Residing in a food desert is associated with an increased risk of readmission following esophagectomy for cancer
Introduction
Despite advancements in treatment practices, the overall prognosis of esophageal cancer remains poor. Neo-adjuvant chemoradiotherapy (nCRT) followed by esophagectomy (tri-modality therapy) remains the standard of care for surgically resectable disease, offering improved overall 5-year survival, compared to non-operative management or surgery alone for patients with greater than T1a lesions (1,2). Nevertheless, perioperative morbidity and long-term mortality rates as a result of tumor recurrence are high (3). While recent advances including the use of adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer are addressing recurrence issues (4), much work remains to be done to improve perioperative morbidity and mortality.
Pre-treatment body mass index (BMI) and nutritional status have been identified as potentially modifiable risk factors in patients with gastrointestinal malignancies undergoing surgical treatment. Extreme BMIs (<18.5 or ≥40 kg/m2) have been linked to higher rates of complications following esophagectomy compared to patients with normal weight and class I/II obesity (5). Poor nutritional status (defined as pre-albumin <35 g/L and weight loss >10% over 6-month) in patients with esophageal cancer has been associated with poor response to chemoradiotherapy, increased respiratory morbidity, increased risk of post-operative complications, and reduced survival following surgery (6,7). When intensive pre-operative nutritional support was employed in this population, either with total parenteral nutrition, feeding tubes, or dietician-delivered support, the rate of treatment complications was significantly reduced (8-12). Thus, nutritional status and malnutrition seem to play a bigger role in determining treatment outcomes than overall body weight (13).
Sarcopenia, defined as the loss of muscle tissue, has been considered a surrogate marker for nutritional status, regardless of the patient’s BMI. Though previously described as part of cancer cachexia (late stage disease), sarcopenia has been shown to be a risk factor for dose limiting toxicity during chemotherapy, major post-operative complications, and reduced survival in esophageal cancer patients who are still considered having only locally advanced disease (14-18).
Food deserts (FDs) are a relatively new area of interest that examines both nutrition-related and patient specific factors including socioeconomic status. Poor nutrition is considered a risk factor for the development of esophageal cancer, and worse treatment outcomes associated with nutrition have been linked to lower socioeconomic status (19-21). The United States Department of Agriculture (USDA) defines a FD as “a low-income census tract where a significant number of people have low access to supermarkets or large grocery stores” (22). Individuals who reside in one of these areas will also have limited access to healthy foods, such as fruits and vegetables, typically carried by these stores. This lack of access has been to shown to result in poor eating habits, and as such individuals in these areas are more likely to have diet-related diseases, including hypertension and diabetes (23). The USDA has compiled a publicly available Food Access Research Atlas that can be searched based on town and zip codes to determine if an individual resides in one of these areas (24). Though emerging evidence suggests FD status is negatively associated with health outcomes (25), only recently has there been research examining this relationship in cancer patients (26). However, the impact of living in a FD and the effects on clinical outcomes in patients undergoing tri-modality therapy for esophageal cancer are unknown.
As the overall morbidity of tri-modality therapy for esophageal cancer remains high, efficient risk assessment and patient optimization are vital to improving patient care. Furthermore, prompt identification of risk factors to proactively deploy targeted resources appropriately and improve treatments will be critical in improving overall outcomes in this patient population. There were two main aims of this study. The first was to investigate the cross-sectional association of living in a FD on perioperative outcomes, and the second aim was to assess the impact of residing in a FD on 30-day postoperative outcomes in a cohort of esophageal cancer patients who underwent tri-modality therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1637/rc).
Methods
Patients
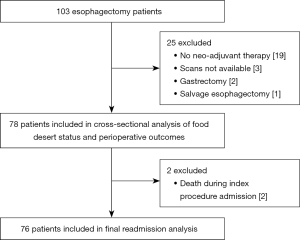
This was a retrospective cohort study of a prospectively maintained thoracic surgery database. Consecutive patients who underwent esophagectomy at our quaternary care hospital between January 1, 2015 and July 31, 2020 were eligible for review. Patients were excluded if they did not receive nCRT prior to surgery (n=19), underwent combined gastrectomy and esophagectomy (n=2), salvage esophagectomy (n=1) or if they did not have both pre and post neo-adjuvant treatment computed tomography (CT) scans available for review (n=3) (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (#30500) at Dartmouth College and Dartmouth-Hitchcock Medical Center and was granted a waiver of consent due to the retrospective nature of the study.
Data collection
Demographic data, geographic location, travel distance to the treatment center, comorbidities, BMI, smoking status, alcohol use, treatment characteristics, weight loss during treatment, nutrition visits throughout treatment, clinical stage of disease, post-operative length of stay, readmissions, 30-day complications and 90-day mortality were obtained. Data fields not available in the database were obtained via medical record review. The primary outcome measure was readmission (yes vs. no) defined as readmission to any hospital within 30 days after discharge. This was assessed in the database as all patients were followed at our institution. Postoperative complications were monitored through 30-day after surgery and graded I–V as classified by Clavien-Dindo (27). Complication data follow Society of Thoracic Surgeons definitions, and are prospectively collected via the thoracic surgery database (28). Travel distance to the treatment center was estimated by the most direct route via Google Maps. To attempt to control for socio-economic status, median household income for individuals (estimated by cross-referencing patient’s home zip code with the United States Census Bureau Quick Facts (29) and insurance status were included. Additionally, in an attempt to control for nutritional related confounders, BMI, psoas muscle index, sarcopenia, albumin levels, weight loss and pre-resection need for a feeding tube were all gathered and analyzed. The primary exposure was residing in a FD, obtained from the USDA Food Access Research Atlas (24) and cross-referenced with patient home zip code to determine FD status (yes vs. no). Covariates were selected among available data, and based on their potential associations with residing in a FD and discharge status.
