Efficacy of the intraoperative opioid-sparing anesthesia on quality of patients’ recovery in video-assisted thoracoscopic surgery: a randomized trial
Introduction
Video-assisted thoracoscopic surgery (VATS) represents a minimally invasive technique that allows for faster recovery and fewer complications after lung surgery. Although less invasive than open thoracotomy, VATS also induces considerable acute or chronic pain because it compresses intercostal nerve, damages muscles, and provokes soft tissue edema around incision sites (1,2). Therefore, VATS is still a painful surgical procedure (3,4), and acute postoperative pain is linked to the late development of neuropathic pain syndrome if not controlled properly (4-6).
Optimizing postoperative pain is crucial to assure a good patient experience, improve postoperative outcomes, and enhance recovery after surgery (7). Opioid-based anesthesia is associated with multiple side effects that impact on patients’ rapid recovery targets, including post-operative nausea and vomiting control, early mobilization and quick returns to oral diet (8). Opioid-sparing anesthesia has been described as the cornerstone of enhanced recovery after lung resection. The current guidelines strongly recommend the use of short-acting anesthetics, regional anesthetic techniques and non-opioid medications to minimize the use of opioids during VATS (9,10). However, the evidence is still sparse in previous published trials that which combination of opioid-sparing anesthesia elements would be more feasible to improve patients’ recovery in thoracic patients.
Thoracic paravertebral blocks have been strongly recommended in thoracic surgery because of their improved side-effect profile and comparable analgesic efficacy to thoracic epidural analgesia (11). Paravertebral blockade, along with tailored doses of short-acting opioids, may reduce long-acting opioids-related adverse effects without compromising analgesia (12). However, it remains unclear whether opioid-sparing anesthesia consisting of paravertebral blockade and intraoperative remifentanil infusion could improve patient-reported recovery of quality, in thoracic patients during the immediate period after surgery.
Therefore, we aimed to investigate the efficacy of the predefined opioid-sparing anesthesia on the quality of patients’ recovery in lung surgeries under VATS compared to routine anesthesia. Specifically, we tested the primary hypothesis that opioid-sparing anesthesia is superior to routine anesthesia on the quality of recovery in postoperative period; secondarily, we tested the hypothesis that opioid-sparing anesthesia reduces opioids-related adverse effects, improves acute pain control and facilitates clinical recovery compared to routine anesthesia. Further, we tested the hypothesis that opioid-sparing anesthesia improves patients’ 30-day quality of recovery after lung surgery. We present the following article in accordance with the CONSORT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-50/rc).
Methods
Ethics and registration
This study was a randomized, parallel, controlled clinical trial. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanghai Chest Hospital Institutional Review Board, Shanghai, China (IRB #KS 2001, approved by Chairman Prof. Ning Zheng) on January 7th, 2020. Written informed consent was obtained from each patient one day before surgery. This trial was registered before subject enrolment began at the Chinese Clinical Trial Registry (ChiCTR2000031609; principal investigator, YQ; date of registration, April 05, 2020).
Patient inclusion and exclusion criteria
Eligible patients were between 18 and 70 years, scheduled for elective video-assisted thoracoscopic lung surgery in Shanghai Chest Hospital, had an American Society of Anesthesiologists (ASA) physical status of I–III.
Patients were excluded if they had clinically important cardiovascular disease, were illiterate to conduct questionnaires, or converted to open thoracotomy, have chronic pain or take pain medicine prior to surgery, or undergone thoracic surgery before. Patients were also excluded when they had contraindications to receive nerve blocks, or received the second surgery because of postoperative haemorrhage or infection immediately in postoperative period.
Randomization and masking
Patients were randomly allocated 1:1 into opioid-sparing anesthesia group and routine anesthesia group, using a consecutive list of computer-generated random numbers kept in sealed envelopes. Envelopes were opened by an investigator not involved in clinical care shortly before anesthesia induction. Blocks were performed after induction of general anesthesia, so the anesthesiologists in the operating room were not masked to treatment. Patients, postoperative outcome assessors and clinicians in surgical ward were masked to treatment allocation.
Protocol
None of the patients received premedication. Patients were monitored with electrocardiography (ECG), invasive blood pressure, pulse oximetry, esophageal temperature, bispectral index (BIS) and surgical pleth index (SPI, S/5 Collect software, GE healthcare, Helsinki, Finland). Invasive blood pressure monitoring was achieved by radial artery cannulation and right internal jugular venous catheterization. Prophylactic antibiotics was given per surgical routine.
