
Surgical lung biopsy for interstitial lung diseases—a single center study of 129 patients
Introduction
Interstitial lung disease (ILD) consists of several lung diseases widely varying in etiology, pathological changes, treatments, and prognosis (1,2). Therefore, it is important to validate the diagnosis and classification of an ILD to select its treatment and determine the prognosis. According to guidelines for the diagnosis and treatment of ILDs, a diagnostic surgical lung biopsy should be used to obtain the differential diagnosis of an ILD in patients with ILDs, which are difficult to distinguish clinically (1). However, with the development of chest high-resolution computed tomography (HRCT) and the high morbidity and mortality associated with a surgical lung biopsy, the utility of surgical biopsy for the diagnosis of an ILD remains controversial (3). Both the development of HRCT and the increasing use of diagnostic cryobiopsy have led to questions on the necessity of surgical lung biopsy (4), since diagnostic cryobiopsy has also been reported to be effective for ILDs (5).
In most cases, a diagnosis can be established from the clinical information, the results of detailed laboratory tests, HRCT, and transbronchial lung biopsy (TBLB), all of which the patient’s physician and radiologist or pathologist will discuss with each other. However, the sensitivity and specificity of such an approach for the diagnosis of idiopathic pulmonary fibrosis (IPF) has been reported to range from 60% to 80% (6). In addition, a TBLB may not be sufficient for a definitive pathological diagnosis, which depends on the sample volume and the biopsy site. A retrospective study of 21 patients with IPF found that only 32% of the TBLB specimens could be accurately diagnosed, compared with 95.4% of the surgical lung biopsy specimens (7).
The postoperative complications of surgical lung biopsy are a serious problem in ILD patients. Patients with a decreased diffusing capacity of the lungs for carbon monoxide [diffusing capacity for carbon monoxide (DLCO) <50% predicted value], who need mechanical ventilation (MV), or who have immunodeficiency or pulmonary hypertension, have been reported to have an increased risk of death after surgical lung biopsy (8-11).
Video-assisted thoracoscopic surgery (VATS) is generally considered a safe procedure for providing lung tissue samples that are sufficient for a definitive histopathological diagnosis (12). The purpose of this study was to evaluate the safety of surgical lung biopsy performed with minimally invasive procedures, including VATS, for patients with ILD. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1773/rc).
Methods
This was a retrospective study of 129 patients with suspected ILD, who consecutively underwent a surgical lung biopsy at Toho University Omori Medical Center between October 2007 and July 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of Toho University Omori Medical Center (No. M20122). Informed consent for VATS was obtained from all patients.
The indications for surgical biopsy were decided through a multidisciplinary discussion. Because this study included patients observed over a long follow-up period, the criteria of patient selection regarding the type of interstitial pneumonia were not consistent. However, worsening respiratory status, such as the appearance of the signs/symptoms of dyspnea or an apparent decrease in a transcutaneous oxygen measurement were not indications for surgical biopsy.
Information was retrospectively gathered from the clinical case notes and included the following: demographic data (age, sex, and smoking status), results of pulmonary function tests (PFTs), estimated pulmonary arterial pressure as measured by echocardiography, surgical approach for biopsy, number of biopsy specimens, location of biopsy site, morbidity, 30- and 60-day mortality after biopsy, length of hospital stay, and postoperative diagnosis. The estimated pulmonary arterial pressure was calculated by adding the transtricuspid pressure gradient to the right atrial pressure (10 mmHg) (13). The transtricuspid pressure gradient was estimated with the use of the modified Bernoulli equation (4V2, where V is the maximum velocity of the regurgitant jet of the tricuspid valve).
Surgical lung biopsy was performed with the patient under general anesthesia and consisted of intubation with a double-lumen catheter and single-lung ventilation. All the patients were placed in the lateral position. VATS was performed with 3 ports. A thoracotomy was performed for patients found to have severe adhesions during endoscopic exploration or for other reasons. The site, side, and the number of biopsy samples were planned after a multidisciplinary discussion (MDD) of physicians who performed careful clinical evaluations and assessments of the distribution of disease as shown on CT. In patients with homogenous bilateral disease and 1 to 3 different lobar targets, the left side was chosen preferentially. The biopsy specimen was obtained by a stapler and consisted of a triangular wedge of pulmonary tissue (Figure 1).
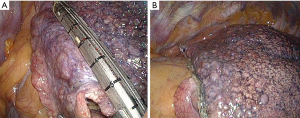
To reinforce the staple line, we covered the staple lines in all patients with both fibrin glue and oxidized cellulose sheets. The patients were enrolled in a rehabilitation program to prevent respiratory complications.
