
A novel angiographic classification of pseudoaneurysms of the pulmonary chronic inflammatory cavity based on selective angiograms and therapeutic implications
Introduction
Hemoptysis is a common clinical symptom. The main causes of hemoptysis include tuberculosis, lung inflammation, and lung tumors. In the chronic tuberculosis cavity and chronic necrotizing pneumonia cavity, pseudoaneurysms (Pas) easily form and are prone to massive hemoptysis and repeated hemoptysis and can even endanger patient’s life. The classic Pa of the chronic pneumonia cavity is the pulmonary artery pseudoaneurysm (PAPa) that Rasmussen first reported in 1886. The PAPa occurs in the tangential direction of the tuberculosis wall, the inflow section and the outflow section of its parent vessel are the same branch of the peripheral pulmonary artery (PA), and the pulmonary blood flows from the proximal side of the parent vessel to the peripheral side. However, Sbano reported that PA angiography may be better for arterial angiography than for pulmonary angiography (1). Among Pa patients with hemoptysis, some can have hemoptysis controlled through bronchial artery (BA) or nonbronchial systemic artery (nBSA) embolization, but others require embolization of the PA (2). To adequately control hemoptysis, some patients who show PAPa on selective pulmonary angiography require additional arterial embolization after target PA embolization (3). Some Pas appear on systemic angiography, and pulmonary embolization is effective (1). Scholars are more likely to explain these phenomena through the role and effects of systemic-pulmonary shunting on peripheral PAPa (1,3,4). However, it remains to be further analyzed whether Pas of the pulmonary chronic inflammatory cavity selectively affect the peripheral pulmonary branches.
For more than 10 years, in the medical practice of interventional examinations of patients undergoing hemoptysis interventions, angiography equipment with the C-arm cone-beam flat-panel detector computed tomography (CBCT) function was used in the interventional operating room, and intubation digital subtraction angiography (DSA) and CBCT angiography (CBCTA) were performed on the target vessels. The purpose of this study was to explore the diversity of Pas of the pulmonary chronic inflammatory cavity and to provide clinical guidance to doctors regarding angiograph typing and corresponding embolization strategies. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1485/rc).
Methods
Patient population
The data of patients with hemoptysis treated by angiography and embolization hemostasis in Guangzhou First People’s Hospital from January 2008 to January 2018 were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Guangzhou First People’s Hospital (No. K-2012-085) and individual consent for this retrospective analysis was waived. The patients continued hemoptysis after undergoing internal medical care to stop bleeding or after interventions in external hospitals and then received interventional embolization in our hospital. The case inclusion criteria were as follows: (I) hemoptysis caused by a Pa of the pulmonary chronic inflammatory cavity and (II) DSA and/or CBCTA clearly showing the pseudoaneurysm systemic artery collateral (Pasac), parental vessels, inflow section, outflow section and details of blood flow direction in the parent vessel. The exclusion criteria were other causes of hemoptysis. Between January 2008 and January 2018, a total of 392 noncancerous hemoptysis patients underwent angiography and embolization hemostasis at Guangzhou First People’s Hospital. Angiography revealed 25 cases of chest Pa, of which 17 were chronic lung inflammatory cavity Pas, and 9 cases met the inclusion criteria for this study.
Definition
Cumulative hemoptysis <100 mL/d was considered a small amount of hemoptysis (S), hemoptysis >100 mL/time or hemoptysis >300 mL/d was considered a large amount of hemoptysis (L), and a total amount of hemoptysis between 100 and 300 mL/d was considered moderate hemoptysis (M). The duration of hemoptysis was the time from the start of hemoptysis to interventional treatment in our hospital.
Data collection
Detailed hospital admission data were collected for each included patient, including demographic information, signs and symptoms, laboratory test results, and imaging results. The treatment status and outcomes were also recorded. The end point of this study was death or discharge of the patients. A trained team of physicians and researchers collaborated to crosscheck the patient data and verify the integrity and correctness of the data.
Pulmonary CT angiography protocol
Angiography was performed using a digital subtraction angiograph: Allura X per FD-20 (Philips, Eindhoven, The Netherlands) and Artis Zee III-celling (Siemens, Forchheim, Germany) for routine DSA, CBCT plain scan and CBCTA. Bronchial artery/nonbronchial systemic artery CBCTA is abbreviated as BA-CBCTA/nBSA-CBCTA.