In both groups, patients were given dexamethasone 5 mg before anesthesia and dolasetron 12.5 mg before the end of surgery as prophylactic treatment for postoperative nausea and vomiting (PONV). Dexmedetomidine 1 µg/kg was infused at least 10 minutes before anesthesia induction. After anesthesia induction, a double-lumen bronchial tube (DLT) was inserted and positioned using flexible bronchoscopy. Mechanical ventilation was initiated with a 100% oxygen and adjusted to maintain end-tidal CO2 pressure of 35–40 mmHg. Ventilator settings were maintained with tidal volume of 6 mL/kg of ideal body weight with or without positive end-expiratory pressure. Propofol administration was adjusted to target BIS between 40 and 50. Remifentanil was infused between 0.5–3 ng/mL, adjusted to keep SPI between 20–50. Cisatracurium 0.2 mg/kg was given to facilitate DLT intubation and 0.12–0.15 mg/kg/hour infused as clinical necessary. Esophageal temperature was maintained above 36.0 °C using forced-air warmers. After surgery, patients were transferred to the post-anesthesia care unit to extubate.
In routine anesthesia group, general anesthesia was induced with 0.6–0.8 µg/kg sufentanil and a target-controlled infusion of propofol set to a plasma concentration of 3–4 µg/mL. Propofol and remifentanil target-controlled infusion was tailored to BIS and SPI. Before the end of surgery, sufentanil 5–10 µg and flurbiprofen axetil 50 mg were added intravenously to prevent pain.
In opioid-sparing anesthesia group, sufentanil was not given throughout the intraoperative period. General anesthesia was induced with target-controlled infusion of propofol set to a plasma concentration of 3–4 µg/mL and remifentanil set to a plasma concentration of 3–4 ng/mL. After anesthesia induction, patients were positioned in the lateral decubitus position to receive paravertebral block. A 1.6–6.0 MHz curved array transducer (KaiLi, E1, Shenzhen, China) was used to identify the paravertebral space at the T4 and T6 vertebral levels. Using in-plane approach, a 10-cm, 21-gauge needle (Pajunk, NanoLine, Geisingen, Germany) was inserted into the paravertebral space, and a total amount of 0.5% ropivacaine 0.6 mL/kg conjunct with 5 mg dexamethasone were injected at T4 and T6 vertebral levels. Propofol and remifentanil target-controlled infusion was tailored to BIS and SPI.
A patient-controlled analgesics (PCA) pump was started immediately after surgery in each patient: sufentanil 1–1.5 µg/kg was diluted into 100 mL saline. The intravenous analgesics pump was infused at a 2 mL/hour rate, with a loading dose of 0.5 mL per request and lock time of 15 minutes. After completion of the surgery, patients were transferred to postanesthesia care unit (PACU) to extubate. Muscle relaxants were routinely reversed with atropine/neostigmine. When patients had an Aldrete score >9 and felt warm-alert-comfortable, then were allowed to discharge to the ward.
In the surgical ward, postoperative pain treatment was managed pragmatically by ward clinicians unaware of group assignment, including routine parecoxib intravenously given twice daily. The PCA was stopped after the second postoperative day or when patients discharged. Patients were discharged home when they: were able to mobilize; achieved adequate pain control with oral medication; were able to eat and drink; had vital signs within normal limits and without severe pulmonary complications.
Outcomes
Demographic, surgical characteristics and intraoperative variables were collected.
The primary outcome was the global score Quality of Recovery-15 scale (QoR-15) at 6 hours after surgery. The QoR-15, a 15-item post-operative QoR scale (range, 0–150, and in which 150 is the best outcome), was used to assess patients’ postoperative recovery (13,14). QoR-15 is a validated questionnaire commonly used in the perioperative setting with good validity, reliability and clinical feasibility (13). Furthermore, we used the Chinese translated version to help patients comprehend well. Similar to the original English version, the QoR-15 Chinese revealed satisfactory psychometric properties (15). The minimal clinically important difference of QoR-15 that patients considered important was estimated at 8 and the patient acceptable symptom state for QoR-15 was 118 (16,17).
The secondary outcomes included QoR-15 at 24 and 48 hours after surgery, Overall Benefit of Analgesia Score Satisfaction with pain treatment (OBAS) and acute pain at 6, 24, and 48 hours after surgery. Total OBAS scores are calculated with 7-item OBAS scales, by summing responses from Q1 through Q6 and adding (4-score from Q7) (18); lower total OBAS scores mean more benefit. The total OBAS score and analgesia-related side effects from Q2–Q6 were recorded. A 11-point Verbal Rating Scale (VRS) ranging from zero (no pain) to 10 (pain as bad as you can imagine) was used to assess acute pain. VRS at most (defined as the maximal pain) was obtained at postoperative 6 hours, during the first 24 hours and the second 24 hours. The secondary outcomes also included time to mobilize, time to first full diet and first flatus.
