
Efficacy of low-dose corticosteroids in patients with acute respiratory distress syndrome: a prospective observational study
Introduction
Acute respiratory distress syndrome (ARDS) is a severe clinical syndrome characterized by refractory hypoxemia that is caused by intrapulmonary and/or extrapulmonary factors (1). Despite progress in ARDS treatment, the mortality of ARDS patients is still high; thus, more effective treatments are needed (2).
Acute inflammatory responses are observed in early ARDS, and they cause an increase in vascular permeability, extravasation of plasma, and the recruitment and activation of immune cells to further induce severe refractory respiratory failure (3). Due to their significant inflammatory response inhibition and immune regulation activity, corticosteroids have been considered as potential drugs for the treatment of ARDS (4).
However, whether ARDS patients can benefit from corticosteroids remains controversial (5-7). Previous studies have determined the effects of different doses of corticosteroids on ARDS patients. Evidence from previous clinical trials has unanimously shown that a high dose of corticosteroids significantly increases the mortality of ARDS patients (8-10). Therefore, in recent years, more studies have aimed to address the effects of low-dose corticosteroids. However, the results of those studies are contradictory. Some randomized clinical trials have suggested that low-dose corticosteroids can reduce mortality or improve pulmonary function; thus, corticosteroids have been recommended for the treatment of ARDS patients (11,12). Conversely, other studies have shown that low-dose corticosteroids do not improve patient outcomes and can cause additional side effects (13,14). In general, more studies are needed to evaluate the effects and indications of low-dose corticosteroids in ARDS patients.
The CHARDS study is a real-world study with the aim of elucidating clinical practice for respiratory support and adjunctive measures for ARDS patients in intensive care units (ICUs) in China. The aim of the present study was to analyse the impact of low-dose corticosteroids on mortality in patients with ARDS based on data from the CHARDS study. Herein, we present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-890/rc).
Methods
Patients
This was a multicentre, prospective, observational study. In this study, we included all patients with ARDS who were admitted to the medical intensive care units/respiratory intensive care units (MICUs/RICUs) of 17 hospitals in mainland China between March 2016 and February 2018. All of the enrolled patients and their family members fully understood and agreed to participate in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committees of all of the participating study centers (No. 2015-77) and informed consent was taken from all the patients. The clinical study registration number is NCT02975908.
Inclusion and exclusion criteria
All the patients met the Berlin definition of ARDS when they were enrolled in the study (1). The patients were managed with noninvasive or invasive ventilation with positive end-expiratory pressure (PEEP) or continuous positive airway control (CPAP) ≥5 cmH2O. The arterial blood gas analysis was repeated 15 min after ventilation and confirmed the PaO2/FiO2 (P/F). Subsequently, the investigators diagnosed the ARDS when the patients met the above criteria and signed consent forms; afterwards, the investigators completed the case report forms. The exclusion criteria were as follows: (I) patients younger than 18 years of age; (II) patients who had structural lung diseases, including moderate-to-severe chronic obstructive pulmonary disease (COPD), bronchiectasis, and pulmonary interstitial fibrosis; (III) patients who died on the day of inclusion; or (IV) informed consent was not received from patients or family members.
Data collection and quality control
All of the patients were evaluated on days 0–14, 21, and 28 after the diagnosis of ARDS. D0 was defined as the day when patients were diagnosed with ARDS. Data were recorded as close to 8 AM as possible on each day. Patient outcomes included ICU and hospital mortality, lengths of stay in the ICU and hospital, and intubation rate. Nosocomial infections included hospital-acquired pneumonia (HAP), catheter-related bloodstream infection and urinary, abdominal and other infections that occurred after 48 h of admission. Any organ failure during an ICU stay was documented.
All of the data entry was double-checked. The completeness and accuracy of the data were checked during and after entry. Two inspectors supervised the quality of the data and provided feedback to the investigators. Missing or poor-quality data were removed before the data analysis was conducted.
Definition of corticosteroid doses
The daily dose of methylprednisolone or an equivalent treatment within 14 days after diagnosis was recorded. For a further description and analysis of corticosteroid usage, we grouped the patients as follows: (I) low-dose group—administration within seven days after ARDS diagnosis, daily dose of methylprednisolone equivalent to within 0.5–1 mg·kg−1·d−1 for more than five consecutive days; (II) high-dose group—administration within seven days after ARDS diagnosis, daily dose of methylprednisolone equivalent to >1 mg·kg−1·d−1 for more than five consecutive days but <500 mg/d methylprednisolone equivalent; and (III) corticosteroid pulse group—dose of methylprednisolone equivalent within 500–1,000 mg/d for five consecutive days, regardless of a dose reduction. Apart from the previously described conditions, other corticosteroid administrations were not discussed further.
