortic atherosclerotic plaque displacement and embolization, reducing microemboli formation and reducing the risk of stroke. The main difference between the Y bridge and the conventional one is that the Y bridge diverts some blood flow, but LIMA has a strong blood flow reserve, so whether there is a difference in blood flow from the aorta and the LIMA branch, and whether the Y bridge reduces LAD perfusion because it diverts the flow from the distal part of the LIMA anastomosis. Indications: (I) patients with angina pectoris that cannot be relieved by drug treatment or frequent occurrence; (II) patients with left main artery disease or severe three-vessel disease confirmed by coronary angiography; (III) patients who failed interventional therapy (PTCA and stents) or had restenosis after CABG; (IV) patients with severe mitral valve insufficiency caused by myocardial rupture, cardiac tamponage, ventricular septal perforation, and papillary muscle rupture after myocardial infarction should be operated on emergency or after systemic stabilization.
In this study, 225 patients with CHD with multiple branches and lesions were treated with either sequential anastomosis or Y-type anastomosis of the great SV for CABG, and the difference of the intermediate patency rate of the postoperative venous bridge was observed and compared according to the postoperative follow-up results of the patients to determine the curative effect (12-14). Our findings will further improve the intermediate patency rate of postoperative venous bridges in patients with CHD. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-497/rc).
Methods
Study subjects and subgroups
A total of 225 CHD patients with multiple branches and lesions were selected from our Hospital from January 2020 to December 2020, including 151 males and 74 females. The patients’ ages ranged from 55 to 75 (64.8±7.8) years. All 225 patients were treated with off-pump CABG (OPCABG), and the internal mammary artery or the great SV were used as the bridging vessels. All the patients had a history of hypertension. Before surgery, they were diagnosed clearly by thoracic echocardiography and THORACIC and abdominal aortic CTA, and the location of the rupture was located, and the range of the dissection tear and the functional status of the aortic valve were evaluated. The follow-up time was 1 year. A total of 113 patients, comprising the control group, underwent sequential anastomosis of the great SV with CABG. Another 112 patients, comprising the experimental group, underwent Y-bridge CABG of the great SV. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Hospital’s Ethics Committee (No. AZ202203210401), and all patients signed the relevant informed consent form.
Inclusion criteria
Patients were included in the study if they met the following inclusion criteria: (I) had been diagnosed with CHD as per the criteria of the World Health Organization; (II) had coronary artery triple vessel disease; (III) had no prohibitions against surgery; (IV) had no serious cerebrovascular disease, liver, or kidney dysfunction; (V) had no calcification in the ascending aorta; and (VI) according to the preoperative coronary angiography (CAG), the patients requiring bypass surgery had target vessels that were distributed in the left anterior descending (LAD) branch system (middle or diagonal branch), circumflex branch system (blunt margin branch), and right coronary artery system (posterior descending branch or left ventricular posterior branch).
Exclusion criteria
Patients were excluded from the study if they met any of the following exclusion criteria: (I) required emergency surgery; (II) had infectious diseases or immune diseases; (III) had cardiac function I–II; (VI) had a history of malignant tumors; and/or (V) had cognitive or mental disorders.
Bridge vascular graft method
The objective of the operation was to revascularize the narrowed or occluded coronary arteries. The left internal mammary artery (LIMA) was anterior to the LAD branch in both groups. For the other vascular lesions, SV Y-type CABG or sequential anastomosis was used, which is also the standard in CABG. The number of SV anastomoses in both groups was 3. In the experimental group, 2 segments of the great SV (i.e., SV1 and SV2) were taken, and SV1 was anastomosed with the ascending aorta (AO). The distal end of the SV1 was anastomosed with the 1st target blood tube (middle or diagonal branch), and the proximal blood tube of the SV2 was then anastomosed with the distal end of SV1. The inverted “Y” shape was formed, and the SV2 was sutured with 7-0 Prolene line. The SV2 was anastomosed with the cyclotron system and the right coronary artery system, respectively, using a cardiac surface vascular fixator. The entire “Y” bridge snaked along the left side of the heart. Sequential anastomosis was used in the control group. The 2 groups were anastomosed from the proximal to distal in the order of the LAD system (middle or diagonal branch), circumflex system (blunt margin branch), and right coronary artery system (the posterior descending branch or the posterior branch of the left ventricle). The furthest anastomosis was far as possible on the target vessels with good conditions and large blood flow, and the target vessels with poor conditions were as far as possible in the middle of the sequential bridge.