Other outcomes were intraoperative consumption of sufentanil, remifentanil, and propofol recorded. Intraoperative hypertension, hypotension, severe bradycardia and supraventricular arrhythmia were collected. Intraoperative hypotension was defined as MAP <65 mmHg lasting at least 2 minutes or vasoactive drug support. Intraoperative hypertension was defined as systolic blood pressure >160 mmHg. Intraoperative severe bradycardia was defined as heart rate <50 beats per minute. Emergence time and DLTs extubation time after surgery were also recorded. Variables about PCA were recorded.
Quality of life on the 30th postoperative day was obtained by phone calls with Short Form-12 Health Survey (SF-12). The SF-12 was designed to assess health from the patients’ point of view and cover eight areas (19). Results were expressed in two meta-scores: a physical component summary and a mental component summary (19). Lower scores indicated worse health-related quality of life.
Statistical analysis
Statistical analyses were performed with SPSS (version 25, IBM Statistics, Chicago, IL, USA) and SAS (version 9.4, Windows version 1.0.19041).
Continuous or discrete baseline characteristics were described using mean and standard deviation (SD) or median and 25th and 75th quartiles. Categorical data were summarized with number or proportions. Categorical variables of binary outcomes were compared using the Chi-square test or Fisher Exact tests. Standardized differences were used to compare imbalance in baseline characteristics. Differences <0.1 in baseline covariates were considered negligible, and differences <0.2 were considered small (17).
The primary outcome was the global score of the QoR-15 measured at 6 hours after surgery. For normally distributed continuous outcomes, we used the student t-test to compare outcomes between the opioid-sparing anesthesia and the routine group. If outcomes were discrete continuous, Mann-Whitney U test was used and outcomes were reported as the Hodges-Lehmann estimate (17).
The secondary outcomes were the global score of QoR-15 at 24 and 48 hours after surgery, OBAS and acute pain at 6, 24, and 48 hours after surgery. For discrete continuous outcome, Mann-Whitney U was used and outcomes were reported as the Hodges-Lehmann estimate.
We calculated sample size according to a previous article in patients undergoing robot-assisted radical prostatectomy (20). A total of 134 patients were needed for a two-sided power of 0.95 and a P value of 0.05. To allow for some individuals not completing the trial, we planned to enrol 80 patients in each treatment group, with 160 patients in total.
Results
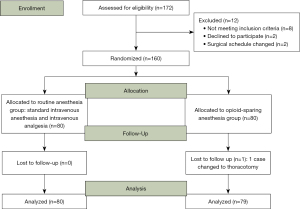
A total of 160 patients were randomized from 7 April 2020 through 12 October 2020 (Figure 1). One patient who had serious intraoperative hemorrhage was excluded, leaving 80 patients in the routine anesthesia group, and 79 patients in opioid-sparing anesthesia group (Figure 1). Baseline characteristics were balanced (Table 1).
Table 1
| Parameters | Routine anesthesia group (n=80) | Opioid-sparing anesthesia group (n=79) | Standardized difference | P value |
|---|---|---|---|---|
| Age (years) | 55±10 | 54±12 | 0.125 | 0.433 |
| Male | 29 (36.2%) | 37 (46.8%) | 0.216 | 0.233 |
| Height (meters) | 1.64±0.08 | 1.65±0.08 | 0.165 | 0.300 |
| Weight (kg) | 61.6±11.7 | 63.1±11.0 | 0.136 | 0.393 |
| Body mass index (kg/m2) | 22.99±3.31 | 23.22±3.04 | 0.070 | 0.658 |
| ASA physical status | ||||
| I–II | 71 (88.8%) | 71 (89.9%) | 0.036 | 1.000 |
| III | 9 (11.2%) | 8 (10.1%) | 0.036 | 1.000 |
| Comorbidities | ||||
| Hypertension | 22 (27.5%) | 24 (30.4%) | 0.064 | 0.822 |
| Diabetic mellitus | 4 (5.0%) | 6 (7.6%) | 0.111 | 0.713 |
| Coronary heart disease | 3 (3.8%) | 3 (3.8%) | 0.002 | 1.000 |
| Arrhythmia | 2 (2.5%) | 1 (1.3%) | 0.091 | 1.000 |
Data are presented as means ± SDs or n (%). The standardized difference is the difference in group means scaled by the pooled SD. Absolute standardized differences <0.1 are considered negligible. Independent t-test was used for two normally distributed continuous outcomes, and Chi-square or Fisher Exact tests for binary outcomes. ASA, American Society of Anesthesiologists; SD, standard deviation.
In the primary analysis, the global score of QoR-15 measured at 6 hours after surgery reported as median (quartiles) was 116 (range, 114–118) in the opioid-sparing anesthesia group and 113 (range, 108–118) in the routine anesthesia group, with median difference reported as opioid-sparing anesthesia minus routine anesthesia for QoR-15 of 4 (95% CI: 1–6) (Table 2).