Measurement of skeletal muscle
Psoas muscle index was calculated via psoas muscle measurements on CT scan. A board-certified radiologist (MM) and a member of the study team trained by the radiologist (KF), obtained bilateral psoas muscle measurements. The cross-sectional area of each psoas muscle at the level of the third lumbar vertebrae (L3) were compared (30). Pre and post neo-adjuvant treatment scans were synced so the same level and used for both measurements. Measurements were obtained on axial CT images in mm, and the cross-sectional area was estimated by multiplying the major axis muscle diameter/2 by the minor axis muscle diameter/2 by π. The area was then normalized for patient height by dividing by height in m2. Sarcopenia was defined using sex-specific cutoff points of <390 mm2/m2 for women and <550 mm2/m2 for men (30).
Statistical analysis
Statistical analysis was performed using Stata/SE 16.1 (StataCorp LLC, College Station, TX, USA). Univariable analysis was conducted using Student’s t-test or Wilcoxon rank-sum for continuous variables, and Fisher’s exact test for categorical variables. Multivariable logistic regression was then used to model readmission status (dependent variable) on FD status (independent variable), adjusted for demographic and clinical measures statistically associated with readmission status at the P<0.10 in univariable analyses. Only those who were alive at discharge were included in this analysis, The final model included FD status, operative time (continuous in 15-minute increments), discharge to a rehabilitation facility, and presence of a grade III/IV complication. Statistical significance of main effects was set at P<0.05 (two-sided).
Results
Of the 103 esophagectomies that occurred during the study period, 78 met inclusion criteria (Figure 1), 23 (29.5%) of whom lived in a FD. There were no significant differences in age, sex, race, pack-years smoked, smoking status, alcohol use, cardiopulmonary comorbidities, pre-treatment BMI, pre-treatment sarcopenia, clinical stage, travel distance, median household income or insurance status between those who lived in a FD and those who did not (Table 1). Interestingly, patients living in a FD were more likely to have a better pre-treatment performance status and female patients had a higher psoas muscle index. Furthermore, no differences were noted in pre-operative dietary interventions, duration of induction therapy, pre-operative performance status, post-nCRT weight loss, post-nCRT psoas muscle index, new post-nCRT sarcopenia, surgical approach, operative time, pathologic stage, index procedure length of stay or total 30-day grade III/IV complication rate based on FD status (Table 2). Patients living in a FD had a significantly higher rate of post-discharge grade III/IV complications (30.4% vs. 10.9%, P=0.05) and readmission within 30 days (39.1% vs. 13.2%, P=0.02). No differences in 30- or 90-day mortality were noted based on FD status.
Table 1
| Characteristics | FD status1 | ||
|---|---|---|---|
| Yes (n=23) | No (n=55) | P value2 | |
| Age (years), mean (SD) | 64.0 (7.4) | 64.9 (8.5) | 0.66 |
| Male, n (%) | 20 (87.0) | 46 (83.6) | >0.99 |
| Caucasian, n (%) | 23 (100.0) | 54 (98.2) | NA |
| Smoking status3, n (%) | 0.65 | ||
| Never | 8 (34.8) | 13 (23.6) | |
| Former | 10 (43.5) | 29 (52.7) | |
| Current | 5 (21.7) | 13 (23.6) | |
| Pack-years, mean (SD) | 42.9 (24.6) | 49.2 (38.8) | 0.56 |
| Alcohol use, n (%) | 0.59 | ||
| None | 6 (26.1) | 16 (29.1) | |
| Current use4 | 11 (47.8) | 22 (40.0) | |
| Current heavy use5 | 1 (4.4) | 0 | |
| Prior use4 | 0 | 2 (3.6) | |
| Prior heavy use5 | 5 (21.7) | 15 (27.3) | |
| Comorbidities, n (%) | |||
| Asthma | 2 (8.7) | 5 (9.1) | >0.99 |
| Chronic obstructive pulmonary disease | 3 (13.0) | 5 (9.1) | 0.69 |
| Hypertension | 11 (47.8) | 26 (47.3) | >0.99 |
| Coronary artery disease | 4 (17.4) | 7 (12.7) | 0.72 |
| Myocardial infarction | 0 | 2 (3.6) | >0.99 |
| Diabetes | 7 (31.8) | 17 (30.9) | >0.99 |
| Cerebrovascular accident | 0 | 3 (5.5) | 0.68 |
| Pre-treatment ECOG performance status, n (%) | 0.04 | ||
| 0 | 13 (56.5) | 16 (29.1) | |
| 1 | 10 (43.5) | 39 (70.9) | |
| Pre-treatment BMI (kg/m2), mean (SD) | 28.2 (6.1) | 29.3 (5.1) | 0.41 |