Statistical analysis
Discrete variables are expressed as counts (percentages), and continuous variables are expressed as the means with standard deviations (SDs) or medians [interquartile ranges (IQRs)]. Proportions were compared by using a χ2 test or Fisher’s exact test. Continuous variables were compared via a t-test or Wilcoxon rank-sum test. Normality was tested by using histograms and the Kolmogorov-Smirnov normality test. Moreover, 1:2 propensity score matching was performed to adjust for differences in baseline characteristics between the low-dose corticosteroid and control groups. A survival analysis was conducted, and survival curves were plotted via a Kaplan-Meier analysis. A multivariate analysis was performed by using a Cox regression analysis. Data processing and statistical analysis were performed by using SPSS 25.0.
Results
Patients enrolled
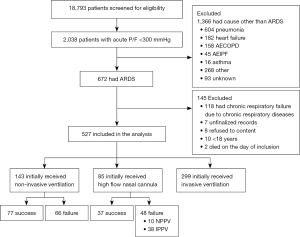
During the study period, 2,038 patients were admitted to the ICU in all of the participating units due to acute respiratory failure; among these patients, 672 patients were diagnosed with ARDS, according to the Berlin definition. A total of 145 patients were excluded, according to the exclusion criteria. Ultimately, 527 patients were enrolled in the study for further analyses (shown in Figure 1). The distribution of patients in different hospitals is shown in Figure S1A.
Corticosteroid administration
A total of 303 patients (57.4%) were treated with corticosteroids, among whom 65 patients (12.3%) received low-dose corticosteroids and 41 (7.8%) received high-dose corticosteroids. In the low-dose group, 55/65 patients (84.6%) were given corticosteroids beginning on the day of diagnosis; the median duration of administration was 10 [7–14] days, and the median daily dose was 0.67 (0.57–0.81) mg/kg methylprednisolone equivalent. In the high-dose group, the median daily dose was 1.50 (1.28–2.42) mg/kg methylprednisolone equivalent. A total of 33/41 patients (80.5%) began receiving corticosteroids on the day of diagnosis, and the median duration of administration was 11 [7–14] days. Eight patients (1.5%) received corticosteroid pulse therapy. All eight patients had moderate or severe ARDS; among them, six patients received 500 mg·d−1 methylprednisolone equivalent, and two patients received 1,000 mg·d−1. Other corticosteroid administrations were not discussed further from a statistical perspective (n=189, 35.8%), due to the pronounced heterogeneity of the dosage and timing. Patients who did not receive corticosteroids (n=224, 42.5%) were treated as the control group. The distribution of the low-dose corticosteroid group and control group in different hospitals is shown in Figure S1B,S1C.
Clinical characteristics
In low-dose corticosteroid and control groups (n=289), most of the patients were male (n=201, 69.6%), and the median age was 57.0 (45.0–69.0) years. The proportions of patients with mild, moderate, and severe ARDS were 30 (10.4%), 142 (49.1%), and 117 (40.5%), respectively. The median P/F ratio was 111.0 (83.5–162.0) mmHg. A total of 83.7% (n=242) of ARDS was induced by intrapulmonary factors. Furthermore, the main risk factors for ARDS were pneumonia (n=219, 75.8%), extrapulmonary sepsis (n=21, 7.3%), aspiration (n=11, 4.2%), and pancreatitis (n=11, 3.8%). Aetiological diagnoses were obtained in 111 patients with pneumonia. The most common pathogens were the influenza virus (n=48, 21.9%) and gram-negative bacilli (n=18, 8.2%) (detailed data are provided in Tables S1,S2).
The clinical characteristics of the patients who were treated or not treated with low-dose corticosteroids are shown in Table 1. The baseline characteristics of the high-dose group and other corticosteroid groups are shown in Tables S3,S4. There was a significant difference in the incidence of immunosuppressed patients (49.2% vs. 17.4%, P=0.000). In addition, the incidence of intrapulmonary ARDS in the low-dose group was higher than that in the control group (92.3% vs. 81.3%, P=0.033). No differences were found among the other variables.