Table 2
| Outcomes | Routine anesthesia group (n=80) | Opioid-sparing anesthesia group (n=79) | Median difference | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| QoR-15 at 6 hours after surgery | 113 (108, 118) | 116 (114, 118) | 4 (1, 6) | 0.000** |
| Secondary outcomes | ||||
| QoR-15 at 24 hours after surgery | 119 (109, 125.75) | 126 (114, 134) | 8 (4, 12) | 0.000** |
| QoR-15 at 48 hours after surgery | 129.7 (122, 133) | 131 (128, 138) | 4.7 (1, 6) | 0.000** |
| OBAS | ||||
| OBAS at 6 hours after surgery | 1 (0, 2) | 0 (0, 1) | 0 (−1, 0) | 0.004** |
| OBAS at 24 hours after surgery | 3 (2, 4) | 2 (1, 3) | −1 (−2, 0) | 0.002** |
| OBAS at 48 hours after surgery | 1 (0, 3) | 0 (0, 2) | 0 (−1, 0) | 0.020* |
| Exploratory outcomes | ||||
| Postoperative pain | ||||
| Pain at most at 6 hours after surgery | 3 (2, 4) | 2 (1, 3) | −1 (−1, 0) | 0.000* |
| Pain at most at 24 hours after surgery | 4 (3, 6) | 3 (2, 5) | −1 (−1, 0) | 0.097 |
| Pain at most at 48 hours after surgery | 3 (2, 5) | 3 (2, 4) | −0.9 (−1, 0) | 0.030* |
| Opioid-related side effects | ||||
| Postoperative nausea | 0 (0, 2) | 0 (0, 0) | 0.002** | |
| Postoperative feeling cold | 0 (0, 0) | 0 (0, 0) | 0.502 | |
| Postoperative dizziness | 1 (0, 1) | 0 (0, 1) | 0.005** | |
| Postoperative sweating | 0 (0, 1) | 0 (0, 1) | 0.779 | |
| Postoperative satisfaction | 4 (3, 4) | 4 (4, 4) | 0.009** | |
| Analgesics consumption of PCA | ||||
| Sufentanil consumption (μg) | 28 (28, 48) | 28 (28, 48) | 0.578 | |
| Postoperative clinical recovery | ||||
| The number of patients with good recovery at 6 hours after surgery | 24 (30.0%) | 28 (35.4%) | 0.464 | |
| The number of patients with good recovery at 24 hours after surgery | 43 (53.8%) | 58 (73.4%) | 0.01* | |
| The number of patients with good recovery at 48 hours after surgery | 69 (86.3%) | 72 (91.1%) | 0.317 | |
| Time to first mobilize | 21.9 (19.5, 25.7) | 20.0 (16.0, 23.0) | 0.000** | |
| Time to first food intake | 18.5 (17.1, 19.5) | 17.7 (16.0, 19.5) | 0.055 | |
| Time to first flatus | 20.0 (11.0, 27.2) | 15.0 (7.0, 21.5) | 0.002** | |
| 30-day outcome | ||||
| SF-12 | ||||
| Physics score | 37 (30, 46) | 40 (31, 47) | 0.337 | |
| Mental score | 60 (57, 62) | 61 (57, 62) | 0.550 | |
| Postoperative length of stay (days) | 2 (2, 3) | 2 (2, 3) | 0.991 |
Data are presented as means ± SDs or median (IQR) or n (%). Mann-Whitney U test was used for nonparametric outcomes, and independent t-test for two normally distributed continuous outcomes. Median difference (reported with 95% confidence intervals) is the median of all pairwise differences between observations in the two groups, not the difference between medians of the groups. Good recovery was defined by QoR-15 equal or above 118. Chi-square test was performed to compare the percentage between two groups. Denotes statistically significant (*, P<0.05; **, P<0.01) differences between two groups. QoR-15, Quality of Recovery-15 scale; OBAS, Overall Benefit of Analgesia Score Satisfaction with pain treatment; PCA, patient-controlled analgesics; SD, standard deviation.
Secondarily, the global score of QoR-15 was 126 (range, 114–134) in opioid-sparing anesthesia group and 119 (range, 109–125.75) in routine anesthesia group at 24 hours, and 131 (range, 128–138) in opioid-sparing anesthesia group versus 129.7 (range, 122–133) in routine anesthesia group at 48 hours after surgery. The median difference in QoR-15 between opioid-sparing and routine anesthesia was 8 (95% CI: 4–12) at 24 hours, and 4.7 (95% CI: 1–6) at 48 hours after surgery (Table 2).