| Pre-treatment psoas muscle index6 (mm2/m2), mean (SD) | |||
| Male | 818.8 (217.8) | 803.2 (294.4) | 0.83 |
| Female | 652.9 (87.8) | 486.07 (106.6) | 0.04 |
| Pre-treatment sarcopenia7, n (%) | |||
| Male | 2 (10.0) | 7 (15.2) | 0.71 |
| Female | 0 | 1 (11.1) | >0.99 |
| Clinical stage, n (%) | 0.38 | ||
| II | 2 (2.9) | 12 (21.8) | |
| III | 15 (65.2) | 30 (54.5) | |
| IV | 6 (26.1) | 13 (23.6) | |
| Driving distance to hospital (km), mean (SD) | 112.3 (45.4) | 106.5 (41.7) | 0.59 |
| Median household income8 (dollars), median (interquartile range) | 48,212 (39,504–56,307) | 55,556 (45,229–68,240) | 0.10 |
| Insurance status, n (%) | >0.99 | ||
| Medicare, military, commercial | 21 (91.3) | 50 (90.9) | |
| Medicaid, none | 2 (8.7) | 5 (9.1) | |
1, as defined by the USDA; 2, P values from Student’s t-test, Wilcoxon rank-sum, or Fisher’s exact test where appropriate; 3, classified at the time of first consultation with a thoracic surgeon; 4, ≤7 drinks per week for females, ≤14 drinks per week for males; 5, >7 drinks per week for females, >14 drinks per week for males; 6, psoas muscle index as defined by psoas muscle area normalized for height; 7, as defined by sex-specific cutoffs; 8, by zip code, as reported by the United States Census Bureau. FD, food desert; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; NA, not applicable; USDA, United States Department of Agriculture.
Table 2
| Characteristics | FD status1 | ||
|---|---|---|---|
| Yes (n=23) | No (n=55) | P value2 | |
| Pre-operative dietitian visits, mean (SD) | 3.1 (2.5) | 4.0 (3.5) | 0.25 |
| Pre-operative albumin <3 g/dL | 0 | 0 | NA |
| Pre-resection feeding tube3, n (%) | 3 (13.0) | 3 (5.5) | 0.35 |
| Induction therapy duration (days), mean (SD) | 37.7 (7.0) | 38.8 (6.5) | 0.52 |
| Pre-operative ECOG performance status, n (%) | 0.10 | ||
| 0 | 3 (13.0) | 12 (21.8) | |
| 1 | 18 (78.3) | 43 (78.2) | |
| 2 | 2 (8.7) | 0 | |
| Pre-operative percent weight loss4 (kg), mean (SD) | −8.4 (8.1) | −7.2 (7.6) | 0.54 |
| Post-treatment psoas muscle index5 (mm2/m2), mean (SD) | |||
| Male | 724.8 (185.1) | 724.8 (296.6) | >0.99 |
| Female | 526.3 (5.2) | 434.9 (114.1) | 0.21 |
| New post-treatment sarcopenia6, n (%) | |||
| Male | 3 (15.0) | 7 (15.2) | >0.99 |
| Female | 0 | 2 (22.2) | >0.99 |
| Operative time (minutes), mean (SD) | 502.7 (86.9) | 478.3 (454.8) | 0.26 |
| Surgical approach, n (%) | 0.12 | ||
| Robotic-assisted | 18 (78.3) | 32 (58.2) | |
| Open | 5 (21.7) | 23 (41.8) | |
| Pathologic stage, n (%) | 0.18 | ||
| I | 11 (47.8) | 21 (38.2) | |
| II | 2 (8.7) | 9 (16.4) | |
| III | 2 (8.7) | 11 (20.0) | |
| IV | 0 | 5 (9.1) | |
| No residual | 8 (34.8) | 9 (16.4) | |
| Index procedure length of stay (days), mean (SD) | 9.5 (4.3) | 10.0 (4.4) | 0.66 |
| Discharge location*, n (%) | 0.64 | ||
| Home | 21 (91.3) | 50 (94.3) | |
| Rehab facility | 2 (8.7) | 3 (5.7) | |
| 30-day grade 3/4 complications7, n (%) | 13 (56.5) | 24 (43.6) | 0.33 |
| In-hospital | 8 (34.8) | 20 (36.4) | >0.99 |
| Anastomotic leak | 1 (4.3) | 2 (3.6) | >0.99 |
| Delayed gastric emptying | 2 (8.7) | 8 (14.5) | 0.74 |
| Pleural effusion | 1 (4.3) | 1 (1.8) | 0.51 |
| Respiratory failure | 0 | 1 (1.8) | NA |
| Pneumothorax | 1 (4.3) | 1 (1.8) | 0.51 |
| After discharge | 7 (30.4) | 6 (10.9) | 0.05 |
| Anastomotic leak | 3 (13.0) | 0 | NA |
| Delayed gastric emptying | 1 (4.3) | 0 | NA |
| Respiratory failure | 1 (4.3) | 0 | NA |
| Pleural effusion | 3 (13.0) | 3 (5.5) | 0.35 |
| 30-day readmission*, n (%) | 11 (39.1) | 8 (14.5) | 0.004 |
| 30-day mortality**, n (%) | 0 | 2 (3.6) | NA |
| 90-day mortality, n (%) | 3 (13.0) | 3 (5.5) | 0.35 |
*, excludes 2 in-hospital mortalities; **, includes 2 individuals who died in-hospital. 1, as defined by the USDA; 2, P values from Student’s t-test or Fischer’s exact test where appropriate; 3, includes j-tube and transnasal feeding tube placement; 4, percent of weight lost from initial visit to day of surgery; 5, psoas muscle index as defined by psoas muscle area normalized for height; 6, as defined by sex-specific cutoffs; 7, grade 3/4 complications as classified by Clavien-Dindo. FD, food desert; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; NA, not applicable; USDA, United States Department of Agriculture.