Table 1
| Variable | Original sample | Matched sample | |||||
|---|---|---|---|---|---|---|---|
| Low-dose corticosteroid (n=65) | Non-corticosteroid (n=224) | P value | Low-dose corticosteroid (n=40) | Non-corticosteroid (n=80) | P value | ||
| Male sex, n (%) | 45 (69.2) | 156 (69.6) | 0.949 | 29 (72.5) | 60 (75.0) | 0.768 | |
| Age, median (IQR), years | 58.0 (44.0–70.0) | 57.0 (45.0–69.0) | 0.874 | 62.0 (44.0–72.3) | 56.0 (46.3–66.8) | 0.488 | |
| BMI, median (IQR) | 24.2 (22.0–26.2) | 24.2 (21.5–26.7) | 0.776 | 23.9 (22.4–26.9) | 24.4 (22.1–26.3) | 0.878 | |
| PaO2/FiO2 at admission (mmHg) | 107.0 (80.0–162.8) | 113.0 (84.1–160.1) | 0.695 | 98.3 (68.3–136.3) | 100.0 (71.9–149.8) | 0.686 | |
| APACHE II score, median (IQR) | 17 [10–23] | 15 [10–21] | 0.240 | 17.5 (10.8–22.3) | 15.5 (11.0–20.0) | 0.457 | |
| SOFA score, median (IQR) | 7 [4–9] | 6 [4–10] | 0.972 | 6.5 (3.8–9.0) | 6.0 (4.0–10.0) | 0.568 | |
| Intrapulmonary ARDS, n (%) | 60 (92.3) | 182 (81.3) | 0.033 | 36 (90.0) | 70 (87.5) | 0.688 | |
| Underlying disease condition, n (%) | |||||||
| Hypertension | 24 (36.9) | 65 (29.3) | 0.241 | 16 (40.0) | 27 (34.2) | 0.532 | |
| Diabetes mellitus | 14 (21.5) | 43 (19.4) | 0.700 | 9 (22.5) | 12 (15.2) | 0.323 | |
| Chronic cardiac insufficiency | 3 (4.6) | 8 (3.6) | 0.716 | 2 (5.0) | 1 (1.3) | 0.220 | |
| Chronic kidney disease | 6 (9.2) | 20 (9.0) | 0.956 | 1 (2.5) | 4 (5.1) | 0.510 | |
| Immunosuppressiona | 32 (49.2) | 39 (17.4) | 0.000 | 7 (17.5) | 15 (18.8) | 0.868 | |
| Laboratory test results at ICU admission | |||||||
| D0 WBC, median (IQR) (×109/L) | 11.3 (8.9–16.4) | 10.0 (6.3–15.3) | 0.150 | 11.1 (7.4–15.5) | 10.0 (5.3–15.0) | 0.334 | |
| D0 PCT, median (IQR) (ng/mL) | 1.1 (0.3–5.0) | 2.0 (0.4–10.5) | 0.077 | 1.8 (0.3–7.0) | 2.1 (0.6–9.6) | 0.221 | |
| D0 CRP, median (IQR) (mg/L) | 130.1 (60.7–211.7) | 124.7 (42.4–200.0) | 0.648 | 160.9 (82.7–222.4) | 133.8 (72.7–189.8) | 0.227 | |
| D0 lactic acid, median (IQR) (mmol/L) | 1.9 (1.2–2.7) | 1.8 (1.1–2.8) | 0.905 | 1.9 (1.2–2.6) | 1.6 (1.1–2.6) | 0.946 | |
| Mechanical ventilation managementb | |||||||
| Intubation rate, n (%) | 51 (78.5) | 158 (70.5) | 0.209 | 33 (82.5) | 57 (71.3) | 0.180 | |
| PEEP median (IQR), cmH2O | 10 [8–12] | 8 [5–10] | 0.002 | 10 [8–12] | 8 [6–10] | 0.008 | |
| VT, median (IQR) (mL/kg PBW) | 6.0 (5.7–6.9) | 6.8 (6.0–7.7) | 0.049 | 6.0 (5.5–6.7) | 6.8 (6.1–7.5) | 0.050 | |
| Plateau pressure, median (IQR), cmH2O | 23 [18–28] | 20 [15–24] | 0.010 | 20 [15–28] | 21 [15–24] | 0.858 | |
| Driving pressure, median (IQR), cmH2O | 13 [8–18] | 12 [7–16] | 0.183 | 11 [7–17] | 13 [7–15] | 0.451 | |
a, immunosuppression was defined as a haematologic malignancy or a solid tumour; or administration of steroids or any immunosuppressive drug within a month; or administration of radiation therapy or chemotherapy within a year. b, only patients with intubation were included. IQR, interquartile range; BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; WBC, white blood cell; PCT, procalcitonin; CRP, C reactive protein; PEEP, positive end expiratory pressure; VT, tidal volume; PBW, predicted body weight.