Patients with opioid-sparing anesthesia demonstrated lower OBAS at 6, 24, and 48 hours after surgery (P<0.05, Table 2). Additionally, VRS at most was significantly lower in opioid-sparing anesthesia group at 6 and 48 hours postoperatively (P<0.05, Table 2). In terms of separate domains of OBAS, patients in the opioid-sparing anesthesia group had less nausea and dizziness than the routine anesthesia group (all P value <0.01, Table 2), but postoperative feeling cold and sweating score didn’t differ between the groups (P>0.05). OBAS’s overall satisfaction was higher in the opioid-sparing anesthesia group (P=0.009, Table 2). The consumption of sufentanil in PCA pump didn’t differ significantly (P=0.578, Table 2).
In opioid-sparing group, 73.4% patients showed good recovery in opioid-sparing group, compared to 53.8% in routine anesthesia group at 24 hours after surgery (P=0.01, Table 2). Patients exhibited faster recovery with opioid-sparing anesthesia on time to mobilize and to first flatus (Table 2). The time to first food intake was not statistically significant (P=0.055). SF-12 at postoperative 30-day didn’t differ between groups (P>0.05, Table 2).
The individual QoR-15 items with results at 6 hours after surgery were shown in Table 3. Three responses to QoR-15 were statistically significant (P<0.05, Table 3).
Table 3
| Individual component of QoR-15 | Routine anesthesia group (n=80) | Opioid-sparing anesthesia group (n=79) | P value |
|---|---|---|---|
| 1. Able to breathe easy | 10 (10, 10) | 10 (10, 10) | 0.790 |
| 2. Been able to enjoy food | 0 (0, 0) | 0 (0, 0) | 0.691 |
| 3. Feeling rested | 10 (8.5, 10) | 10 (10, 10) | 0.025* |
| 4. Have had a good sleep | 10 (8, 10) | 10 (10, 10) | 0.052 |
| 5. Able to look after personal toilet and hygiene | 0 (0, 0) | 0 (0, 0) | 0.031* |
| 6. Able to communicate with family and friends | 10 (10, 10) | 10 (10, 10) | 0.146 |
| 7. Getting support from hospital doctors and nurses | 10 (10, 10) | 10 (10, 10) | 0.173 |
| 8. Able to return to work or usual home activities | 0 (0, 0) | 0 (0, 0) | 0.276 |
| 9. Feeling comfortable and in control | 10 (5, 10) | 10 (8, 10) | 0.002** |
| 10. Having a feeling of general well-being | 5 (5, 8) | 8 (5, 8) | 0.112 |
| 11. Moderate pain | 10 (10, 10) | 10 (10, 10) | 0.136 |
| 12. Severe pain | 10 (10, 10) | 10 (10, 10) | 0.316 |
| 13. Nausea and vomiting | 10 (10, 10) | 10 (10, 10) | 0.300 |
| 14. Feeling worried or anxious | 10 (10, 10) | 10 (10, 10) | 0.564 |
| 15. Feeling sad or depressed | 10 (10, 10) | 10 (10, 10) | 0.564 |
Data are presented as median (IQR). Mann-Whitney test was used for individual component of QoR-15 between the two groups. Denotes statistically significant (*, P<0.05; **, P<0.01) differences between two groups. QoR-15, Quality of Recovery-15 scale.
Intraoperative consumption of sufentanil, remifentanil and propofol was significantly less in the opioid-sparing anesthesia group compared to routine anesthesia group (Table 4). Incidence of intraoperative hypertension (P=0.000) and severe bradycardia (P=0.028) were less common in opioid-sparing anesthesia group (Table 4). Time to emergence and extubate were significantly shorter in patients with opioid-sparing anesthesia (P=0.000, Table 4).