In order to more fully investigate other potential risk factors for readmission, we stratified the patients by readmission status. Excluding 2 in-hospital mortalities, 19 patients (25.0%) were readmitted within 30 days of discharge from their index procedure. Demographic characteristics of patients who were and were not readmitted were similar (Table 3). Both groups had an average age of 64, and were primarily male (94.7% vs. 80.7%, P=0.27). There were no differences in smoking history, pack-years smoked, alcohol use, cardiopulmonary co-morbidities or pre-treatment performance status, BMI, psoas muscle index, sarcopenia, or clinical stage. Both groups had similar average travel distance to the institution, median household income and insurance status. Patients who resided in a FD were significantly more likely to be readmitted than those who resided in areas with access to adequate nutritional sources (57.9% vs. 21.1%, P=0.002).
Table 3
| Characteristics | Readmission within 30 days of discharge* | ||
|---|---|---|---|
| Yes (n=19) | No (n=57) | P value1 | |
| Age (years), mean (SD) | 64.7 (8.1) | 64.3 (8.2) | 0.85 |
| Male, n (%) | 18 (94.7) | 46 (80.7) | 0.27 |
| Caucasian, n (%) | 19 (100.0) | 56 (98.3) | NA |
| Smoking status2, n (%) | 0.94 | ||
| Never | 5 (26.3) | 15 (26.3) | |
| Former | 9 (47.4) | 30 (52.6) | |
| Current | 5 (26.3) | 12 (21.1) | |
| Pack-years, mean (SD) | 45.6 (31.4) | 48.6 (37.4) | 0.79 |
| Alcohol use, n (%) | 0.52 | ||
| None | 4 (21.1) | 16 (28.1) | |
| Current use3 | 9 (47.4) | 24 (42.1) | |
| Current heavy use4 | 1 (5.3) | 0 | |
| Prior use3 | 0 | 2 (3.5) | |
| Prior heavy use4 | 5 (26.3) | 15 (26.3) | |
| Comorbidities, n (%) | |||
| Asthma | 2 (10.5) | 5 (8.8) | >0.99 |
| Chronic obstructive pulmonary disease | 2 (10.5) | 6 (10.5) | >0.99 |
| Hypertension | 9 (47.4) | 27 (47.4) | >0.99 |
| Coronary artery disease | 3 (15.8) | 8 (14.0) | >0.99 |
| Myocardial infarction | 0 | 2 (3.5) | >0.99 |
| Diabetes | 6 (31.6) | 18 (31.6) | 0.56 |
| Cerebrovascular accident | 1 (5.3) | 2 (3.5) | 0.62 |
| Pre-treatment ECOG performance status, n (%) | 0.42 | ||
| 0 | 9 (47.4) | 20 (35.1) | |
| 1 | 10 (52.6) | 37 (64.9) | |
| Pre-treatment BMI (kg/m2), mean (SD) | 28.1 (4.1) | 29.1 (5.7) | 0.46 |
| Pre-treatment psoas muscle index5 (mm2/m2), mean (SD) | |||
| Male | 874.7 (271.4) | 792.0 (267.0) | 0.27 |
| Female | 567.5 | 524.2 (129.3) | NA |
| Pre-treatment sarcopenia6, n (%) | |||
| Male | 1 (5.6) | 7 (15.2) | 0.42 |
| Female | 0 | 1 (9.1) | >0.99 |
| Clinical stage, n (%) | 0.23 | ||
| II | 4 (21.1) | 11 (19.3) | |
| III | 13 (68.4) | 29 (50.9) | |
| IV | 2 (10.5) | 17 (29.8) | |
| Driving distance to hospital (km), mean (SD) | 98.9 (45.2) | 112.2 (41.8) | 0.24 |
| Median household income7 (dollars), median (interquartile range) | 55,556 (41,029–59,745) | 55,201 (45,229–65,879) | 0.52 |
| Insurance status, n (%) | 0.36 | ||
| Medicare, military, or commercial | 16 (84.2) | 53 (93.0) | |
| Medicaid, or none | 3 (15.8) | 4 (7.0) | |
| Lives in a FD8, n (%) | 11 (57.9) | 12 (21.1) | 0.002 |
*, excludes 2 in-hospital mortalities. 1, P values from Student’s t-test, Wilcoxon rank-sum, or Fisher’s exact test where appropriate; 2, classified at the time of first consultation with a thoracic surgeon; 3, ≤7 drinks per week for females, ≤14 drinks per week for males; 4, >7 drinks per week for females, >14 drinks per week for males; 5, psoas muscle index as defined by psoas muscle area normalized for height; 6, as defined by sex-specific cutoffs; 7, by zip code, as reported by the United States Census Bureau; 8, as defined by the USDA. SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; FD, food desert; NA, not applicable; USDA, United States Department of Agriculture.