Surgical method
The patients in both groups were successfully operated on using OPCABG. The anesthesia method was tracheal intubation and general anesthesia. The methods of target vessel exposure in the OPCABG were as follows: all distal anastomoses were performed using the 7-0 pro-LENE single suture technique with local carbon dioxide exposure during anastomosis. A rhomboid anastomosis (in which the long axis of the grafted vessels was perpendicular to the coronary incision) was used for the side-to-side anastomosis. End-to-end anastomosis was parallel to the long axis (Figure 1).
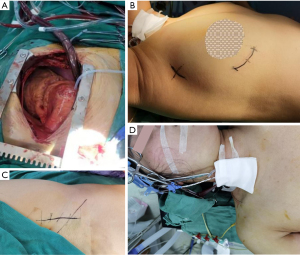
Bridge vascular flow measurement
After the arterial blood pressure was stabilized, the blood flow and waveform of the transplanted vessels were recorded by a blood flow meter, and the measurement position was the venous bridge blood flow before each anastomosis.
Postoperative follow-up
To compare the 2 methods more clearly, we measured the real-time blood flow at each anastomosis of the intraoperative vein bridge. The left ventricular ejection fraction (LVEF) of the control group and the experimental group was reviewed 3 months, 6 months, and 1 year after surgery. The left ventricular diastolic diameter (LVEDD), incidence of major adverse cardiovascular events, and CAG re-examination after re-admission due to similar symptoms showed the rate of bridging vessel blockage. If a patient recovered well after surgery and did not have recurrence, CAG was recommended for >1 year after surgery to determine the patency rate of the bridge vessels. The evaluation indexes of the transplanted vessels were divided into the following 3 levels: (I) patency, without stenosis <50%; (II) stenosis (partial patency) from 50% to 99%; and (III) complete occlusion. The patency rates of the vascular bridges were calculated using the following formula: patency rate = (the total number of bridge vessels − the number of bridge vessel occlusions)/100% of the total bridge vessels.
Statistical analysis
SPSS 23.0 statistical software was used for the data analysis. The count data are described as the example and percentage (%). The statistical inference differences between the groups were compared using t-test or Fisher’s exact test. Counting data were represented by “n (%)”, and the t-test was performed. Measurement data were represented by “mean ± SD”, and the t-test was performed. P<0.05 was considered statistically significant.
Results
General clinical features of perioperative patients
A total of 450 bridged vessels were transplanted (250 in the experimental group and 200 in the control group), with an average of 2 bridged vessels per patient, and a total of 400 anastomoses. LIMA anastomosis was found in all patients. There was no death in the perioperative period, and there were 18 postoperative complications, including 3 cases of recurrent angina pectoris, 5 cases of myocardial infarction, 3 cases requiring vascular intervention, 2 cases of cardiogenic death, 1 case of thoracotomy for hemostasis, 2 cases of poor wound healing, and 2 cases of cerebral infarction. A total of 6 patients were treated with intra-aortic balloon pump counter pulsation after surgery, including 2 in the experimental group, and 4 in the control group. All the patients with the above complications were cured and discharged after receiving the appropriate treatment.
General situation comparison
There were no statistically significant differences among the patients in terms of gender, age, body mass index (BMI), preoperative LVEF value, underlying diseases, smoking rate, and the other general data between the 2 groups (P>0.05); that is, the baseline data of the 2 groups were consistent and comparable (Table 1). There patients comprised 151 males and 74 females, and were aged from 55 to 75 (64.8±7.8) years.
Table 1
| General information | Experimental group (n=112) | Control group (n=113) | P |
|---|---|---|---|
| Male/female, n | 68/44 | 83/30 | 0.318 |
| Age (year), mean ± SD | 64.5±8.6 | 62.2±7.1 | 0.108 |
| BMI (kg/m2), mean ± SD | 25.4±4.7 | 26.6±4.2 | 0.223 |
| LVEF (%), mean ± SD | 54.4±5.4 | 54.6±4.9 | 0.552 |
| Hypertension, n (%) | 66 (58.93) | 62 (54.87) | 0.551 |
| Diabetes mellitus, n (%) | 42 (37.50) | 38 (33.63) | 0.625 |
| Smoke, n (%) | 45 (40.18) | 52 (46.02) | 0.624 |
BMI, body mass index; LVEF, left ventricular ejection fraction.
