
Risk factors for impaired neurological outcome after thoracic aortic surgery
Introduction
Although surgical techniques and outcome in thoracic aortic surgery have improved over the last decade, stroke remains a common complication with rates around 15% (1). Previously described risk factors for postoperative stroke include female gender (2), emergency surgery (3), preoperative malperfusion syndrome (4,5), total arch replacement (1), increased cardiopulmonary bypass (1,2,6), and circulatory arrest time (3). Postoperative stroke can substantially affect patient’s quality of life and is associated with further complications, like renal or multi-organ failure, prolonged ventilation (5), longer duration of hospitalization (7), and mortality (3,4,8). Identification of unknown and verification of known risk factors in a large cohort is urgently needed. Considering these factors in the preoperative patient evaluation may lead to an improved patient selection and reduce perioperative complication rates. Risk factor analyses have primarily been performed in specific pathologies like acute type A aortic dissection. A more generalized approach is needed to improve patient care in both, elective and emergent thoracic aortic surgery. We aimed to identify independent risk factors associated with new postoperative stroke in a cohort that underwent the whole spectrum of thoracic aortic surgery. Moreover, we compared early and late survival rate between the stroke and no stroke group. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1591/rc).
Methods
Study design, setting and participants
Data from patients undergoing thoracic aortic surgery at the German Aortic Center Hamburg were collected using our dedicated institutional aortic database and evaluated to identify independent risk factors for the occurrence of postoperative stroke. Data acquisition was performed anonymized and retrospectively.
The German Aortic Center Hamburg is a high-volume aortic center in Northern Germany offering the whole spectrum of thoracic aortic procedures. Our registry comprises all patients who were planned to undergo thoracic aortic surgery before starting the procedure, since we included patients based on our digital operating room schedule. Therefore, the rare cases in which the decision to operate on the thoracic aorta was taken intraoperatively were not included into our database. Besides that, our registry is fully comprehensive. Follow-up data was collected retrospectively from the patient’s chart if available. All patients undergoing thoracic aortic surgery from January 2010 to February 2020 were included. All procedure types in thoracic aortic surgery were included, such as root replacement, supracoronary ascending replacement, and hemi- or total arch repair including extension into the proximal descending aorta using the frozen elephant trunk (FET) procedure. These procedures were performed by a total of 25 different cardiac surgeons. This implies all urgency statuses (elective, urgent, emergent, salvage) with or without hypothermic circulatory arrest (HCA) and selective antegrade cerebral perfusion (SACP) as well as concomitant procedures.
As the study was performed anonymized and retrospectively, in accordance with German law, no ethical approval is needed and informed patient consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Definitions of variables
Based on the Valve Academic Research Consortium-2 criteria (9), stroke was defined as a new focal or global postoperative neurological deficit (ND) of ischemic or hemorrhagic origin, persisting at least 7 days. ND was diagnosed by clinical signs and symptoms confirmed by a neurologist and brain imaging using computed tomography (CT) or magnetic resonance imaging (MRI). In case the patient already presented with a ND upon admission, the endpoint of postoperative ND was only triggered, if a change in symptoms lead to a higher modified Rankin Scale (mRS) (10) of at least one point difference before and after the procedure. Stroke was further divided into embolic/territorial, hemodynamic, hemorrhagic, and lacunar by brain imaging and as reported by a neurologist or neuroradiologist.
Focal ND was defined as symptoms affecting specific body areas. Global ND was defined as coma or ND affecting the whole brain.
Urgency statuses were defined according to the EuroSCORE II as (11):
- elective: routine admission for operation;
- urgent: patients not electively admitted for operation but who require surgery on the current admission for medical reasons and cannot be discharged without a definitive procedure;
- emergency: operation before the beginning of the next working day after decision to operate;
- salvage: patients requiring cardiopulmonary resuscitation (external cardiac massage) en route to the operating theatre or before induction of anaesthesia. This does not include cardiopulmonary resuscitation after induction of anaesthesia.