No differences were noted in pre-operative nutritional visits or interventions, duration of neo-adjuvant therapy, pre-operative performance status, post-nCRT psoas muscle index, %-change in psoas muscle index, new post-nCRT sarcopenia, surgical approach or pathologic stage between those who were readmitted and those who were not (Table 4). Only 1 male with new post-nCRT treatment sarcopenia was readmitted. In addition, readmitted patients had a larger average psoas muscle index and less %-change in psoas muscle index compared to those who were not readmitted, though this was not statistically significantly different.
Table 4
| Characteristics | Readmission within 30 days of discharge* | ||
|---|---|---|---|
| Yes (n=19) | No (n=57) | P value1 | |
| Pre-operative dietitian visits, mean (SD) | 3.4 (2.6) | 3.7 (3.4) | 0.71 |
| Pre-operative albumin <3.0 g/dL | 0 | 0 | NA |
| Pre-resection feeding tube2, n (%) | 1 (6.3) | 5 (8.3) | >0.99 |
| Induction therapy duration (days), mean (SD) | 39.5 (7.5) | 38.1 (6.4) | 0.46 |
| Pre-operative ECOG performance status, n (%) | 0.36 | ||
| 0 | 5 (26.3) | 10 (17.5) | |
| 1 | 13 (68.4) | 46 (80.7) | |
| 2 | 1 (5.3) | 1 (1.8) | |
| Pre-operative percent weight loss3 (kg), mean (SD) | −5.3 (5.4) | −7.5 (7.3) | 0.24 |
| Post-treatment psoas muscle index4 (mm2/m2), mean (SD) | |||
| Male | 790.9 (253.4) | 709.5 (266.0) | 0.27 |
| Female | 524.6 | 451.6 (108.7) | NA |
| Change in psoas muscle index4 (%), mean (SD) | −9.2 (10.8) | −11.1 (11.1) | 0.54 |
| New post-treatment sarcopenia5, n (%) | |||
| Male | 1 (6.3) | 9 (18.8) | 0.26 |
| Female | 0 | 2 (18.2) | >0.99 |
| Operative time (minutes), mean (SD) | 537.6 (74.9) | 468.1 (85.8) | 0.002 |
| Surgical approach, n (%) | 0.41 | ||
| Robotic-assisted | 14 (81.3) | 35 (60.0) | |
| Open | 5 (18.8) | 22 (40.0) | |
| Pathologic stage, n (%) | 0.98 | ||
| I | 9 (47.4) | 22 (38.5) | |
| II | 2 (10.5) | 9 (15.8) | |
| III | 3 (15.8) | 9 (15.8) | |
| IV | 1 (5.3) | 4 (7.0) | |
| No residual | 4 (21.1) | 13 (22.8) | |
| Index procedure length of stay (days), mean (SD) | 9.6 (3.8) | 10.6 (5.6) | 0.39 |
| Discharge location, n (%) | 0.01 | ||
| Home | 15 (79.0) | 56 (98.3) | |
| Rehab facility | 4 (21.1) | 1 (1.8) | |
| 30-day grade 3/4 complications6, n (%) | 17 (89.5) | 18 (31.6) | <0.001 |
| In-hospital | 8 (42.1) | 18 (31.6) | 0.42 |
| Anastomotic leak | 1 (5.2) | 6 (10.5) | 0.67 |
| Delayed gastric emptying | 2 (10.5) | 8 (14.0) | >0.99 |
| Pleural effusion | 2 (10.5) | 3 (5.3) | 0.59 |
| Respiratory failure | 0 | 2 (3.5) | NA |
| Pneumothorax | 0 | 2 (3.5) | NA |
| After discharge | 13 (68.4) | 0 | NA |
| Anastomotic leak | 4 (21.1) | 0 | NA |
| Delayed gastric emptying | 1 (5.3) | 0 | NA |
| Respiratory failure | 1 (5.3) | 0 | NA |
| Pleural effusion | 7 (36.8) | 0 | NA |
| 30-day mortality, n (%) | 0 | 0 | NA |
| 90-day mortality, n (%) | 2 (10.5) | 2 (3.5) | 0.26 |
*, excludes 2 in-hospital mortalities. 1, P values from Student’s t-test or Fischer’s exact test where appropriate; 2, includes j-tube and transnasal feeding tube placement; 3, percent of weight lost from initial visit to day of surgery; 4, psoas muscle index as defined by psoas muscle area normalized for height; 5, as defined by sex-specific cutoffs; 6, grade 3/4 complications as classified by Clavien-Dindo. SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
Patients that were readmitted had an average longer operative time than those who were not (537.6 vs. 468.1 minutes, P=0.002). Post-operative length of stay was not different based on readmission status. Rates of in-hospital grade III/IV complications were similar between groups (45.1% vs. 31.6%, P=0.42). Not surprisingly, those who were readmitted were significantly more likely to experience an outpatient grade III/IV complication within 30-day of their index procedure, compared to those who were not readmitted (89.5% vs. 31.6%, P<0.001). While a higher proportion of patients who were readmitted experienced any grade III/IV complication, only patients with a post-discharge grade III/IV complications were readmitted, precluding statistical analysis. The most common reasons for readmission were pleural effusion (n=7), anastomotic leak (n=4), wound infection (n=4), aspiration pneumonia (n=2), hypotension (n=1) and deep vein thrombosis (n=1). No differences were noted in 30- or 90-day mortality based on readmission status.