Angiographic and embolization techniques
After informed consent was obtained from the patients and their families, the Seldinger technique was used to intubate the thoracic aorta, BA and related nonbronchial systemic arteriography through the right femoral artery approach. Some patients were supplemented with pulmonary angiography via the right femoral vein. Angiography was performed using the posterior-anterior position thoracic DSA method, and in some cases, CBCTA was also performed on the BA and/or nBSA target vessels. The raw volume data acquired by CBCTA were transferred to the corresponding postprocessing workstations. The postprocessing methods included volume rendering (VR), multiplanar reconstruction (MPR), maximum density projection reconstruction (MIP), and dual volume reconstruction (DVR).
Results
General data
Clinical diagnosis was made by clinical history, fiber optic bronchoscopy, thoracic CT examination, sputum examination, etc.; this study included 8 cases of chronic pulmonary tuberculous cavity Pa and 1 case of Pseudomonas aeruginosa pneumonia chronic cavity Pa. Two of the nine patients (cases 4 and 5) continued to have hemoptysis or increased hemoptysis after interventional embolization at another hospital. Nine chest CT examinations showed chronic inflammatory cavities in the lungs, of which cavity gas in 6 patients showed cavity mural nodules (CMNs), and pulmonary chronic inflammatory cavity in 3 patients were filled with liquid. The enhanced CT of 6 of the 7 patients showed significant enhancement of the CMN. CBCTA was performed on the main blood supply artery of the Pa of cases 3–9 while performing DSA. Table 1 summarizes the general condition and features of the 8 patients with Pas.
Table 1
| Case no./sex/age/successful hemostasis | Hemoptysis Sev./Dura. |
Clinical diagnosis | Cavity [Site/size (mm)] | Pa [Size (mm)/shape] | Pa vessels (FBV/IBV/OBV) | DBF (Within OBV) | Embolism (T.V.) |
|---|---|---|---|---|---|---|---|
| 1/male/34/yes | M-L/15 days | STB (CC) | RS3/38×45 | 38×45/oval | PAb/PAb/PAb | Anterograde | PAb |
| 2/male/61/yes | M/20 days | PAP (CC) | LS1+2/74×45 | 8×7/oval | 3SAb/PAb/PAb | Retrograde | SA + PAb |
| 3/female/46/yes | S/15 days | STB (CC) | RS3/29×25 | 2.8×3.1/oval | BA/BA/BA | Anterograde | SA |
| 4/male/45/yes | S-M/24 days | STB (CC) | RS2/33×25 | 26×15/gourd | 2ICA/SAC/SAC | Anterograde | SA |
| 5/male/45/yes | M/33 days | STB (CC) | RS2/15×15 | 6×4/oval | LCA/LCA/LCA | Anterograde | SA |
| 6/male/59/yes | M/6 days | STB (CC) | LS1+2/45×32 | 6×4/oval | BA/SAC/PAb | Anterograde | SA |
| 7/female/45/yes | S-M/31 days | STB (CC) | LS6/25×32 | 5×5/oval | 2BA + IFA/SAC/Pab | Anterograde | SA |
| 8/male/18/lobectomy | S-M/6 days | STB (CC) | RS2/25×30 | 16×9/gourd | 2ICA/SAC/PAb | Retrograde | SA |
| 9/male/22/yes | S-M/55 days | STB (CC) | RS1/19×26 | 9×13/“7” | 2ICA/2SAC/PAb | Retrograde | SA + PAb |
Pa, pseudoaneurysm; FBV, feeding blood vessel; IBV, inflowing blood vessel; OBV, outflowing blood vessel; DBF, direction of blood flow within outflowing blood vessel; T.V., transvascular embolization; M-L, medium-large; STB (CC), secondary pulmonary tuberculosis (chronic cavity); PAb, pulmonary artery branch; M, medium; PAP (CC), Pseudomonas aeruginosa pneumonia (chronic cavity); SAb, systemic artery branch; SA, systemic artery; S, small; BA, bronchial artery; S-M, small-medium; ICA, intercostal artery; SAC, systemic artery collateral; LCA, lateral costal artery; IFA, inferior pulmonary ligament artery.