Statistical analysis
Statistical analysis consisted of basic statistical analysis, with the two-tailed Fisher exact test used to compare complications. All statistical computations were performed with the use of a statistical software package (JMP, version 10.0.0; SAS Institute, Cary, NC, USA).
Results
The characteristics of the 129 patients who underwent surgical lung biopsy are summarized in Table 1. The estimated pulmonary artery pressure as determined by echocardiography was determined in 111 (86%) patients.
Table 1
| Variables | Number or numerical value | Q1 and Q3 or % |
|---|---|---|
| Age (mean) | 62.6 years | 59.0 and 68.5 |
| Male/Female | 63/66 | 49%/51% |
| Smoking (pack-year: mean) | 16.5 | 0 and 30.5 |
| Preoperative pulmonary function | ||
| VC (mean) | 2.65 L | 2.06 and 3.15 |
| VC, % (mean) | 91.1% | 77.8 and 105.3 |
| FEV1% (mean) | 81.9% | 77.4 and 86.7 |
| DLCO, % | 71.0% | 55.5 and 82.9 |
| Estimated pulmonary arterial pressure (mean) | 30.4 mmHg | 27.6 and 33.8 |
| Serum KL-6 (mean) | 1,217.6 U/mL | 628 and 1,499 |
| Postoperative diagnosis based on MDD | ||
| Idiopathic interstitial pneumonia | 72 | 55.8% |
| Unclassifiable idiopathic interstitial pneumonia | 34 | 26.4% |
| Idiopathic pulmonary fibrosis | 18 | 14.0% |
| Nonspecific interstitial pneumonia | 15 | 11.6% |
| Pleuroparenchymal fibroelastosis | 5 | 3.9% |
| CVD-IP | 42 | 32.6% |
| Environmental lung disease | 9 | 7.0% |
| Others | 6 | 4.7% |
VC, vital capacity; FEV1%, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; KL-6, Krebs von den Lungen-6; MDD, multidisciplinary discussion; CVD-IP, collagen vascular disease-associated interstitial pneumonia; Q1, quartile 1; Q3, quartile 3.
Table 2 summarizes the surgical parameters and complications after surgery. Biopsies of 2 or more sites were performed in 124 (96%) patients. The most common sites for biopsy were the left lower lobe (44%), left upper lobe (23%), and right lower lobe (22%). For the left upper lobe, the lingular division (17%) was selected more frequently than the upper division (6%) for biopsy. The mean operative time was 85.6 min. The mean duration of chest tube drainage and hospital stay were 1.2 and 5.1 days, respectively.
Table 2
| Variables | Number | Percentage, % |
|---|---|---|
| Surgical approach | ||
| VATS/thoracotomy | 120/9 | 93/7 |
| Number of biopsy sites | ||
| 1/2/3 | 5/112/12 | 4/87/9 |
| Location of biopsy site (total 266 specimens) | ||
| Right upper lobe (apex) | 19 (4) | 7 |
| Right middle lobe | 10 | 4 |
| Right lower lobe | 59 | 22 |
| Upper division of left upper lobe (apex) | 17 (9) | 6 |
| Lingular division of left upper lobe | 44 | 17 |
| Left lower lobe | 117 | 44 |
| Complications | 13 | 10.1 |
| Acute exacerbation of interstitial pneumonia | 1 | 0.8 |
| Pneumothorax after removal of drainage tube | 8 | 6.2 |
| Postoperative pneumonia | 2 | 1.6 |
| Hemothorax | 1 | 0.8 |
| Wound infection | 1 | 0.8 |
| Postoperative mortality | ||
| 30 days | 0 | 0 |
| 60 days | 0 | 0 |
VATS, video-assisted thoracoscopic surgery.
The 30- and 60-day mortality was 0%. Postoperative complications occurred in 13 of 129 patients (10.1%), which included 8 patients who developed pneumothorax after removal of the chest tube (6.2%), 2 who developed postoperative pneumonia, and 1 who developed postoperative acute exacerbation (AE) of interstitial pneumonia (0.8%).
There were no hospital deaths after the biopsy procedure. The patient who developed postoperative AE of interstitial pneumonia and subsequently recovered after surgery died 76 days after surgery after being discharged because of worsening ILD.