To further evaluate FD status as an independent risk factor for readmission, a multivariable logistic regression model was developed. Unadjusted analysis (Table 5) demonstrated that those who reside in a FD were 5 times more likely to be readmitted [odds ratio (OR): 5.16; 95% confidence interval (CI): 1.70–15.67] compared to those who did not. Residing in a FD remained a significant risk factor for readmission in a multivariate model which adjusted for operative time, discharge to a rehab and presence of a grade III/IV complication (OR: 6.38, 95% CI: 1.45–28.08). This model yielded a c-statistic of 0.90.
Table 5
| Characteristics | Unadjusted OR (95% CI) | Adjusted1 OR (95% CI) |
|---|---|---|
| FD status2 | 5.16 (1.70–15.67) | 6.38 (1.45–28.08) |
| Operative time (per 15 minutes) | 1.16 (1.05–1.29) | 1.17 (1.02–1.35) |
| Discharged to a rehab | 14.93 (1.55–143.71) | 4.04 (0.37–44.33) |
| Grade 3/4 complication3 | 18.4 (3.84–88.35) | 18.6 (2.97–116.32) |
1, adjusted for FD status, operative time, discharge to a rehab, and presence of a grade 3/4 complication; 2, as defined by the USDA; 3, as classified by Clavien-Dindo. FD, food desert; OR, odds ratio; CI, confidence interval.
Discussion
The American Cancer Society estimates over 18,000 new esophageal cancer cases are diagnosed in the United States each year, with only half considered potentially curative (31,32). Despite treatment advances, esophageal cancer continues to be associated with high morbidity and mortality. Re-admissions following treatment are associated with higher cost and decreased patient quality of life. In addition, hospital readmissions following esophagectomy have been associated with significant reductions in long-term survival (33,34). Therefore, pre-treatment risk assessment and patient optimization to reduce complications and readmissions is crucial in this population. Our study examined a potential new prognostic factor for esophageal cancer patients undergoing tri-modality treatment. We found that patients who resided in a FD were 5 times more likely to be re-admitted following their esophagectomy compared to patients who did not. Furthermore, after adjustment, the OR for readmission not only remained approximately the same, but also remained significant, suggesting that the covariates were not mediators on the casual pathway. This easily identifiable information may help clinicians identify which patients may benefit from intensive preoperative interventions.
Our findings indicate that nutritional status may not currently be well-captured in previously established risk factors like BMI or sarcopenia, as has been previously reported. A recent study by Fong et al. noted worse survival for patients with stage II/III breast and colorectal cancers living in urban FDs (26). However, as this was a large administrative database study, the authors were unable to evaluate the quality of care patients received as data on margin status, surgical complications, and duration of therapy were not available. The current study, though involving a single-center and a relatively small number of patients, demonstrates that FD status is an independent prognostic factor for readmission following esophagectomy in patients who received tri-modality therapy without other obvious differences in baseline characteristics and provided care. In addition, residing in a FD represents an easily identifiable combination of the likely related issues of nutrition, access disparities, and socioeconomic status that does not require additional laboratory testing or non-clinical imaging measurements, such as sarcopenia index. Additionally, this study produced similar findings to other studies that assessed malnutrition and post-operative complications. Specifically, that malnourished cancer patients experience more severe post-operative complications and the most common complications to occur were infections and healing disorders (35). And furthermore, that malnutrition in esophagectomy patients is one of the most common causes for readmission (36,37). While evidence suggests a link between FD status and poor health outcomes, this study adds to the emerging literature that it negatively affects esophageal cancer treatment outcomes.
In this study, patients who lived in a FD were significantly more likely to experience a postoperative grade III/IV complication and be readmitted following their esophagectomy. This occurred despite no difference in in-hospital complications or initial postoperative length of stay, indicating they are more apt to have a post-discharge complication. This suggests that all patients fare similarly when provided equal resources in the hospital. However, once discharged, patients who must return to a nutritional and asset-limited environment (i.e., a FD) do worse than their counterparts who have more abundant resources. Social and geographic disparities have been shown to negatively impact access to healthcare, resulting in worse treatment and survival outcomes for multiple types of cancers (38,39). Though this is a small series of patients, our data indicates that residing in a FD may represent another type of socio-geographic disparity that negatively impacts post-treatment outcomes for patients with esophageal cancer undergoing tri-modality therapy.