Table 4
| Parameters | Routine anesthesia group (n=80) | Opioid-sparing anesthesia group (n=79) | P value |
|---|---|---|---|
| Duration of surgery, minutes | 90.5 (74.25, 120) | 87.0 (70.0, 123) | 0.885 |
| Duration of anesthesia, minutes | 70.5 (54.2, 91.75) | 59.0 (45.0, 95.0) | 0.317 |
| Sufentanil consumption, μg | 40 (40, 45) | 0 (0, 0) | 0.000** |
| Remifentanil consumption, μg | 600 (410, 900) | 500 (400, 700) | 0.030* |
| Propofol consumption, mg | 640 (440, 920) | 560 (400, 800) | 0.041* |
| Surgical direction | 0.697 | ||
| Left-side | 29 (36.2%) | 31 (39.2%) | |
| Right-side | 51 (63.8%) | 48 (60.8%) | |
| Surgical type | 0.736 | ||
| Wedge resection | 24 (30.0%) | 27 (34.2%) | |
| Segmentectomy | 24 (30.0%) | 25 (31.6%) | |
| Lobectomy | 32 (40.0%) | 27 (34.2%) | |
| The number of drainage tubes | 0.682 | ||
| 1 tube | 67 (83.8%) | 68 (86.1%) | |
| 2 tubes | 13 (16.2%) | 11 (13.9%) | |
| The number of incisions | 0.462 | ||
| 1 incision | 18 (22.5%) | 22 (27.8%) | |
| 2 incisions | 53 (66.3%) | 52 (65.8%) | |
| 3 incisions | 9 (11.2%) | 5 (6.3%) | |
| Intraoperative total fluid volume, mL | 1,000 (1,000, 1,000) | 1,000 (1,000, 1,100) | 0.079 |
| Intraoperative hypotension | 19 (23.8%) | 24 (30.4%) | 0.347 |
| Intraoperative hypertension | 25 (31.3%) | 4 (5.1%) | 0.000 |
| Intraoperative supraventricular arrhythmia | 1 (1.3%) | 1 (1.3%) | 1.000 |
| Intraoperative bradycardia | 6 (7.5%) | 0 (0.0%) | 0.028* |
| Emergence time, minutes | 39 (28, 58) | 15 (5, 25) | 0.000** |
| Extubation time, minutes | 40 (28, 59) | 15 (5, 28) | 0.000** |
Data are presented as median (IQR) or n (%). *, P<0.05; **, P<0.01.
Discussion
In this randomized assessor- and patient-masked trial, we found that our opioid-sparing anesthesia regime was not superior to routine anesthesia on the global score of QoR-15 at 6 hours after surgery in patients having VATS lung resection. The median difference in the global score of QoR-15 between groups was 4 and it appeared unimportant given the minimum clinically important difference for QoR-15 of 8 (16). Nevertheless, our opioid-sparing anesthesia regime demonstrated a clinically meaningful improvement in quality of recovery at 24 hours after surgery.
Indeed, thoracic patients experienced a series of postoperative symptoms such as pain, dyspnea, fatigue, emotional distress, pain and limited activity (21,22), which significantly affected patients’ outcomes. That was consistent with our data. In both groups, the QoR-15 score decreased as expected during the postoperative period. At 6 hours after surgery, QoR-15 had the lowest global score and the percentage of patients having good recovery was only one third. To improve QoR-15 at immediate postoperative period was of significant value. However, our opioid-sparing anesthesia regime failed to reach the clinical significance on QoR-15 at 6 hours, and we speculated that it might be accountable for several reasons: among the questionnaires, pain, physical comfort, physical independence, emotional distress, and psychological support all constituted the components of QoR-15. Even pain and nausea were under control, the other domains of QoR-15 still had lower scores; second, being an indicator reflecting patients’ recovery, change of 8 in QoR-15 as the minimally clinically important difference were based on an observational study of 204 patients, none of whom underwent thoracic surgery (23). Therefore, it remained unclear whether QoR-15 could detect the minor changes in the immediate postoperative period for thoracic patients. Third, it should emphasize patients’ education before the surgery and in the recovery room as the initial few hours rarely reflect longer term outcomes.
Interestingly, our opioid-sparing anesthesia regime demonstrated clinically meaningful improvement in quality of recovery compared to the routine anesthesia at 24 hours after surgery, which was consistent with a recent study (24). Yao found preoperative single-injection thoracic erector spinae plane block with ropivacaine improved QoR-40 at 24 hours in VATS (24). Our opioid-sparing anesthesia using paravertebral blocks conjunct with remifentanil enabled 73.4% of patient with good recovery, which was defined as QoR-15 above 118. Furthermore, the median of QoR-15 reached to 126 at 24 hours in our trial, which was higher than 114 in patients who received erector spinae plane block with fentanyl anesthesia (25). Although there was no consensus for a gold standard of analgesia for VATS, paravertebral blocks were predominantly used in thoracic surgeries owing to its better analgesic efficacy (26,27). The combined paravertebral blocks with short-acting remifentanil, without intraoperative use of sufentainil, provided patients’ better recovery. Under paravertebral blocks and guided by SPI, the remifentanil infusion rate was not greater than 0.2 µg/kg/minute to induce postoperative hyperalgesia (28).
Our data also demonstrated that OBAS and acute pain score reduced in opioid-sparing group during the postoperative period, which was similar to a previous study (29). Opioid-related adverse effects such as nausea, vomiting and dizziness posed great challenges in the postoperative setting, which may limit patients’ early ambulation and food-intake. Our opioid-sparing anesthesia reduced postoperative nausea and dizziness, which may account for the fact that the opioid-sparing regime shortened the time to mobilize by 2 hours, and promoted the recovery for first flatus by 5 hours (30-32).