To minimize the influence of the different baseline characteristics, 1:2 propensity score matching was performed. A total of 120 patients were included in the matching analysis. The baseline characteristics of the matched sample are shown in Table 1. To ensure an adequate sample size, mechanical ventilation-related variables were not included in the matching protocol, and these variables had no significant effect on the risk of death in the matched sample (data not shown).
Mortality analysis
The outcomes of the matched sample between the two groups are displayed in Table 2 (the original sample is displayed in Table S5). The hospital patient mortality in the two groups was 27.5% and 42.5% (P=0.110). This difference in mortality was not significantly different. The median ICU and hospital lengths of stay were increased by 7.0 days (18.0 vs. 11.0, P=0.001) and 7.0 days (24.0 vs. 17.0 , P=0.002), respectively. In the survivors, the length of hospital stay between the two groups was 25.0 (19.0–37.0) vs. 23.0 days (14.0–29.5), P=0.106, and the length of ICU stay was 18.0 (10.5–26.8) vs. 11.0 days (7.0–22.0), P=0.026. In the nonsurvivors, the length of hospital stay between the two groups was 21.0 (15.0–25.0) vs. 12.0 days (4.8–20.3), P=0.009, and the length of ICU stay was 19.0 (13.0–24.0) vs. 9.0 days (4.0–17.3), P=0.011. The incidence rates of nosocomial infection and other organ failure events were not significantly different. The outcomes of the high-dose group and other corticosteroid groups are shown in Tables S6,S7.
Table 2
| Outcome | Low-dose corticosteroid (n=40) | Non-corticosteroid (n=80) | P value |
|---|---|---|---|
| Duration of mechanical ventilation* (days) | 11.0 (6.0–14.0) | 6.5 (0.0–11.0) | 0.007 |
| Nosocomial infection, n (%) | 12 (30.0) | 27 (33.8) | 0.679 |
| New organ failure, n (%) | 11 (27.5) | 34 (42.5) | 0.110 |
| Ventilator free days at day 28, d | 16.0 (2.0–22.0) | 14.0 (0.0–28.0) | 0.980 |
| ICU length of stay (days) | 18.0 (12.0–24.0) | 11.0 (5.0–19.0) | 0.001 |
| Hospital length of stay (days) | 24.0 (19.0–33.0) | 17.0 (10.0–28.0) | 0.002 |
| ICU mortality, n (%) | 11 (27.5) | 33 (41.3) | 0.141 |
| Hospital mortality, n (%) | 11 (27.5) | 34 (42.5) | 0.110 |
*, only patients with intubation were included. ICU, intensive care unit.
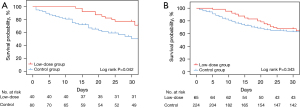
Kaplan-Meier survival curves were constructed to illustrate the 30-day survival rates in the two groups (shown in Figure 2A,2B). The mortality decreased significantly in the low-dose corticosteroid group in the matched sample (P=0.042). In addition, the curve showed that corticosteroids had no effect on the intubation rate (shown in Figure S2A,S2B).
To eliminate potential confounding factors, univariate and multivariate Cox regression analyses were performed in matched samples (Figures 3,4) and original samples (Tables S8,S9), respectively. The multivariate Cox regression analysis of hospital mortality identified low-dose corticosteroids as being a protective factor in matched samples (HR: 0.48; 95% CI: 0.24–0.97; P=0.040). Administration of low-dose corticosteroids showed a lower risk of death in patients with intrapulmonary ARDS in the matched sample (shown in Table 3, data of original sample shown in Table S10).