Angiographic findings and classification of Pa
As shown in Table 1, 7 patients showed a single Pa in a single cavity, and case 2 was closely adjacent to the same parent vessel in a cavity (4.2 mm × 4.6 mm, 3.5 mm × 3.9 mm). In case 9, two adjacent Pas in a cavity wall nodule merged into a “7” shape (positive projection side length 9 mm × 13 mm). Cases 4, 5, and 8 of Pa from the arterial blood supply were revealed during thoracic aorta angiography. In cases 1, 2, 3, 8, and 9, cavity side pulmonary angiography showed that the perfusion decreased in the pulmonary area where the cavity was located, and the branching of the PA was filled as a stump cutoff. The parental vessels that formed the Pa in 9 patients included pulmonary arteries (cases 1, 2), systemic arteries (cases 3–5), and systemic-pulmonary anastomotic vessels (cases 6–9). The blood supply vessels of the Pas included 1 pulmonary artery branch (PAb) (case 1) and 15 systemic arteries (cases 2–9), namely, 4 bronchial arteries (3 cases), 4 subclavian arteries (2 cases), 6 intercostal arteries (3 cases), and 1 lower lung ligament artery (1 case), and there were 3 cases (cases 3, 5, 6) with one blood supply artery, 3 cases (cases 4, 8, 9) with 2 blood supply arteries, and 2 cases (cases 2, 7) with 3 blood supply arteries. The systemic arteries form a complex aberrant hyperplasia network in the lung surface adjacent to the cavity and in the lung lesions. The branches of the systemic arteries enter the diseased lung tissue, wherein cases 3–5 formed Pas along the cavity wall. The systemic arteries in cases 6–9 and the branches of the pulmonary arteries on the cavity wall were anastomosed to form Pas. In case 2, the systemic arteries and the peripheral branches of the PA were anastomosed to form Pas.
According to the difference between the inflow and outflow sections of the parent vessels and the direction of blood flow in the parent vessels, the 9 Pas in our study can be divided into the following types I: PAPa, the inflow and outflow sections of the parent vessel that form the Pa are the same peripheral PAb; Ia: pulmonary artery Pa supplied by the pulmonary artery (PA-PAPa), blood supply is derived from the PA, and the direction of blood flow is from the proximal side of the PA to the distal side, as in case 1; Ib: pulmonary artery pseudoaneurysm supplied by the systemic artery (SA-PAPa), the peripheral end of the parent vessel is anastomosed to the lateral branch of the systemic artery, and the blood flows retrograde from the peripheral side of the parent vessel to the proximal side, as in case 2 (Figure 1); II: systemic arterial pseudoaneurysm (SAPa), the parent vessel of the Pa consists of systemic artery branches or collaterals; IIa: bronchial artery pseudoaneurysm (BA-SAPa), the parent vessel is the branch of the BA, as in case 3 (Figure 2); IIb: non-bronchial systemic artery pseudoaneurysm (nBSA-SAPa), the parent vessels are nonbronchial arterial collaterals, as in cases 4, 5 (Figure 3); III: systemic-pulmonary anastomotic pseudoaneurysm (SPPa), the parent vessels of the Pa are anastomotic vessels composed of the collateral branch of the systemic artery and the peripheral branches of the PA on the cavity wall, and vascular damage occurs at the anastomosis; IIIa: systemic-pulmonary anastomotic pseudoaneurysm with anterograde centrifugal filling of the outflowing pulmonary artery branches (SP antero Pa), in the outflow section of the parent artery, the blood flow in the peripheral branch of the PA is anterograde and centrifugally filled, as in cases 6, 7 (Figure 4); and IIIb: systemic-pulmonary anastomotic pseudoaneurysm with retrograde centripetal filling of outflow pulmonary artery branches (SP retro Pa), the blood flow of the PAbs in the outflow section of the parent artery is retrograde and centripetal , as in cases 8, 9 (Figures 5,6).