Patients who had been diagnosed with pleuroparenchymal fibroelastosis (PPFE) developed a significantly higher number of postoperative complications than other patients (Table 3), which included 3 cases of pneumothorax. Postoperative pneumothorax was observed in 4 of 13 (30.7%) patients who underwent biopsy of the apex of the lung (right S1, left S1+2) (Figure 2, Table 4). The rate of pneumothorax occurring after biopsy of the apex was higher than the rate of biopsy occurring after biopsy of other sites (P=0.0086). Biopsy of the upper lobe was performed in all 5 patients with PPFE. Multivariate analysis did not identify a significant risk factor for pneumothorax.
Table 3
| Preoperative factors (case number) | Case number of postoperative complications | P value |
|---|---|---|
| Male (n=63): female (n=66) | 8:5 | 0.8000 |
| <70-year-old (n=103): ≥70-year-old (n=26) | 11:2 | 0.4897 |
| Smoker (n=69): non-smoker (n=60) | 5:8 | 0.3176 |
| KL-6 <500 (n=15): KL-6 ≥500 (n=113) | 1:12 | 0.5314 |
| %DLCO <50 (n=21): %DLCO ≥50 (n=107) | 1:12 | 0.3314 |
| IPF (n=17): non-IPF (n=112) | 0:13 | 0.2946 |
| PPFE (n=5): non-PPFE (n=124) | 3:10 | 0.0072 |
| Apex (S1, S1+2) (n=13): others (n=116) | 4:9 | 0.9961 |
| 1 or 2 sites (n=116): 3 sites (n=13) | 11:2 | 0.8739 |
Data are missing for one case each of %DLCO and KL-6 (total 128 cases). DLCO, diffusing capacity for carbon monoxide; KL-6, Krebs von den Lungen-6; IPF, Idiopathic pulmonary fibrosis; PPFE, pleuroparenchymal fibroelastosis.
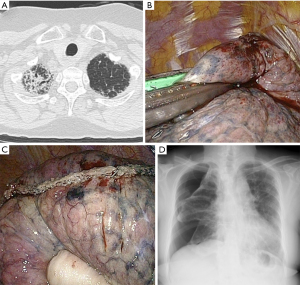
Table 4
| Biopsy site | Number of patients with postoperative pneumothorax/total case number | P value |
|---|---|---|
| Apex (S1, S1+2): others | 4/13:4/116 | 0.0086 |
| Lower lobe: others | 1/41:7/88 | 0.2129 |
Discussion
The usefulness of surgical lung biopsy for the diagnosis of ILD remains controversial, because of the use of chest HRCT. In addition, surgical lung biopsy has high morbidity and mortality. The latest IPF guidelines provide a conditional recommendation of VATS for the definitive diagnosis of patients without a typical UIP pattern on chest HRCT (1). VATS is generally considered to be a safe procedure that provides lung tissue samples that are sufficient for a definitive histopathological diagnosis. However, the postoperative complications are severe in patients with ILD, because the postoperative acute exacerbation of ILD is critical (11). The incidence of postsurgical lung biopsy complications has been reported to be 16–71%, with an overall postoperative mortality of 0–24% (14,15). It was reported that patients with decreased DLCO <50% of the predicted value, with requirement of MV, with immunocompromised status, and with pulmonary hypertension might be at increased risk of death after surgical lung biopsy (9-11). For selected patients with no risk factors, the overall postsurgical lung biopsy mortality rate was reported to be 1.5% (15). Sigurdsson et al. reported 30-day mortality and complication rate after surgical lung biopsy of 2.7% and 16%, respectively, for 73 patients with ILD (16). Moreover, Kreider et al. studied 68 patients with ILD who underwent VATS lung biopsy and found a 60-day mortality and morbidity rate of 4.4% and 19%, respectively (15). A comprehensive literature review by Nguyen and Meyer reported that 9.6% of patients with ILD who underwent VATS developed 1 or more postoperative complications. They also reported an overall 30-day mortality of 2.1% for the VATS lung biopsy (17). In our study, the 30- and 60-day mortality was 0%.
In general, the risk factors of surgical lung biopsy in patients with ILD have been reported to be the following: male sex, increased age, increased number of comorbidities, unstable condition such as rapidly progressing ILD requiring mechanical ventilation, severely impaired pulmonary function, and coexisting pulmonary hypertension (9-11).
Previous reports have focused mainly on the status of a patient (8-11). We have improved the procedure of surgical biopsy for patients with ILDs to reduce their postoperative complications. Therefore, this study focused mainly on the surgical procedure.