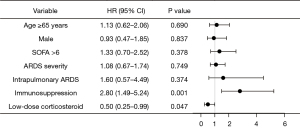
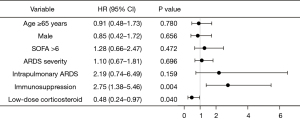
Table 3
| Subgroup | Hospital mortality | |
|---|---|---|
| HR (95% CI) | P | |
| All patients (n=120) | 0.48 (0.24–0.97) | 0.040 |
| Patients with intrapulmonary ARDS (n=106) | 0.36 (0.17–0.77) | 0.009 |
| Patients with mechanical ventilation (n=90) | 0.50 (0.25–1.03) | 0.062 |
| Patients with shock (n=39) | 0.92 (0.23–1.47) | 0.250 |
| Patients without immunosuppression (n=98) | 0.53 (0.23–1.23) | 0.142 |
ARDS, acute respiratory distress syndrome.
Adverse effects of low-dose corticosteroids
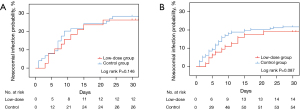
The adverse effects of corticosteroids may cause the conditions of patients to deteriorate and even influence outcomes, and secondary infection was observed to be the most severe threat. To investigate the acute adverse events associated with corticosteroids, the incidence of nosocomial infection was analysed via a Kaplan-Meier analysis in matched samples and original samples (shown in Figure 5A,5B). No significant difference was found in the nosocomial infection rate between the two groups (P=0.146). Hyperglycaemia and ICU-acquired weakness were not recorded.
Discussion
To date, the present study is the largest observational study focusing on corticosteroid administration in ARDS patients in China. In this study, we determined two main conclusions about the administration of low-dose corticosteroids in patients with ARDS. First, our results show that low-dose corticosteroid therapy may play a protective role in ARDS patients, although the lengths of ICU and hospital stays are prolonged. Second, no significant relationship was found between low-dose corticosteroids and the nosocomial infection rate.
The efficacy and safety of low-dose corticosteroids (≤1 mg·kg−1·d−1) in ARDS patients is still controversial. A randomized controlled trial (RCT) reported by Meduri et al. suggested that the administration of low-dose (1 mg·kg−1·d−1) methylprednisolone in the early stage could significantly reduce mortality, compared to that in the placebo group (20.6% vs. 42.9%, P=0.03) (11). These researchers subsequently published numerous meta-analyses that all supported the notion that the administration of a low dose of corticosteroids could reduce the mortality rate and shorten the length of hospital stay for ARDS patients (15,16). Furthermore, a double-blind, single-centre RCT conducted by Tongyoo et al. suggested that, although corticosteroids do not reduce mortality (22.5% vs. 27.3%, P=0.51), secondary endpoints, such as the P/F ratio and lung injury score, were improved (P=0.01) (12). In contrast, a meta-analysis by Ruan et al. showed that corticosteroids did not significantly affect ICU mortality or the 60-day mortality rate (13). Additionally, the results of a meta-analysis by Horita et al. showed that the benefit of corticosteroids for ARDS patients was uncertain (14); therefore, corticosteroids should not be used as a routine treatment.
The studies referred above have shown inconsistent conclusions, largely due to the heterogeneity of the ARDS patients. Therefore, more subgroup studies are needed to reduce heterogeneity. In recent years, many large, controlled studies and retrospective studies have confirmed that the administration of corticosteroids in influenza-related ARDS patients significantly increases mortality or the risk of nosocomial infections (9,17,18).
Our findings are partially consistent with previous results. Although there was no significant difference in mortality, according to the univariate analysis (27.5% vs. 42.5%, P=0.110), the Cox regression analysis revealed that a low-dose corticosteroid administration may reduce ICU and hospital mortality. This potential benefit can be explained by the anti-inflammatory function of corticosteroids, by which the systemic inflammation experienced in ARDS was relieved. Although we did not collect serum for cytokine determinations, previous in vivo experiments have also demonstrated this point (19,20). Furthermore, the reason for the prolonged ICU and hospital stays may be that the administration of corticosteroids increases the risk of ICU-acquired weakness and may delay the clearance of pathogens (21,22). Thus, it is necessary to realize that the potential benefits and risks are equally important.
In the present study, the purpose of corticosteroid administration was not recorded on the case report form. Due to the use of oral corticosteroids in patients with other autoimmune diseases or in ARDS patients, immunosuppression may overlap with low-dose corticosteroid administration. Meanwhile, immunosuppression has been shown to increase mortality due to superinfections and ICU-acquired infection (23,24). Our study also supports this notion. Therefore, we performed propensity score matching to eliminate confounding factors. The results from the matched samples further support this conclusion.