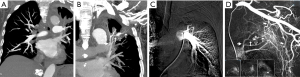
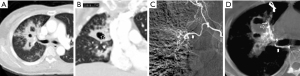
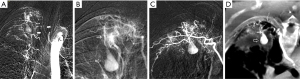
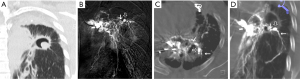
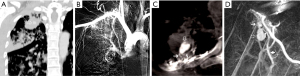
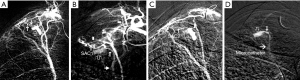
Vascular embolization treatment
All bronchial and nonbronchial systemic arteries in supply cavity lesions were embolized using PVA particles and/or gelatin sponge particles. For pulmonary cavity Pa, the corresponding embolization method was used according to the angiographic findings. In case 1, the PA was embolized with stainless steel coils near the neck of the Pa. In case 2, 3 left subclavian branches of the Pa were embolized with PVA particles, and via the pulmonary route, a 1:3 concentration of an NBCA-lipiodol mixture was injected into the Pasac and parent vessels. In case 3, the parent vessel and Pasac were embolized with a 1:5 concentration of the NBCA-lipiodol mixture. In cases 4–9, PVA particles were used to embolize the blood supply artery of the parent vessel. In case 9, the outflow PAb of the Pa was successfully embolized with a micro-steel coil near the neck of the Pa through super selective PA intubation (Figure 6D). In case 8, the super selective PA intubation of the Pa outflow was unsuccessful. Two severe hemorrhages occurred within 10 days after embolization, and the right upper lobe was excised. The remaining 8 cases of embolization hemostasis were technically and clinically successful. The patients were stable after surgery and were discharged in a short time. Symptomatic treatment continued in all cases after surgery, and there was no recurrent hemoptysis during the follow-up period (4–38 months, 18.2±10. 8 months). In case 2, on CT scan of the chest at 4 months after surgery, the high-density NBCA-lipiodol mixture cast remained in the Pasac and the parent vessels. In case 4, the chest CT after 4 months showed that the Pa disappeared, the cavity was closed, and local fibrous plaque was repaired.
Discussion
This study showed that there is a diversity of Pas of the pulmonary chronic inflammatory cavity. This diversity was mainly manifested in 3 aspects: (I) the parent vessels of Pas can be BA branches and nBSA collaterals, body-pulmonary anastomosis vessels, or the peripheral branches of the PA; (II) the blood supply source of PA Pas could be the PA or systemic arteries; and (III) in Pas of systemic-PA anastomosis vessels, the blood flowing out from the PAb may flow in the anterograde centrifugal direction or in the retrograde centripetal direction. In the 9 patients in this study, there was only 1 case of pulmonary Pa in a typical sense. There were 4 cases of Pa that occurred in systemic-pulmonary anastomosis vessels, and the parent vessels in 3 cases were BA branches and nBSA collaterals. This finding suggests that Pas of systemic arteries and systemic-PA anastomosis were common forms of Pas of the pulmonary chronic inflammatory cavity. Even in the PA Pa in case 2, the peripheral end branch of the parent PA could also obtain blood by anastomosis with the collateral branch of the systemic artery.
Almost all of the PAs of the pulmonary chronic inflammatory cavity were regarded as PA Pas, and based on this classification, corresponding angiographic typing and embolization treatment programs have been proposed (3-6). In practice, however, inflammatory and infectious lesions in the lungs can obtain blood from the BA, nBSA, and PA systems (7,8). The source of hemoptysis can come from one or more of these three systems (9,10). Destructive lesions of the lung, regardless of pathogenesis, can destroy and corrode any blood vessels in and around the lesion, whether the PA or a systemic artery (2). During the formation of chronic lesion cavities in the lung with necrotizing inflammation and tuberculous inflammation, hyperplastic and anastomotic vessels adjacent to the cavity or blood vessels originally located in the cavity-forming region can be wrapped or dragged into the cavity wall, which not only selectively involves the PA (3). Blood vessels invading the cavity wall of chronic inflammation are infiltrated and destroyed by inflammation and inflammatory granuloma, and granulation tissue is gradually replaced by fibrin. Under the synergy of intravascular pressure, the local blood vessel wall is progressively weak and thin and can break into the cavity to form a Pa (1,11-13). This study suggests that the diversity of Pas of pulmonary chronic inflammatory cavities is difficult to summarize alone in terms of “pulmonary artery Pa”.