Instant blood flow measurement of intraoperative venous bridge anastomosis
We selected the LAD system (middle or diagonal branch) as the 1st target vessel for anastomosis, the circumflex system (blunt margin branch) as the 2nd target vessel, and the right coronary artery system (posterior descending branch or posterior left ventricular branch) as the 3rd target vessel. The blood flow of the main vein bridge of the 2 groups was denoted as A1, and the blood flow before the 2nd anastomosis was denoted as A2 when measuring the blood flow of the sequential anastomosis, and so on. A coronary artery flow meter was used to measure the blood flow in the main trunk of the sequential bridge and the anterior bridge of each anastomosis, and A1–A2 was used to measure the blood flow of the 1st anastomosis (i.e., the flow into the anastomosis was calculated rather than the flow through). The blood flow of the main vein bridge of the two groups was denoted as A1, and the blood flow before the second anastomosis was denoted as A2 when measuring the blood flow of the sequential anastomosis, and so on. Coronary artery flow meter was used to measure the blood flow of the main trunk of the sequential bridge and the anterior bridge of each anastomosis. In addition, A1–A2 was used to measure the blood flow of the first anastomosis, and the flow of Y-type composite bridge into each anastomosis was calculated in the same way.
In the same way, the flow of the Y-shaped composite bridge into each anastomosis was calculated. According to the statistics, there was no significant difference in intraoperative immediate blood flow into each anastomosis between the 2 groups (P>0.05; Table 2).
Table 2
| Groups | Cases (N) | A1 | A2 | A3 | A4 |
|---|---|---|---|---|---|
| Experimental group | 112 | 37.2±5.1 | 17.5±4.4 | 11.3±3.2 | 9.6±2.2 |
| Control group | 113 | 38.5±4.5 | 17.7±4.5 | 11.4±3.4 | 10.4±2.9 |
| P | – | 0.235 | 0.725 | 0.535 | 0.167 |
A1, artery 1; A2, artery 2; A3, artery 3; A4, artery 4.
Postoperative study, LVEF and LVDD
The LVEF and LVEDD of the patients in the 2 groups were followed-up 3 months, 6 months, and 1 year after surgery. The results showed that there was no significant difference in LVEF and LVEDD between the 2 groups at 3 months and 6 months after surgery (P>0.05). The LVEF of the experimental group was significantly higher than that of the control group 1 year after surgery, and the difference was statistically significant (P<0.05). There was no statistically significant difference in the LVEDD between the 2 groups 1 year after surgery (P>0.05; Table 3).
Table 3
| Observational index | Time | Experimental group (n=112) | Control group (n=113) | P |
|---|---|---|---|---|
| LVEF (%) | 3 months after the surgery | 54.2±4.8 | 53.4±5.2 | 0.556 |
| 6 months after the surgery | 53.4±4.5 | 54.2±4.6 | 0.522 | |
| 1 year after the surgery | 51.6±5.1 | 67.6±5.6 | 0.001 | |
| LVEDD (mm) | 3 months after the surgery | 48.5±5.5 | 48.5±5.5 | 0.935 |
| 6 months after the surgery | 45.4±5.6 | 46.7±4.8 | 0.115 | |
| 1 year after the surgery | 46.5±2.2 | 46.5±1.8 | 0.845 |
LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension.
The incidence of major adverse cardiovascular events
Major adverse cardiovascular events were defined as the recurrence of angina pectoris, recurrent myocardial infarction, revascularization, including intervention and CABG, and cardiogenic death. According to the statistics, the occurrence of major adverse cardiovascular events in the 2 groups mainly occurred in the 1st year or later after surgery, and the total incidence of the major adverse cardiovascular events in the experimental group was significantly lower than that in the control group, and the difference was statistically significant (P<0.05; Table 4).
Table 4
| Cardiovascular events | Experimental group (n=112) | Control group (n=113) | P |
|---|---|---|---|
| Recurrence of angina | 4 | 3 | 0.328 |
| Another myocardial infarction | 8 | 4 | 0.625 |
| Re-vascular intervention therapy | 6 | 0 | 0.425 |
| Cardiac death | 2 | 2 | 0.125 |
| Total major adverse cardiovascular events | 20 | 9 | 0.035 |
Difference in graft patency rates
A comparison of the total patency rates of the postoperative venous bridge between the 2 groups revealed that the rate of the experimental group was significantly higher than that of the control group (P<0.05), and the difference was statistically significant (Table 5).