Any atherosclerotic entity was defined as either coronary artery disease in the patient’s history and/or extracardiac arteriopathy as defined by the EuroSCORE II. Extracardiac arteriopathy includes claudication, carotid stenosis >50%, amputation for arterial disease or preceding or prospective intervention on the abdominal aorta, carotids or arteries of extremities (11).
Further definitions can be found in Appendix 1.
Study size
Of all 1,392 consecutive patients in the study period, patients with missing data on clinical parameters (n=7) and endocarditis (n=32) were excluded. Furthermore, we excluded patients who died intraoperatively (n=19) since occurrence of intraoperative stroke could not be determined.
Statistical analysis
Categorical variables were reported as frequencies and percentages and compared between study groups (ND vs. no ND group) using chi-square test or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean ± standard deviation (SD) and compared between groups using the two-sided Student’s t-test. Continuous variables such as age and surgical times were converted into binary variables using cut-off values between the 70th and 85th percentile of each value based on clinical experience. Temperature was considered as low below the 25th percentile. Potential pre- and intraoperative factors (candidates) identified by significant differences in the univariate analysis were further tested using multivariate logistic regression to explore independent variables associated with postoperative stroke. Variables in the logistic regression model were selected by a stepwise backward elimination process. Adjusted odds ratio (OR), 95% confidence intervals (CIs) and P values were reported.
To identify specific risk factors, risk factor analysis was repeated for the secondary endpoints embolic and hemodynamic stroke. Additionally, we performed subgroup analyses for patients presenting with acute type A dissection (ATAD), presenting with aneurysm, undergoing root surgery and undergoing total arch replacement.
To retrospectively evaluate long-term survival, Kaplan-Meier estimations were calculated. In landmark analyses, patients that died within 30 or 90 days were excluded, respectively. To compare ND and no ND groups, Cox regression analysis was conducted, and adjusted hazard ratio (HR) and P value were reported. Apart from stroke, covariates entered in this model were age, gender, peripheral arterial disease, ejection fraction under 30%, chronic lung disease, renal function, preoperative critical state, ATAD and high extracorporeal circulation (ECC) time, as these factors are known to be associated with increased postoperative mortality after aortic surgery (2,12-14).
Patients with missing data on cannulation technique (n=7) were excluded from our study since they could not be included in the logistic regression analysis.
The level of significance for all analyses was set at alpha =0.05.
Statistical analyses were performed using IBM SPSS version 26.0.0.0 (IBM Corp., Armonk, NY, USA).
Results
Preoperative patients’ characteristics
The mean age of all 1,334 analyzed patients was 61.4±14.2 years with 34.9% being female. The mean EuroSCORE II was 8.6±10.1. Genetic aortic syndrome was diagnosed in 123 patients (9.2%). Underlying aortic pathologies mainly were thoracic aortic aneurysms (71.1%, n=948) and aortic syndromes (27.8%, n=371). Of all patients presenting with aortic syndrome, most patients suffered from ATAD (n=240, 18.0% of the total cohort). Further baseline characteristics are depicted in Table 1.