Imaging knowledge of Pas of the pulmonary chronic inflammatory cavity has been derived from two-dimensional angiography, chest enhanced CT scan, and computed tomographic angiography (CTA). However, image overlay of two-dimensional angiography and multislice spiral CT has limited ability to resolve small blood vessels in the lung, limiting the complete display of the details of pulmonary vascular lesions. CBCTA has the ability to seamlessly integrate volumetric CT imaging and DSA imaging into volumetric angiography, and for the volume data obtained by the injection of the contrast agent through transvascular catheterization, the isotropic voxel volumetric dataset was visualized at any angle using MPRs, maximum intensity projections and VR techniques at any angle, which greatly enhances the diagnostic ability of angiography and the guiding role of interventional surgery (14). The combination of DSA and CBCTA helps to identify the type of angiography of a Pa of the pulmonary chronic inflammatory cavity. Pas are composed of Pasacs and parent vessels. The key factor to clarifying the type of Pa is to clarify the composition of the parent vessel. In this study, we divided the parent vessels into inflow sections and outflow sections, and combined with the blood flow direction in the parent vessel, the true state of the Pa of the pulmonary chronic inflammatory cavity in vivo was revealed. Cases 3–9 showed that, although DSA angiography can clearly show cavity Pas, in most cases, two-dimensional angiography had difficulty distinguishing the inflow section and outflow section of the parent vessel, as well as the details of the damage to the parent vessel forming the Pa, from a bunch of cluttered arterial branch hyperplasia images. The DSA two-dimensional dynamic sequence and CBCTA rotation acquisition sequence images help us to observe the dynamic imaging process of Pas. CBCTA concentrates angiographic information in one volumetric data set, and postprocessing reconstruction provides a panoramic view of the parent artery and Pa, allowing physicians to confidently diagnose and type Pa. As far as we know, there have been no reports of the use of CBCTA to guide embolize hemostasis in patients with hemoptysis. Our study showed that CBCTA can provide more accurate diagnostic information on the basis of DSA, which is worthy of popularization and application.
Understanding that Pas of the pulmonary chronic inflammatory cavity are diverse helps to explain angiographic findings and select the most appropriate embolization strategy. Based on angiographic findings, Shin classified Pas of the peripheral pulmonary artery (PAPs), which were associated with infectious lung disease, into four types of angiography (4). Tsukada used a classification system proposed by Shin et al. for selective pulmonary and arterial embolization and achieved good results (3). Based on the clear display of the Pasac and the parent vessels by angiography and CBCTA, our study considered the composition of the parent vessels of the Pa, the blood flow direction in the parent vessels, and the blood supply source as a whole, taking the imaging morphology and hemodynamics into account at the same time, rendering the description of the body state of Pa closer to reality, and it is more feasible to perform angiographic classification on this basis. The actual embolization effect of the cases in our study shows that the embolization strategy for Pas of the pulmonary chronic inflammatory cavity can be more organized and simplified according to the angiographic classification. (I) For Pas shown by systemic arterial angiography, if there is no peripheral PAb as the outflow section of the parent vessel (i.e., type II: SAPa) or if the parent vessel outflows from the PAb to reveal anterograde centrifugal filling (i.e., type IIIa: SP antero Pa), it is only necessary to firmly embolize all of the arterial blood vessels involved in the Pa blood supply. (II) For Pas shown on systemic artery angiography, if the peripheral PAb of the parent vessel (such as type Ib: SA-PAPa) or the outflow PAb of the parent vessel (such as type IIIb: SP retro Pa) is filled in a retrograde manner, it is necessary to firmly embolize all of the systemic arteries involved in the blood supply to the Pa and to catheterize and embolize the peripheral branch of PA in the outflow section of the parent vessels through the PA. (III) For pulmonary artery Pas supplied by the pulmonary artery (such as type Ia: PA-PAPa), it is only necessary to embolize the Pasac and/or parent vessels via the PA. (IV) For cases in which successful intubation can be performed in the parent vessel via the PA, the sac is thrombosed in situ, and front and back door techniques or a continuous embolization technique can be used to occlude parent vessels and aneurysms (15).