Table 5
| Groups | Cases (N) | Part of the smooth, n (%) | Vascular occlusion, n (%) | Patency rate, n (%) |
|---|---|---|---|---|
| Experimental group | 112 | 21 (18.75) | 16 (14.29) | 96 (85.71) |
| Control group | 113 | 18 (15.93) | 39 (34.51) | 74 (65.49) |
| P | 0.023 |
Discussion
The use of the great SV [in the internal breast artery (ITA)] in CABG is prone to progressive intimal hyperplasia, resulting in mid-term sclerosis and obstruction, and has a patency rate of only 30–35% 10 years after surgery (15). As a bypass vessel, the ITA has a constant anatomical position, shows no progressive intimal hyperplasia, sclerosis, or obstruction after surgery, has a 10-year patency rate of up to 9.0%~5%, and has a similar blood supply to the great SV, which promotes the clinical use of ITA as a heart bypass vessel (16-19).
The safety and efficacy of the total artery vascular Y-type composite bridge vessel (the bilateral internal mammary artery or the LIMA-radial artery) have been widely confirmed, and it is considered the most ideal choice of bridge vessel (20). However, the use of the bilateral internal mammary artery may cause poor sternal union after surgery, especially in elderly patients with diabetes (21-23). The great SV has long been used as the standard bypass material and is still used in 80% of CABG worldwide (24). The clinical application of the great SV bridge mainly includes single anastomosis, sequential anastomosis. However, the mid-term clinical curative effect has rarely been reported (24). Additionally, the vascular lesion of the bridge is the main cause of myocardial ischemia after CABG, which is of great significance in studying the mid-term clinical efficacy of the specific bypass operation of the great SV bridge (25).
Different conclusions have been reached about the degree of risk of sequential bridge occlusion (26). Pavei et al. (27) followed-up with 428 patients for 15 years and found that while sequential bridges were more completely vascularized than single-vessel bridges, patients with sequential bridges also had a higher incidence of myocardial infarction and other cardiac events (28,29). We believe that sequential great SV bridges and Y-type bridges share the following common characteristics: (I) compared to single-branch bridges (point-to-point bypass bridges), SV bridges sand Y-type bridges have a larger mean blood flow, and a lower mean pulsatile index; (II) SV bridges sand Y-type bridges reduce the length of bridge vessels, reduce the operation time of anastomotic shortening, reduce the number of aortic anastomoses, which in turn can reduce the perforation and the number and time of aortic clamping, which is more meaningful for patients with more calcified plaques in the ascending aorta, and reduce postoperative arrhythmia low cardiac output and other complications. These advantages and characteristics have been widely reported and confirmed (30).
This study mainly analyzed the advantages and disadvantages of using the great SV as a sequential bridge or Y-type bridge, and focused on comparing the differences in clinical efficacy of the 2 great SV bypass bridges for postoperative patients. We found that there was no significant difference in cardiac function between the 2 groups in the short term (i.e., 3 and 6 months after surgery), and the incidence of major adverse cardiovascular events mainly occurred in the middle-postoperative period (i.e., 1 year after surgery) or later. Additionally, we found no statistical differences in body weight, age, heart function, and other general conditions, or in the results of the real-time blood flow measurements for each anastomosis of the intraoperative vein bridge. Compared to the control group, the LVEF, vascular bridge and anastomotic patency rate in the experimental group were also significantly higher at 1, 2, and 3 years after operation (P<0.05). Finally, the incidence of major adverse cardiovascular events was significantly decreased (P<0.05) (31-33).
We are of the view that the Y-type composite bridge improves the clinical effect experienced by patients in the middle-postoperative period for a number of reasons. First, the Y-type bridge prevents anastomosis and the possibility of massive myocardial ischemia, and the relationship is not easy to prevent the next anastomosis and the bridge (34). Second, in the Y-type bridge, the shape of the vessels is smoother, which prevents the possibility of blockages in the bridge vessel (35). Third, in the Y-type anastomosis, the distance between the 2nd and 3rd anastomoses and the AO is shorter than that of a sequential bridge (36).