Table 1
| Characteristics | All (n=1,334) | No stroke (n=1,240) | Stroke (n=94) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.4±14.2 | 61.0±14.3 | 67.2±11.5 | <0.001 |
| Female gender, n (%) | 466 (34.9) | 434 (35.0) | 32 (34.0) | 0.851 |
| Obesity (BMI >35 kg/m²), n (%) | 81 (6.1) | 79 (6.4) | 2 (2.1) | 0.097 |
| Arterial hypertension, n (%) | 1,040 (78.0) | 958 (77.3) | 82 (87.2) | 0.024 |
| Any atherosclerotic entity, n (%) | 493 (37.0) | 445 (35.9) | 48 (51.1) | 0.003 |
| Coronary artery disease | 380 (28.5) | 341 (27.5) | 39 (41.5) | 0.004 |
| Extracardiac arteriopathy | 184 (13.8) | 164 (13.2) | 20 (21.3) | 0.029 |
| Atrial fibrillation, n (%) | 209 (15.7) | 192 (15.5) | 17 (18.1) | 0.504 |
| IDDM, n (%) | 31 (2.3) | 28 (2.3) | 3 (3.2) | 0.476 |
| Chronic lung disease, n (%) | 134 (10.0) | 117 (9.4) | 17 (18.1) | 0.007 |
| Smoker, n (%) | 525 (39.4) | 484 (39.0) | 41 (43.6) | 0.380 |
| Chronic kidney disease, n (%) | 256 (19.2) | 224 (18.1) | 32 (34.0) | <0.001 |
| Previous cardiac surgery, n (%) | 136 (10.2) | 126 (10.2) | 10 (10.6) | 0.883 |
| EuroSCORE II, mean ± SD | 8.6±10.1 | 8.1±9.5 | 15.0±13.9 | <0.001 |
| Preoperative stroke, n (%) | 114 (8.5) | 103 (8.3) | 11 (11.7) | 0.256 |
| Linked to aortic syndrome | 34 (2.5) | 33 (2.7) | 1 (1.1) | 0.508 |
| Not linked to aortic syndrome | 80 (6.0) | 70 (5.6) | 10 (10.6) | 0.049 |
| Preoperative critical state, n (%) | 134 (10.0) | 110 (8.9) | 24 (25.5) | <0.001 |
| Preoperative cerebral malperfusion, n (%) | 56 (4.2) | 44 (3.5) | 12 (12.8) | <0.001 |
| Genetic aortic syndrome, n (%) | 123 (9.2) | 121 (9.8) | 2 (2.1) | 0.014 |
| Malfunction or infection of TEVAR or aortic graft, n (%) | 14 (1.0) | 11 (0.9) | 3 (3.2) | 0.070 |
| Aneurysm, n (%) | 948 (71.1) | 914 (73.7) | 34 (36.2) | <0.001 |
| ATAD, n (%) | 240 (18.0) | 202 (16.3) | 38 (40.4) | <0.001 |
| IMH, n (%) | 43 (3.2) | 36 (2.9) | 7 (7.4) | 0.027 |
| PAU, n (%) | 13 (1.0) | 11 (0.9) | 2 (2.1) | 0.232 |
| Chronic dissection, n (%) | 56 (4.5) | 61 (4.6) | 5 (5.3) | 0.613 |
| Aortic rupture, n (%) | 68 (5.1) | 56 (4.5) | 12 (12.8) | 0.002 |
| Maximum thoracic aortic diameter (mm), mean ± SD | 5.49±1.18 | 5.49±1.19 | 5.45±1.09 | 0.766 |
| Urgency status, n (%) | ||||
| Elective | 999 (74.9) | 960 (77.4) | 39 (41.5) | <0.001 |
| Urgent | 47 (3.5) | 42 (3.4) | 5 (5.3) | 0.375 |
| Emergent or salvage | 286 (21.4) | 236 (19.0) | 50 (53.2) | <0.001 |
SD, standard deviation; BMI, body mass index; IDDM, insulin-dependent diabetes mellitus; TEVAR, thoracic endovascular aortic repair; ATAD, acute type A dissection; IMH, intramural hematoma; PAU, penetrating atherosclerotic ulcer.
Procedural data
Of all aortic procedures, 999 (74.9%) were elective, 47 (3.5%) were urgent, and 286 (21.4%) were emergent or salvage. Aortic root replacement was performed in 451 patients (33.8%). Of these, 221 (16.6% of the total cohort) underwent valve sparing root replacements. Isolated supracoronary ascending replacement without HCA was performed in 82 (6.1%) patients. Hemiarch and total arch replacement using HCA was performed in 419 (31.4%) and 203 patients (15.2%), respectively. Of these, most patients underwent aortic surgery using HCA with SACP for cerebral protection (n=530, 86.0%). The mean HCA, SACP and ECC times were 36.8±25.3 min, 44.1±32.8 min, and 185.9±79.8 min, respectively. The most common site of cannulation was the ascending aorta or aortic arch (n=903, 67.7%), followed by the subclavian (n=211, 15.8%) and the innominate artery (n=182, 13.6%). In only 34 (2.5%) patients, the femoral artery was cannulated for cardiopulmonary bypass.