Many cancer patients note that their nutritional status has a significant impact on their overall quality of life (40,41). Esophagectomy patients specifically note a reduced quality of life post-operatively related to eating and the physiologic effects of the resection (42,43). While nutritional risk-screening is recommended in these patients, no consensus on the best assessment tool exists. Proponents argue that dietary intake, physical activity, and body composition should be critical components of this assessment, including measuring sarcopenia (44,45). However, patients cite a lack of coordination and conflicting messages from their healthcare providers regarding nutritional care as causes of their own uncertainty and confusion (41). Despite limited consensus on the optimal assessment or interventions, early nutritional intervention has been demonstrated to improve treatment outcomes in esophageal cancer patients (45,46). The pre-induction therapy and pre-operative periods are critical times to ensure patients are nutritionally optimized in order to prevent the postoperative period from being a continuation of declining quality of life. Our data suggest that clinicians should consider whether patients live in a FD as part of their nutrition risk screening assessment and use this to help direct resources to limit treatment related adverse outcomes.
There are several limitations to our study. This is a retrospective review from a single-institution with a homogenous, rural patient population deemed healthy enough to undergo esophageal resection. The potential for selection bias exists, and the results may not be generalizable to a more heterogeneous or urban population. Evidence suggests that there is a distinction between urban and rural FDs, and perhaps, despite sharing a similar definition, these two types of deserts may not be directly comparable. Urban FDs are more likely to be characterized by low-income neighborhoods, affecting primarily racial minorities. Rural FDs are characterized by limited access, including a significant decrease in the number of food retailers compared to urban areas (23). Second, though we attempted to control for demographics, co-morbidities, travel distance, median household income and insurance status, potential confounders that we were unable to address, such as patient education level, and activity/exercise level, and likely mediators such as information from detailed diet histories, may exist. The relationship between FD status and these other potential indicators of worse outcomes remain ill-defined and will be the topic of future research. Despite these limitations, this study adds to the limited literature that pre-treatment risk assessment in this population is increasingly important. To our knowledge, this is the first study to examine the relationship of FDs and treatment outcomes in esophageal cancer patients undergoing tri-modality therapy. Results from this study will be used to inform a larger, multi-institutional study that will address many of these limitations and further examine the relationship between FD and treatment outcomes.
Conclusions
Living in a FD is a significant prognostic factor for readmission after esophagectomy for patients receiving tri-modality therapy for esophageal cancer and remained an independent factor after multivariable analysis. Awareness of FD status may help clinicians improve pre-treatment risk assessment and focus interventions to improve patient treatment and outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1637/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1637/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1637/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1637/coif). JDP is supported by the Dartmouth-Hitchcock Cancer Research Fellows Program and by the NCI Cancer Center Support Grant 5P30CA023108 to the Dartmouth-Hitchcock Norris Cotton Cancer Center as well as the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (#30500) at Dartmouth College and Dartmouth-Hitchcock Medical Center and was granted a waiver of consent due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Fang HY, Chao YK, Chang HK, et al. Survival outcomes of consolidation chemoradiotherapy in esophageal cancer patients who achieve clinical complete response but refuse surgery after neoadjuvant chemoradiotherapy. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Mitzman B, Schipper PH, Edwards MA, et al. Complications After Esophagectomy Are Associated With Extremes of Body Mass Index. Ann Thorac Surg 2018;106:973-80. [Crossref] [PubMed]
- Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557-63. [Crossref] [PubMed]
- Yoshida N, Baba Y, Shigaki H, et al. Preoperative Nutritional Assessment by Controlling Nutritional Status (CONUT) is Useful to estimate Postoperative Morbidity After Esophagectomy for Esophageal Cancer. World J Surg 2016;40:1910-7. [Crossref] [PubMed]
- Wu GH, Liu ZH, Wu ZH, et al. Perioperative artificial nutrition in malnourished gastrointestinal cancer patients. World J Gastroenterol 2006;12:2441-4. [Crossref] [PubMed]
- Ligthart-Melis GC, Weijs PJ, te Boveldt ND, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587-93. [Crossref] [PubMed]
- Cong MH, Li SL, Cheng GW, et al. An Interdisciplinary Nutrition Support Team Improves Clinical and Hospitalized Outcomes of Esophageal Cancer Patients with Concurrent Chemoradiotherapy. Chin Med J (Engl) 2015;128:3003-7. [Crossref] [PubMed]
- Fujita T, Daiko H, Nishimura M. Early enteral nutrition reduces the rate of life-threatening complications after thoracic esophagectomy in patients with esophageal cancer. Eur Surg Res 2012;48:79-84. [Crossref] [PubMed]