Y-type bridge surgery followed by a sequential bypass was found to reduce the postoperative rate of major adverse cardiac events, delay the time in which bridge vascular blockages occurs, improve the bridge vascular patency rate of postoperative patients, prolong patient survival time, and improve patient quality of life (37). We intend to conduct long-term follow-up studies on sequential and Y-type composite bridges to develop a more reasonable surgical approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-497/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-497/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-497/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Hospital’s Ethics Committee (No. AZ202203210401), and all patients signed the relevant informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bademci MS, Kocaaslan C, Bayraktar FA, et al. Great saphenous vein sparing techniques in lower extremity venous insufficiency treatment. Phlebology 2021;36:331. [Crossref] [PubMed]
- Kikuchi K, Mori M. Less-invasive coronary artery bypass grafting international landscape and progress. Curr Opin Cardiol 2017;32:715-21. [Crossref] [PubMed]
- Naouli H, Lathelize H, Bouarhroum A. Leiomyosarcoma of the Great Saphenous Vein: Case Report and Literature Review. Ann Vasc Surg 2019;56:353.e1-6. [Crossref] [PubMed]
- Rosenblum JM, Binongo J, Wei J, et al. Priorities in coronary artery bypass grafting: Is midterm survival more dependent on completeness of revascularization or multiple arterial grafts? J Thorac Cardiovasc Surg 2021;161:2070-8.e6. [Crossref] [PubMed]
- Pagano M, Passaro G, Flore R, et al. Inferior selective crossectomy for great saphenous vein incompetence: Our experience. Vascular 2021;29:290-6. [Crossref] [PubMed]
- Rodrigues AJ, Evora PR, Tubino PV. On-pump versus off-pump coronary artery bypass graft surgery: what do the evidence show? Rev Bras Cir Cardiovasc 2013;28:531-7. [Crossref] [PubMed]
- Kockaert M, de Roos KP, van Dijk L, et al. Duplication of the great saphenous vein: a definition problem and implications for therapy. Dermatol Surg 2012;38:77-82. [Crossref] [PubMed]
- Khan MS, Islam MY, Ahmed MU, et al. On pump coronary artery bypass graft surgery versus off pump coronary artery bypass graft surgery: a review. Glob J Health Sci 2014;6:186-93. [Crossref] [PubMed]
- Aggarwal V. Pathogenesis and management of superficial venous aneurysms through a case of thrombosed large great saphenous vein aneurysm. Vascular 2021;29:297-300. [Crossref] [PubMed]
- Bainbridge D, Cheng DC. Minimally invasive direct coronary artery bypass and off-pump coronary artery bypass surgery: anesthetic considerations. Anesthesiol Clin 2008;26:437-52. [Crossref] [PubMed]
- Balint R, Farics A, Parti K, et al. Which endovenous ablation method does offer a better long-term technical success in the treatment of the incompetent great saphenous vein? Vascular 2016;24:649-57. Review. [Crossref] [PubMed]
- Houlind K. On-pump versus off-pump coronary artery bypass surgery: what is the status after ROOBY, DOORS, CORONARY and GOPCABE? Future Cardiol 2013;9:569-79. [Crossref] [PubMed]
- Cangiano R, Orlandino G, Patitucci G, et al. Primary Leiomyosarcoma of the Great Saphenous Vein: Case Report and Review of the Literature. Ann Vasc Surg 2017;38:315.e1-7. [Crossref] [PubMed]
- Puskas JD, Gaudino M, Taggart DP. Experience Is Crucial in Off-Pump Coronary Artery Bypass Grafting. Circulation 2019;139:1872-5. [Crossref] [PubMed]
- Leo M, Stefano R, Raffaele AI. Foam sclerotherapy of the great saphenous vein in association with pre-terminal saphenous junction ligation/division as an office-based procedure: 12-Month results. Phlebology 2018;33:321-9. [Crossref] [PubMed]
- Virk SA, Bowman SRA, Chan L, et al. Equivalent outcomes after coronary artery bypass graft surgery performed by consultant versus trainee surgeons: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016;151:647-54.e1. [Crossref] [PubMed]
- Alat I. A malpractice: Stripping of the great saphenous vein without the division of the side-branches at the saphenofemoral junction. Bratisl Lek Listy 2020;121:242-7. [Crossref] [PubMed]