Stroke rate
In 7.0 % (n=94) of all patients who underwent thoracic aortic surgery, a new permanent postoperative ND was detected. Of all patients, 2.5% suffered transient ND. In 48.9% (n=46) of the permanent strokes, the ND was considered focal. In 51.1% (n=48) it was considered global. In cranial MRI or CT imaging, lesions were observed in the left and right hemisphere in 17 (20.0%) and 19 patients (22.4%), respectively. In 49 patients (57.6%) both hemispheres were involved. In 9 patients, MRI or CT images weren’t available for retrospective analysis anymore. Of all patients suffering from postoperative stroke we could evaluate the mRS score of 63 patients at discharge. Of these, 79.4% (n=50) had a score of 3 or higher. The highest stroke rate with 15.8% occurred in patients undergoing surgery for ATAD. In elective procedures the overall stroke rate was 3.9%. Figure 1 shows postoperative stroke rates for different procedures. Of these, patients who underwent total arch surgery had the highest rate with 10.7%. However, if the total arch was replaced using a simplified FET technique performed by an experienced aortic surgeon in an elective setting, no stroke was observed. The lowest surgeon-independent stroke rate was found after elective valve-sparing root replacement (0.5%).
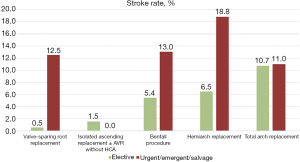
Risk factor analysis of the total cohort
Univariate analysis
Table 1 shows baseline characteristics of the analyzed patient cohort. Patients suffering from postoperative ND were significantly older, had a higher EuroSCORE II, and were more likely to have comorbidities such as coronary artery disease, chronic kidney disease, or history of cerebrovascular accident compared to patients without neurological impairment after surgery. Patients with postoperative ND presented with a higher rate of ATAD, preoperative critical state, preoperative cerebral malperfusion, and aortic rupture. Genetic aortic syndromes and elective procedures were less common in the ND group (Table 1).
Table 2 shows surgical procedures performed in our cohort. Patients suffering from postoperative stroke more often underwent aortic arch replacement and concomitant coronary artery bypass grafting (CABG). Valve-sparing root replacements and Wheat procedures were less common in the ND group.
Table 2
| Procedures | All (n=1,334) | No stroke (n=1,240) | Stroke (n=94) | P value |
|---|---|---|---|---|
| Aortic root surgery, n (%) | 451 (33.8) | 431 (34.8) | 20 (21.3) | 0.008 |
| Bentall procedure | 230 (17.2) | 214 (17.3) | 16 (17.0) | 0.953 |
| Valve-sparing root replacement | 221 (16.6) | 217 (17.5) | 4 (4.3) | 0.001 |
| Isolated ascending replacement, n (%) | 82 (6.1) | 80 (6.5) | 2 (2.1) | 0.092 |
| Wheat (supracoronary + AVR), n (%) | 251 (18.8) | 241 (19.4) | 10 (10.6) | 0.035 |
| Arch procedure, n (%) | 622 (46.6) | 446 (44.0) | 76 (80.9) | <0.001 |
| Hemiarch | 419 (31.4) | 365 (29.4) | 54 (57.4) | <0.001 |
| Total arch | 203 (15.2) | 181 (14.6) | 22 (23.4) | 0.022 |
| Conventional | 69 (5.2) | 61 (4.9) | 8 (8.5) | 0.143 |
| FET | 134 (10.0) | 120 (9.7) | 14 (14.9) | 0.105 |
| Simplified FET technique | 70 (5.2) | 67 (5.4) | 3 (3.2) | 0.475 |
| Descending procedure, n (%) | 9 (0.7) | 8 (0.6) | 1 (1.1) | 0.483 |
| Concomitant surgery, n (%) | ||||
| CABG | 193 (14.5) | 169 (13.6) | 24 (25.5) | 0.002 |
| TVR | 21 (1.6) | 20 (1.6) | 1 (1.1) | >0.990 |
| MVR | 58 (4.3) | 55 (4.4) | 3 (3.2) | 0.793 |
| VAD implantation | 2 (0.1) | 2 (0.2) | 0 (0.0) | >0.990 |
AVR, aortic valve replacement; FET, frozen elephant trunk; CABG, coronary artery bypass grafting; TVR, tricuspid valve replacement; MVR, mitral valve repair; VAD, ventricular assist device.