This study has the following deficiencies: (I) our study was a small-scale case study belonging to a single center, and other types of angiographic findings may be observed as the number of cases increases. For example, in some cases, when arterial blood with systemic-pulmonary anastomosis fills the PA trees of the cavity area centripetally, it can laterally flow into the adjacent PAb and forward to perfuse the Pa on the PAb (PA Pa of bypass systemic artery supply). (II) Similar to the vast majority of studies in the literature on pulmonary Pas, the cases in our study are also limited to imaging studies. We believe that, for hemoptysis patients with Pas of the pulmonary chronic inflammatory cavity, a comprehensive chest vascular system assessment, including PA, BA, and nBSA assessments (16), is required, and according to the type of angiography, the corresponding appropriate vascular embolization strategy can be adopted.
Acknowledgments
The authors would like to express their gratitude to the following colleagues for their support and participation in this study: Xishan Li, Shuoyi Ma, Long Huang, Jiaqiu Liang, Chaoping Luo and Xizhen Wen. The authors would also like to express their gratitude to Miss Yanwan Liao for her assistance in revising this article.
Funding: This work was supported by the Guandong Natural Science Foundation (2017A030313860). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1485/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1485/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1485/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sbano H, Mitchell AW, Ind PW, et al. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005;184:1253-9. [Crossref] [PubMed]
- Remy J, Lemaitre L, Lafitte JJ, et al. Massive hemoptysis of pulmonary arterial origin: diagnosis and treatment. AJR Am J Roentgenol 1984;143:963-9. [Crossref] [PubMed]
- Tsukada J, Hasegawa I, Torikai H, et al. Interventional therapeutic strategy for hemoptysis originating from infectious pulmonary artery pseudoaneurysms. J Vasc Interv Radiol 2015;26:1046-51.e1. [Crossref] [PubMed]
- Shin S, Shin TB, Choi H, et al. Peripheral pulmonary arterial pseudoaneurysms: therapeutic implications of endovascular treatment and angiographic classifications. Radiology 2010;256:656-64. [Crossref] [PubMed]
- Kierse R, Jensen U, Helmberger H, et al. Value of multislice CT in the diagnosis of pulmonary artery pseudoaneurysm from Swan-Ganz catheter placement. J Vasc Interv Radiol 2004;15:1133-7. [Crossref] [PubMed]
- Shin TB, Yoon SK, Lee KN, et al. The role of pulmonary CT angiography and selective pulmonary angiography in endovascular management of pulmonary artery pseudoaneurysms associated with infectious lung diseases. J Vasc Interv Radiol 2007;18:882-7. [Crossref] [PubMed]
- Sevin CM, Light RW. Microscopic anatomy of the pleura. Thorac Surg Clin 2011;21:173-5. vii. [Crossref] [PubMed]
- English JC, Leslie KO. Pathology of the pleura. Clin Chest Med 2006;27:157-80. [Crossref] [PubMed]
- Patankar T, Prasad S, Deshmukh H, et al. Fatal hemoptysis caused by ruptured giant Rasmussen's aneurysm. AJR Am J Roentgenol 2000;174:262-3. [Crossref] [PubMed]
- Larici AR, Franchi P, Occhipinti M, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol 2014;20:299-309. [Crossref] [PubMed]
- Jayet PY, Denys A, Zellweger JP, et al. Successful embolization of Rasmussen's aneurysm for severe haemoptysis. Swiss Med Wkly 2004;134:705-6. [PubMed]
- Gesuete V, Corzani A, Bronzetti G, et al. Rasmussen's aneurysm in childhood: a case report. Congenit Heart Dis 2013;8:E41-4. [Crossref] [PubMed]
- Jaureguizar Oriol A, Ayala Carbonero AM, Gorospe Sarasúa L. Life-threatening hemoptysis secondary to Rasmussen's aneurysm in an HIV patient. Arch Bronconeumol 2016;52:439-40. [Crossref] [PubMed]
- Budai C, Cirillo L, Patruno F, et al. Flat panel angiography images in the post-operative follow-up of surgically clipped intracranial aneurysms. Neuroradiol J 2014;27:203-6. [Crossref] [PubMed]
- Krokidis M, Spiliopoulos S, Ahmed I, et al. Emergency endovascular management of pulmonary artery aneurysms and pseudoaneurysms for the treatment of massive haemoptysis. Hellenic J Cardiol 2014;55:204-10. [PubMed]
- Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. Multi-detector row CT of hemoptysis. Radiographics 2006;26:3-22. [Crossref] [PubMed]