- Niebauer J. Is There a Role for Cardiac Rehabilitation After Coronary Artery Bypass Grafting? Treatment After Coronary Artery Bypass Surgery Remains Incomplete Without Rehabilitation. Circulation 2016;133:2529-37. [Crossref] [PubMed]
- Zhang SZ, Wang GX, Zhou XT. The clinical application of microincision vein harvesting of the great saphenous vein in coronary artery bypass grafting. BMC Cardiovasc Disord 2020;20:297. [Crossref] [PubMed]
- Saito A, Hirahara N, Motomura N, et al. Current status of cardiovascular surgery in Japan, 2013 and 2014: A report based on the Japan Cardiovascular Surgery Database 3. Coronary artery bypass surgery. Gen Thorac Cardiovasc Surg 2018;66:8-12. [Crossref] [PubMed]
- Shao C, Yan J, Zhang N, et al. Single-stage treatment with iliac vein stenting and stripping of the great saphenous vein for patients with left iliac vein compression syndrome. Asian J Surg 2022;45:257-64. [Crossref] [PubMed]
- Sellke FW. Do radiopaque markers make a difference after coronary artery bypass grafting? J Thorac Cardiovasc Surg 2018;155:1573. [Crossref] [PubMed]
- Chen P, Chen H, Yang M. Comparison of high ligation of great saphenous vein using pneumatic tourniquets and conventional method for great saphenous vein varicosis. Medicine (Baltimore) 2020;99:e21975. [Crossref] [PubMed]
- Ramakrishna H. Off-Pump Versus On-Pump Coronary Artery Bypass Grafting: Should This Debate Even Continue? J Cardiothorac Vasc Anesth 2019;33:1195-6. [Crossref] [PubMed]
- Stücker M, Moritz R, Altmeyer P, et al. New concept: different types of insufficiency of the saphenofemoral junction identified by duplex as a chance for a more differentiated therapy of the great saphenous vein. Phlebology 2013;28:268-74. [Crossref] [PubMed]
- Lawton JS. Off-pump coronary artery bypass grafting: Do it often, do it well, and do it completely-or don't do it at all. J Thorac Cardiovasc Surg 2016;152:1331-2. [Crossref] [PubMed]
- Pavei P, Spreafico G, Bernardi E, et al. Favorable long-term results of endovenous laser ablation of great and small saphenous vein incompetence with a 1470-nm laser and radial fiber. J Vasc Surg Venous Lymphat Disord 2021;9:352-60. [Crossref] [PubMed]
- Gaudino M, Mack MJ, Taggart DP. Additional Arterial Conduits in Coronary Artery Bypass Surgery: Finally Coming of Age. J Am Coll Cardiol 2018;71:2974-6. [Crossref] [PubMed]
- Mühlberger D, Brenner E, Frings N, et al. Functional repair of the great saphenous vein by external valvuloplasty reduces the vein's diameter: 6-month results of a multicentre study. J Int Med Res 2021;49:3000605211014364. [Crossref] [PubMed]
- Fan G, Wang X, Chen C, et al. Surgeons' Preference for Off-Pump or On-Pump Coronary Artery Bypass Grafting Surgery. Heart Surg Forum 2021;24:E422-6. [Crossref] [PubMed]
- Shadid N, Nelemans P, Lawson J, et al. Predictors of recurrence of great saphenous vein reflux following treatment with ultrasound-guided foamsclerotherapy. Phlebology 2015;30:194-9. [Crossref] [PubMed]
- Carrel TP, Eckstein FS, Englberger L, et al. Clinical experience with devices for facilitated anastomoses in coronary artery bypass surgery. Ann Thorac Surg 2004;77:1110-20. [Crossref] [PubMed]
- Hach-Wunderle V, Hach W. Invasive therapeutic options in truncal varicosity of the great saphenous vein. Vasa 2006;35:157-66. [Crossref] [PubMed]
- Raja SG, Dreyfus GD. Off-pump coronary artery bypass surgery: to do or not to do? Current best available evidence. J Cardiothorac Vasc Anesth 2004;18:486-505. [Crossref] [PubMed]
- Pagano M, Bissacco D, Flore R, et al. Great saphenous vein reflux treatment in patients with femoral valve incompetence, the Excluded Saphenous Vein Technique (ESVT): a pilot study. Eur Rev Med Pharmacol Sci 2018;22:7453-7. [PubMed]
- Tully PJ, Baker RA, Kneebone AC, et al. Neuropsychologic and quality-of-life outcomes after coronary artery bypass surgery with and without cardiopulmonary bypass: a prospective randomized trial. J Cardiothorac Vasc Anesth 2008;22:515-21. [Crossref] [PubMed]
- Tauraginskii RA, Lurie F, Simakov S, et al. Venous reflux in the great saphenous vein is driven by a suction force provided by the calf muscle pump in the compression-decompression maneuver. J Vasc Surg Venous Lymphat Disord 2021;9:1282-90. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