The second key finding is that low-dose corticosteroid administration had no relationship with nosocomial infections within 30 days after diagnosis (30.0% vs. 33.8%, P=0.679). This conclusion is in accordance with a report by Cao et al. (17), which suggested that rates of HAP were higher in the high-dose corticosteroid group than in the control group, but not in the low-dose group. Notably, our data encompassed only 14 days after the diagnosis; thus, we cannot conclusively state the effects of long-course corticosteroid treatment (>14 days) on nosocomial infections.
In clinical practice, the variation in corticosteroid dosages is large. Therefore, it is difficult to describe the strategy of corticosteroid administration in an observational study. To achieve the number of patients and a balance of consistency, we finally defined a representative dose (daily doses of 0.5–1.0 mg·kg−1 for at least 5 days) as the low-dose group, based on clinical practice in China. This standard guaranteed the consistency of the corticosteroid dose to a certain extent; however, many patients were not analysed. Although the standardization of the corticosteroid dose was not fully achieved, we considered that it still has positive implications.
There were limitations to this study. First, this study was an observational study; therefore, ensuring the internal consistency of patients in the different groups is problematic. Although propensity score matching was performed, there may also be some potentially hard-to-eliminate differences between the two groups. In addition, incomplete data on adjunctive measures in the two groups may be a major deficiency. Second, a lack of purpose regarding corticosteroid use made our grouping scheme less accurate (to a certain extent), which may be why a portion of the data was not truly utilized. Third, the sample size was small, especially in the propensity score matching sample. It also restricted further analysis about the role of high-dose corticosteroid group. Last, the missing data for aetiologic diagnoses in some patients may lead to potential diagnosis biases in ARDS. Therefore, further random clinical trials are needed.
Conclusions
Based on our limited observational data, the administration of low-dose corticosteroids may reduce mortality in patients with ARDS, and the lengths of ICU and hospital stays are prolonged. Moreover, no significant relationship was found between low-dose corticosteroids and the nosocomial infection rate.
Acknowledgments
We thank all the staff in the participating ICUs who helped us to collect the data of the patients.
Funding: This work was supported by the Capital Clinical Features Applied Research and Achievement Promotion Project of Beijing, China (Z161100000516116); the National Key Research and Development Program of China (2016YFC1304300); the CAMS Innovation Fund for Medical Sciences (CIFMS) (2018-I2 M-1-003); and the Non-profit Central Research Institute Fund of CAMS (2019TX320006).
Footnote
Investigators of CHARDSnet: Lixin Xie, Ying Wang, Department of Respiratory and Critical Care Medicine, The General Hospital of the People’s Liberation Army; Li Weng, Medical Intensive Care Unit, Peking Union Medical College Hospital; Guangfa Zhu, Yan Liu, Man Song, Department of Respiratory and Critical Care Medicine, Beijing Anzhen Hospital, Capital Medical University; Yanming Zhao, Jing Chen, Department of Respiratory and Critical Care Medicine, Beijing Hospital; Hongwen Zhao, Haijia Hou, Department of Respiratory and Critical Care Medicine, The First Hospital of China Medical University; Jingping Yang, Rina Wu, Xiyuan Xu, Department of Respiratory and Critical Care Medicine, Inner Mongolia Baogang Hospital; Xixin Yan, Haibo Xu, Department of Respiratory and Critical Care Medicine, The Second Hospital of Hebei Medical University; Dawei Wu, Haining Lu, Department of Critical Care Medicine, Qilu Hospital of Shandong University (Qingdao); Gengyun Sun, Dan Zhang, Department of Respiratory and Critical Care Medicine, The First Hospital of Anhui Medical University; Beilei Zhao, Binhai Pan, Department of Respiratory and Critical Care Medicine, Nanjing General Hospital of Nanjing Military Command, PLA; Jialin Liu, Ruoming Tan, Department of Critical Care Medicine, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine; Pinhua Pan, Rongli Lu, Department of Respiratory and Critical Care Medicine, Xiangya Hospital of Central South University; Hong Luo, Han Zhang, Respiratory Department, The Second Xiangya Hospital of Central South University; Daoxin Wang, Wang Deng, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Chongqing Medical University; Yusheng Chen, Fengfeng Lu, Department of Respiratory and Critical Care Medicine, Fujian Provincial Hospital; Sicheng Xu, Xia Luo, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Xinjiang Medical University; Hong Teng, Lijuan Chen, Department of Respiratory and Critical Care Medicine, Sichuan Provincial People’s Hospital; Lihua Xing, Shilei Wang, Junlu Li, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Zhengzhou University; Tongwen Sun, Shaohua Liu, Bing Han, Intensive Care Unit, The First Affiliated Hospital of Zhengzhou University.