There were no serious adverse events in all patients. Incidence of intraoperative hypertension decreased from 31% in routine anesthesia to 5% in opioid-sparing anesthesia, presumably due to sympathetic inhibition by paravertebral block. Furthermore, anesthetics-sparing explained the less time to emergence and extubate. The postoperative length of stay didn’t differ, in line with a previous study which also showed fast-track interventions reduced time to extubate but did not reduce length of stay (33).
Our study has several limitations. First, the treatments were delivered at a single major thoracic center with annual 19,000 thoracic cases. In the study population, 60% patients undergone sub-lobar resections so patients may not benefit from more complicated thoracic surgeries. Lobectomy may be a further interesting hypothesis to address the benefits of our opioid-sparing scheme, due to its more invasive procedures. Second, our included patients were relatively younger, limiting the generalization of our result to older patients or patients with severe comorbidities; furthermore, this opioid-sparing anesthesia masked patients, surgeons and nurses in surgical ward, so it may lack more efforts to involve in postoperative mobilization. The addition of PCA pumps to both groups may skew the results somewhat, and not adding the PCA to both groups may enlarge the difference between groups, with respect to pain control or recovery. Fourth, our study mainly assessed patients’ questionnaires. Although the QoR questionnaires used are useful, it would have been more objective to use other measures to quantify patient outcomes such as the 6-minute walk distance mentioned. Finally, we didn’t assess baseline QoR-15. A recent review concluded that the lack of baseline QoR-15 values was a disadvantage (34). But some experts argued that the baseline values taken <24 hours before surgery can’t reflect the true baseline scores owing to fear and anxiety (35,36).
In summary, this randomized trial demonstrated that our opioid-sparing anesthesia was not superior to routine anesthesia on patient-reported QoR-15, at 6 hours after surgery in patients having VATS lung resection. Nevertheless, we found our opioid-sparing anesthesia improved patients’ quality of recovery at 24 hours after surgery. The protocols of our opioid-sparing anesthesia provided reduced OBAS score, optimal acute pain control and better clinical recovery during the postoperative period. New approaches need incorporate multidiscipline efforts to fasten patients’ postoperative recovery.
Acknowledgments
The authors thank Jun Yang, Wentao Li, Yunhai Yang, Wentao Fang in the Department of Thoracic Surgery, and Haixia Yao, Qing Miao, Yunyun Zhang in the Department of Anesthesiology at Shanghai Chest Hospital, for their assistance in patients’ enrolment and following up. We also thank Dr. Mauro Bravo, in the Department of Outcomes Research, Cleveland Clinic, Cleveland, OH, USA, for his help in polishing our paper.
Funding: This work was supported by the funding of Shanghai Municipal Commission of Health (202040200), Shanghai Shen Kang Hospital Development Center Project (SHDC2020CR4063); and National Natural Science Foundation of China (82071233).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-50/rc
Trial Protocol: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-50/tp
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-50/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-50/coif). YQ reports funding from Shanghai Municipal Commission of Health [202040200]. JW reports funding from Shanghai Shen Kang Hospital Development Center Project (SHDC2020CR4063) and National Natural Science Foundation of China [82071233]. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanghai Chest Hospital Institutional Review Board, Shanghai, China (IRB #KS 2001) on January 7th, 2020. Written informed consent was obtained from each patient one day before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: A noninferiority randomised trial. Eur J Anaesthesiol 2021;38:S97-105. [Crossref] [PubMed]
- Bayman EO, Parekh KR, Keech J, et al. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology 2017;126:938-51. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Umari M, Carpanese V, Moro V, et al. Postoperative analgesia after pulmonary resection with a focus on video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2018;53:932-8. [Crossref] [PubMed]
- Lim J, Chen D, McNicol E, et al. Risk factors for persistent pain after breast and thoracic surgeries: a systematic literature review and meta-analysis. Pain 2022;163:3-20. [Crossref] [PubMed]
- Hollmann MW, Rathmell JP, Lirk P. Optimal postoperative pain management: redefining the role for opioids. Lancet 2019;393:1483-5. [Crossref] [PubMed]