- Garth AK, Newsome CM, Simmance N, et al. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet 2010;23:393-401. [Crossref] [PubMed]
- Grotenhuis BA, Wijnhoven BP, Hötte GJ, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 2010;34:2621-7. [Crossref] [PubMed]
- Anandavadivelan P, Brismar TB, Nilsson M, et al. Sarcopenic obesity: A probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr 2016;35:724-30. [Crossref] [PubMed]
- Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann Surg 2017;266:822-30. [Crossref] [PubMed]
- Paireder M, Asari R, Kristo I, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol 2017;43:478-84. [Crossref] [PubMed]
- Zargar H, Almassi N, Kovac E, et al. Change in Psoas Muscle Volume as a Predictor of Outcomes in Patients Treated with Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Bladder Cancer 2017;3:57-63. [Crossref] [PubMed]
- Park SY, Yoon JK, Lee SJ, et al. Postoperative change of the psoas muscle area as a predictor of survival in surgically treated esophageal cancer patients. J Thorac Dis 2017;9:355-61. [Crossref] [PubMed]
- Wang N, Cao F, Liu F, et al. The effect of socioeconomic status on health-care delay and treatment of esophageal cancer. J Transl Med 2015;13:241. [Crossref] [PubMed]
- Camp NL. Food insecurity and food deserts. Nurse Pract 2015;40:32-6. [Crossref] [PubMed]
- Lis CG, Gupta D, Lammersfeld CA, et al. Role of nutritional status in predicting quality of life outcomes in cancer--a systematic review of the epidemiological literature. Nutr J 2012;11:27. [Crossref] [PubMed]
- Dutko P, Ver Ploeg M, Farrigan T. Characteristics and influential factors of food deserts. United States Department of Agriculture, 2012. Available online: https://www.ers.usda.gov/webdocs/publications/45014/30940_err140.pdf
- Karpyn A, Manon M, Treuhaft S, et al. Policy solutions to the 'grocery gap'. Health Aff (Millwood) 2010;29:473-80. [Crossref] [PubMed]
- United States Department of Agriculture Economic Research Service. Food Access Research Atlas. Accessed September 1, 2020. Available online: https://www.ers.usda.gov/data-products/food-access-research-atlas
- Morris AA, McAllister P, Grant A, et al. Relation of Living in a "Food Desert" to Recurrent Hospitalizations in Patients With Heart Failure. Am J Cardiol 2019;123:291-6. [Crossref] [PubMed]
- Fong AJ, Lafaro K, Ituarte PHG, et al. Association of Living in Urban Food Deserts with Mortality from Breast and Colorectal Cancer. Ann Surg Oncol 2021;28:1311-9. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
-
The Society of Thoracic Surgeons - United States Census Bureau. Quick Facts. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045219
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- American Cancer Society. Key Statistics for Esophageal Cancer. 2022. Available online: https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html
- Cancer Stat Facts: Esophageal Cancer. Accessed September 1, 2020. Available online: https://seer.cancer.gov/statfacts/html/esoph.html
- Fernandez FG, Khullar O, Force SD, et al. Hospital readmission is associated with poor survival after esophagectomy for esophageal cancer. Ann Thorac Surg 2015;99:292-7. [Crossref] [PubMed]
- Stitzenberg KB, Chang Y, Smith AB, et al. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol 2015;33:455-64. [Crossref] [PubMed]
- Caburet C, Farigon N, Mulliez A, et al. Impact of nutritional status at the outset of assessment on postoperative complications in head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis 2020;137:393-8. [Crossref] [PubMed]
- Makiura D, Ono R, Inoue J, et al. Impact of Sarcopenia on Unplanned Readmission and Survival After Esophagectomy in Patients with Esophageal Cancer. Ann Surg Oncol 2018;25:456-64. [Crossref] [PubMed]
- Hu Y, McMurry TL, Stukenborg GJ, et al. Readmission predicts 90-day mortality after esophagectomy: Analysis of Surveillance, Epidemiology, and End Results Registry linked to Medicare outcomes. J Thorac Cardiovasc Surg 2015;150:1254-60. [Crossref] [PubMed]
- Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer 2001;91:178-88. [Crossref] [PubMed]
- Ambroggi M, Biasini C, Del Giovane C, et al. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist 2015;20:1378-85. [Crossref] [PubMed]
- Martin L. Identifying the Barriers and Enablers to Nutrition Care in Head and Neck and Esophageal Cancers: An International Qualitative Study. JPEN J Parenter Enteral Nutr 2016;40:355-66. [Crossref] [PubMed]
- Alberda C, Alvadj-Korenic T, Mayan M, et al. Nutrition Care in Patients With Head and Neck or Esophageal Cancer: The Patient Perspective. Nutr Clin Pract 2017;32:664-74. [Crossref] [PubMed]
- McCorry NK, Dempster M, Clarke C, et al. Adjusting to life after esophagectomy: the experience of survivors and carers. Qual Health Res 2009;19:1485-94. [Crossref] [PubMed]
- Jaromahum J, Fowler S. Lived experiences of eating after esophagectomy: a phenomenological study. Medsurg Nurs 2010;19:96-100. [PubMed]
- Steenhagen E, van Vulpen JK, van Hillegersberg R, et al. Nutrition in peri-operative esophageal cancer management. Expert Rev Gastroenterol Hepatol 2017;11:663-72. [Crossref] [PubMed]
- Steenhagen E. Preoperative nutritional optimization of esophageal cancer patients. J Thorac Dis 2019;11:S645-53. [Crossref] [PubMed]
- Chen F, Fang J, Wang H, et al. Effects of nutritional support on short-term clinical outcomes and immune response in unresectable locally advanced oesophageal squamous cell carcinoma. Eur J Cancer Care (Engl) 2018;27:e12818. [Crossref] [PubMed]