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-890/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-890/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-890/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-890/coif). All authors report funding from Beijing Municipal Science & Technology Commission, National Natural Science Foundation, and Chinese Academy of Medical Sciences.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committees of all of the participating study centers (No. 2015-77) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Suter PM. Lung Inflammation in ARDS--friend or foe. N Engl J Med 2006;354:1739-42. [Crossref] [PubMed]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med 2005;353:1711-23. [Crossref] [PubMed]
- Blot M, Salmon-Rousseau A, Chavanet P, et al. Do we know enough to recommend corticosteroids in acute respiratory distress syndrome. Crit Care 2017;21:327. [Crossref] [PubMed]
- Meduri GU, Siemieniuk RAC, Ness RA, et al. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care 2018;6:53. [Crossref] [PubMed]
- Briegel J, Bein T, Möhnle P. Update on low-dose corticosteroids. Curr Opin Anaesthesiol 2017;30:186-91. [Crossref] [PubMed]
- Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 1987;317:1565-70. [Crossref] [PubMed]
- Sivanandy P, Zi Xien F, Woon Kit L, et al. A review on current trends in the treatment of human infection with H7N9-avian influenza A. J Infect Public Health 2019;12:153-8. [Crossref] [PubMed]
- Kido T, Muramatsu K, Asakawa T, et al. The relationship between high-dose corticosteroid treatment and mortality in acute respiratory distress syndrome: a retrospective and observational study using a nationwide administrative database in Japan. BMC Pulm Med 2018;18:28. [Crossref] [PubMed]
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131:954-63. [Crossref] [PubMed]
- Tongyoo S, Permpikul C, Mongkolpun W, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care 2016;20:329. [Crossref] [PubMed]
- Ruan SY, Lin HH, Huang CT, et al. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care 2014;18:R63. [Crossref] [PubMed]
- Horita N, Hashimoto S, Miyazawa N, et al. Impact of Corticosteroids on Mortality in Patients with Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis. Intern Med 2015;54:1473-9. [Crossref] [PubMed]
- Meduri GU, Bridges L, Shih MC, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med 2016;42:829-40. [Crossref] [PubMed]
- Peter JV, John P, Graham PL, et al. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ 2008;336:1006-9. [Crossref] [PubMed]
- Cao B, Gao H, Zhou B, et al. Adjuvant Corticosteroid Treatment in Adults With Influenza A (H7N9) Viral Pneumonia. Crit Care Med 2016;44:e318-28. [Crossref] [PubMed]
- Singh A, Khera K, Agarwal J, et al. Descriptive Analysis of Mortality Predictors in H1n1 Influenza in South Indian Patients. Infect Disord Drug Targets 2017;17:106-15. [Crossref] [PubMed]
- Aoyagi T, Sato Y, Toyama M, et al. Etoposide and Corticosteroid Combination Therapy Improves Acute Respiratory Distress Syndrome in Mice. Shock 2019;52:83-91. [Crossref] [PubMed]
- Song LC, Chen XX, Meng JG, et al. Effects of different corticosteroid doses and durations on smoke inhalation-induced acute lung injury and pulmonary fibrosis in the rat. Int Immunopharmacol 2019;71:392-403. [Crossref] [PubMed]
- Yang T, Li Z, Jiang L, et al. Corticosteroid use and intensive care unit-acquired weakness: a systematic review and meta-analysis. Crit Care 2018;22:187. [Crossref] [PubMed]
- Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009;200:492-500. [Crossref] [PubMed]
- Demoule A, Antonelli M, Schellongowski P, et al. Respiratory Mechanics and Outcomes in Immunocompromised Patients With ARDS: A Secondary Analysis of the EFRAIM Study. Chest 2020;158:1947-57. [Crossref] [PubMed]
- Cortegiani A, Madotto F, Gregoretti C, et al. Immunocompromised patients with acute respiratory distress syndrome: secondary analysis of the LUNG SAFE database. Crit Care 2018;22:157. [Crossref] [PubMed]