Table 3 shows intraoperative data. Here, ECC time was significantly increased in patients showing postoperative neurological impairment. Aortic clamp time, HCA and cerebral perfusion (CP) time did not significantly differ between the groups. The ND group more frequently underwent HCA with SACP and was cooled to lower body temperatures intraoperatively.
Table 3
| Intraoperative data | All (n=1,334) | No stroke (n=1,240) | Stroke (n=94) | P value |
|---|---|---|---|---|
| Aortic surgeon (>20 aortic procedures per year), n (%) | 771 (57.8) | 721 (58.2) | 50 (53.2) | 0.344 |
| Time of surgery, n (%) | ||||
| Weekend surgery | 63 (4.7) | 52 (4.2) | 11 (11.7) | 0.003 |
| Night-time surgery | 197 (14.8) | 165 (13.3) | 32 (34.0) | <0.001 |
| ECC time (min), mean ± SD | 185.9±79.8 | 181.6±76.3 | 242.3±101.5 | <0.001 |
| Aortic clamp time (min), mean ± SD | 105.3±50.5 | 104.6±49.6 | 115.3±61.3 | 0.108 |
| HCA with SACP, n (%) | 530 (39.7) | 466 (37.6) | 64 (68.1) | <0.001 |
| Unilateral CP, n (%) | 53 (4.1) | 46 (3.8) | 7 (7.8) | 0.087 |
| Bilateral CP, n (%) | 546 (41.7) | 481 (39.5) | 65 (72.2) | <0.001 |
| Cerebral hypothermic arrest, n (%) | 19 (1.5) | 18 (1.5) | 1 (1.1) | 1.000 |
| CP flow (mL/min), mean ± SD | 1,240.8±425.8 | 1,240.5±435.4 | 1,242.4±353.7 | 0.973 |
| CP volume (liter), mean ± SD | 58.1±54.5 | 57.5±53.8 | 62.9±59.4 | 0.465 |
| CP temperature (℃), mean ± SD | 22.4±4.5 | 22.5±4.6 | 21.8±3.2 | 0.215 |
| Cerebral monitoring, n (%) | 576 (49.1) | 515 (47.2) | 61 (73.5) | <0.001 |
| Circulatory arrest time (min), mean ± SD | 36.8±25.3 | 36.1±24.7 | 41.6±29.5 | 0.082 |
| CP time (min), mean ± SD | 41.6±31.7 | 40.9±31.0 | 46.4±36.1 | 0.158 |
| Deepest body temperature (℃), mean ± SD | 28.7±4.0 | 28.9±4.0 | 25.9±3.5 | <0.001 |
| Site of arterial cannulation, n (%) | ||||
| Ascending aorta or aortic arch | 903 (67.7) | 857 (69.1) | 46 (48.9) | <0.001 |
| Innominate artery | 182 (13.6) | 169 (13.6) | 13 (13.8) | 0.956 |
| Subclavian artery | 211 (15.8) | 181 (14.6) | 30 (31.9) | <0.001 |
| Femoral artery | 34 (2.5) | 29 (2.3) | 5 (5.3) | 0.085 |
ECC, extracorporeal circulation; SD, standard deviation; HCA, hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion; CP, cerebral perfusion.