The low mortality rate seen in our study may be related to appropriate patient selection and specific surgical procedures such as applying fibrin glue to the partially resected stump and attaching an oxidized cellulose sheet for reinforcement of the residual resected stump. In addition to specific surgical procedures, we provide a perioperative rehabilitation program to reduce the risk of postoperative respiratory infections. A surgical biopsy performed for a patient with worsening respiratory status leads to postoperative respiratory complications and should be avoided.
As the guidelines have implied, the more biopsies are performed, the higher the yield of diagnoses, but the higher the rate of complications. Therefore, only 9% of the patients were biopsied at 3 sites, and these patients were carefully selected based on preoperative discussions.
The postoperative complication rate was 10.1%, including 8 patients with pneumothorax after removal of the chest tube (6.2%), and 1 patient with acute exacerbation (0.8%). Four of 13 (30.8%) patients biopsied in the apex developed complications of pneumothorax after removal of the drainage tube. On the other hand, of the patients undergoing biopsy of the lower lobe only, one 1 showed air leakage. It is thought that the reasons why air leakage is frequently detected after biopsy of the apex of the lung are that the “plane” region is strongly compressed and that a large number of patients had PPFE. Although an increased expression of KL-6 is an indicator of interstitial pneumonia, an assessment of our data did not confirm a significant association between the finding and the frequency of postoperative complications.
In this study, 26% of the patients were diagnosed with unclassifiable idiopathic interstitial pneumonia (IIP). The reasons for the relatively large number of cases of unclassifiable IIP may be that (I) surgical biopsies were not performed in patients with typical HRCT images, and (II) many patients had multiple pathological findings.
This study has limitations. First, the study was retrospective. Second, this study included patients with various diseases. However, despite these limitations, we believe that our findings are important. Currently, cryobiopsy has become popular for the diagnosis of ILD. Despite the retrospective data, our findings should help in deciding between surgical biopsy and cryobiopsy. However, according to our data, surgical biopsy in the apex should be avoided, if possible.
In conclusion, surgical lung biopsy for patients with diffuse lung disease was performed safely. However, biopsy sites for ILD need to be carefully selected to avoid postoperative complications such as seen in patients undergoing biopsy of the apex of the lung, who had a significantly higher rate of postoperative pneumothorax than patients undergoing biopsy of other sites. When performing biopsies in the apex, we should add reinforcements such as fibrin glue and oxidized cellulose sheets.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1773/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1773/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1773/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1773/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of Toho University Omori Medical Center (No. M20122). Informed consent for VATS was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Maher TM. A clinical approach to diffuse parenchymal lung disease. Immunol Allergy Clin North Am 2012;32:453-72. [Crossref] [PubMed]
- Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 2018;6:138-53. [Crossref] [PubMed]
- Halkos ME, Gal AA, Kerendi F, et al. Role of thoracic surgeons in the diagnosis of idiopathic interstitial lung disease. Ann Thorac Surg 2005;79:2172-9. [Crossref] [PubMed]
- Dhooria S, Mehta RM, Srinivasan A, et al. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J 2018;12:1711-20. [Crossref] [PubMed]
- Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193-6. [Crossref] [PubMed]
- Berbescu EA, Katzenstein AL, Snow JL, et al. Transbronchial biopsy in usual interstitial pneumonia. Chest 2006;129:1126-31. [Crossref] [PubMed]
- Hutchinson JP, Fogarty AW, McKeever TM, et al. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. [Crossref] [PubMed]
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. [Crossref] [PubMed]
- Park JH, Kim DK, Kim DS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardiothorac Surg 2007;31:1115-9. [Crossref] [PubMed]
- Bando M, Ohno S, Hosono T, et al. Risk of Acute Exacerbation After Video-assisted Thoracoscopic Lung Biopsy for Interstitial Lung Disease. J Bronchology Interv Pulmonol 2009;16:229-35. [Crossref] [PubMed]
- Sugino K, Otsuka H, Matsumoto Y, et al. The role of video-assisted thoracoscopic surgery in the diagnosis of interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis 2019;36:148-56. [PubMed]
- Otto CM. Left and ventricular systolic function: In: Snyder A, eds. Textbook of clinical echocardiography. 4th ed. Philadelphia: Saunders, 2009:125-56.
- Lettieri CJ, Veerappan GR, Helman DL, et al. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest 2005;127:1600-5. [Crossref] [PubMed]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg 2007;83:1140-4. [Crossref] [PubMed]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, et al. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg 2009;88:227-32. [Crossref] [PubMed]
- Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:3-16. [PubMed]