- Guay J, Nishimori M, Kopp SL. Epidural Local Anesthetics Versus Opioid-Based Analgesic Regimens for Postoperative Gastrointestinal Paralysis, Vomiting, and Pain After Abdominal Surgery: A Cochrane Review. Anesth Analg 2016;123:1591-602. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice. J Thorac Dis 2018;10:S542-54. [Crossref] [PubMed]
- Helander EM, Webb MP, Kendrick J, et al. PECS, serratus plane, erector spinae, and paravertebral blocks: A comprehensive review. Best Pract Res Clin Anaesthesiol 2019;33:573-81. [Crossref] [PubMed]
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131-57. [Crossref] [PubMed]
- Kleif J, Waage J, Christensen KB, et al. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth 2018;120:28-36. [Crossref] [PubMed]
- Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88-105. [Crossref] [PubMed]
- Bu XS, Zhang J, Zuo YX. Validation of the Chinese Version of the Quality of Recovery-15 Score and Its Comparison with the Post-Operative Quality Recovery Scale. Patient 2016;9:251-9. [Crossref] [PubMed]
- Myles PS, Myles DB, Galagher W, et al. Minimal Clinically Important Difference for Three Quality of Recovery Scales. Anesthesiology 2016;125:39-45. [Crossref] [PubMed]
- Barrington MJ, Seah GJ, Gotmaker R, et al. Quality of Recovery After Breast Surgery: A Multicenter Randomized Clinical Trial Comparing Pectoral Nerves Interfascial Plane (Pectoral Nerves II) Block With Surgical Infiltration. Anesth Analg 2020;130:1559-67. [Crossref] [PubMed]
- Lehmann N, Joshi GP, Dirkmann D, et al. Development and longitudinal validation of the overall benefit of analgesia score: a simple multi-dimensional quality assessment instrument. Br J Anaesth 2010;105:511-8. [Crossref] [PubMed]
- Frumovitz M, Obermair A, Coleman RL, et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): a secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2020;21:851-60. [Crossref] [PubMed]
- Koning MV, de Vlieger R, Teunissen AJW, et al. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot-assisted radical prostatectomy: a randomised controlled trial. Anaesthesia 2020;75:599-608. [Crossref] [PubMed]
- Kneuertz PJ, Moffatt-Bruce SD. Search for Meaningful Use of Patient-Reported Outcomes in Thoracic Surgery. Ann Thorac Surg 2020;109:1317-8. [Crossref] [PubMed]
- Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 2015;150:613-9.e2. [Crossref] [PubMed]
- Abid S, Magee D, Jaggar SI. A comparison of fascial plane blocks on quality of recovery for minimally invasive thoracic surgery. Comment on Br J Anaesth 2020; 125: 802-10. Br J Anaesth 2021;127:e14-5. [Crossref] [PubMed]
- Yao Y, Fu S, Dai S, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: A prospective, randomized, controlled trial. J Clin Anesth 2020;63:109783. [Crossref] [PubMed]
- Finnerty DT, McMahon A, McNamara JR, et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth 2020;125:802-10. [Crossref] [PubMed]
- D'Ercole F, Arora H, Kumar PA. Paravertebral Block for Thoracic Surgery. J Cardiothorac Vasc Anesth 2018;32:915-27. [Crossref] [PubMed]
- Turhan Ö, Sivrikoz N, Sungur Z, et al. Thoracic Paravertebral Block Achieves Better Pain Control Than Erector Spinae Plane Block and Intercostal Nerve Block in Thoracoscopic Surgery: A Randomized Study. J Cardiothorac Vasc Anesth 2021;35:2920-7. [Crossref] [PubMed]
- Yu EH, Tran DH, Lam SW, et al. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia 2016;71:1347-62. [Crossref] [PubMed]
- Jin Y, Zhao S, Cai J, et al. Erector Spinae Plane Block for Perioperative Pain Control and Short-term Outcomes in Lumbar Laminoplasty: A Randomized Clinical Trial. J Pain Res 2021;14:2717-27. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Khandhar SJ, Schatz CL, Collins DT, et al. Thoracic enhanced recovery with ambulation after surgery: a 6-year experience. Eur J Cardiothorac Surg 2018;53:1192-8. [Crossref] [PubMed]
- Mayor MA, Khandhar SJ, Chandy J, et al. Implementing a thoracic enhanced recovery with ambulation after surgery program: key aspects and challenges. J Thorac Dis 2018;10:S3809-14. [Crossref] [PubMed]
- Wong WT, Lai VK, Chee YE, et al. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev 2016;9:CD003587. [Crossref] [PubMed]
- Bowyer A, Jakobsson J, Ljungqvist O, et al. A review of the scope and measurement of postoperative quality of recovery. Anaesthesia 2014;69:1266-78. [Crossref] [PubMed]
- Chazapis M, Walker EM, Rooms MA, et al. Measuring quality of recovery-15 after day case surgery. Br J Anaesth 2016;116:241-8. [Crossref] [PubMed]
- Finnerty DT, Buggy DJ. Comparison of fascial plane blocks on quality of recovery for minimally invasive thoracic surgery. Response to Br J Anaesth 2021; 127: e14-5. Br J Anaesth 2021;127:e99-100. [Crossref] [PubMed]