Table 4 shows outcome parameters. The overall 30-day mortality was 5.9% (n=79) and was significantly increased in patients with ND compared to patients without ND (34.0% vs. 3.8%, P<0.001). Rates of postoperative paraplegia, and reanimation or shock were significantly higher in the ND group. ND patients showed a higher rate of resternotomy.
Table 4
| Parameters | All (n=1,334) | No stroke (n=1,240) | Stroke (n=94) | P value |
|---|---|---|---|---|
| In-hospital/30-day mortality, n (%) | 79 (5.9) | 47 (3.8) | 32 (34.0) | <0.001 |
| One-year mortality, n (%) | 106 (17.8) | 65 (12.2) | 41 (65.1) | <0.001 |
| Transient ND, n (%) | 34 (2.5) | 34 (2.7) | – | – |
| Resternotomy for bleeding or tamponade, n (%) | 139 (10.4) | 116 (9.4) | 23 (24.5) | <0.001 |
| Reanimation or shock, n (%) | 77 (5.8) | 59 (4.8) | 18 (19.1) | <0.001 |
| Paraplegia, n (%) | 14 (1.0) | 9 (0.7) | 5 (5.3) | 0.002 |
| Recurrent nerve palsy, n (%) | 29 (2.2) | 27 (2.2) | 2 (2.1) | >0.999 |
| Pneumonia or other respiratory insufficiency, n (%) | 174 (13.0) | 143 (11.5) | 31 (33.0) | <0.001 |
| Sepsis, n (%) | 30 (2.2) | 18 (1.5) | 12 (12.8) | <0.001 |
| New-onset atrial fibrillation, n (%) | 265 (19.9) | 243 (19.6) | 22 (23.4) | 0.372 |
| Myocardial infarction, n (%) | 10 (0.7) | 9 (0.7) | 1 (1.1) | 0.520 |
ND, neurological deficit.
Multivariate risk factor analysis
The results of the multivariate risk factor analysis are shown in Table 5. Advanced age (>70 years; adjusted OR, 1.83; 95% CI, 1.16–2.88; P=0.009), ATAD (adjusted OR, 1.69; 95% CI, 1.00–2.84; P=0.0495), aortic arch surgery (adjusted OR, 3.24; 95% CI, 1.78–5.88; P<0.001), and concomitant CABG (adjusted OR, 2.19; 95% CI, 1.27–3.79; P=0.005) were identified as independent risk factors for postoperative stroke. Of all surgical times, only high ECC time (>230 min) was identified as independent risk factor (adjusted OR, 1.70; 95% CI, 1.04–2.77; P=0.034).
Table 5
| Risk factors | OR | 95% CI | P value |
|---|---|---|---|
| Advanced age (>70 years) | 1.83 | 1.16–2.88 | 0.009 |
| Arterial hypertension | – | – | 0.662 |
| Any atherosclerotic entity | – | – | 0.397 |
| Chronic kidney disease | – | – | 0.061 |
| History of cerebrovascular accident | – | – | 0.230 |
| Preoperative cerebral malperfusion | – | – | 0.126 |
| Preoperative critical state | – | – | 0.101 |
| ATAD | 1.69 | 1.00–2.84 | 0.0495 |
| Aortic rupture | – | – | 0.096 |
| Aortic surgeon | – | – | 0.421 |
| Weekend surgery | – | – | 0.300 |
| Night-time surgery | – | – | 0.200 |
| Valve-sparing root replacement | – | – | 0.071 |
| Aortic arch surgery | 3.24 | 1.78–5.88 | <0.001 |
| FET | – | – | 0.315 |
| Concomitant CABG | 2.19 | 1.27–3.79 | 0.005 |
| High ECC time (>230 min) | 1.70 | 1.04–2.77 | 0.034 |
| High aortic clamp time (>155 min) | – | – | 0.479 |
| HCA with SACP | – | – | 0.843 |
| High circulatory arrest time (>60 min) | – | – | 0.752 |
| High CP time (>90 min) | – | – | 0.235 |
| Low body temperature (<25 ℃) | – | – | 0.286 |
| Site of arterial cannulation | |||
| Ascending aorta or aortic arch | – | – | 0.512 |
| Innominate artery | – | – | 0.114 |
| Subclavian artery | – | – | 0.121 |
| Femoral artery | – | – | 0.123 |
P value of acute type A dissection is reported with 4 decimals to show significance. ATAD, acute type A dissection; FET, frozen elephant trunk; CABG, coronary artery bypass grafting; ECC, extracorporeal circulation; HCA, hypothermic circulatory arrest; SACP, selective antegrade cerebral perfusion; CP, cerebral perfusion; OR, odds ratio; CI, confidence interval.
Concomitant CABG
As concomitant CABG was identified as independent risk factor, we further analyzed CABG specific procedural data. Of all patients that underwent concomitant CABG, 99 (55.0%) underwent complete arterial bypass grafting. In 81 patients (45.0%) a central anastomosis to the aorta was performed. Within the group of patients undergoing concomitant CABG, the rate of patients receiving central anastomoses to the aorta was significantly higher in the stroke group (no stroke 41.8% vs. stroke 68.2%, P=0.020).
Risk factor analysis of secondary endpoints and subgroups
Further risk factor analyses were performed to identify specific risk factors for the occurrence of embolic or hemodynamic stroke, respectively (Tables S1-S10).
Of all postoperative strokes, 59 (62.8%) were of embolic and 23 (24.5%) of hemodynamic origin. Two strokes were caused by hemorrhage. One stroke was considered lacunar. In nine patients, strokes could not be clearly classified.
Risk factor analysis for the occurrence of an embolic stroke identified aortic arch surgery (adjusted OR, 3.95; 95% CI, 2.08–7.51; P<0.001), preoperative cerebral malperfusion (adjusted OR, 2.52; 95% CI, 1.10–5.77; P=0.028), and concomitant CABG (adjusted OR, 2.09; 95% CI, 1.11–3.96; P=0.023) as independent risk factors.
For the occurrence of a hemodynamic stroke, risk factor analysis identified advanced age (adjusted OR, 2.46; 95% CI, 1.02–5.92; P=0.046), any atherosclerotic entity (adjusted OR, 2.66; 95% CI, 1.10–6.42; P=0.030), ATAD (adjusted OR, 3.92; 95% CI, 1.59–9.66; P=0.003) and aortic arch surgery (adjusted OR, 14.26; 95% CI, 1.82–111.74; P=0.011) as independent risk factors.
Further subgroup analyses are shown in Tables S11-S30. Risk factors identified in the subgroup of patients presenting with aneurysm were any atherosclerotic entity, chronic kidney disease, preoperative critical state. In patients with ATAD no risk factors could be identified. Risk factors in patients undergoing root surgery were advanced age and ATAD. In patients undergoing total arch replacement, concomitant CABG was a risk factor. Furthermore, in this subgroup, the FET procedure using aortic arch zone 2 as landing zone was revealed to be a protective factor against stroke.
Long-term survival
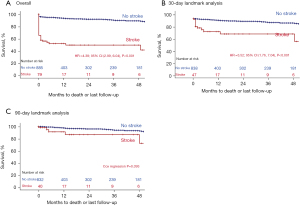
Figure 2 shows the estimated Kaplan-Meier curves of patients with and without postoperative stroke for the overall cohort including early mortality, a 30-day, and a 90-day landmark analysis. Adjusted Cox regression analysis revealed a significantly increased long-term mortality in stroke patients when evaluating the overall cohort (Cox regression: HR, 4.38; 95% CI, 2.99–6.04; P<0.001). The 30-day landmark analysis still showed a decreased survival in stroke patients (Cox regression: HR, 3.52; 95% CI, 1.76–7.04; P<0.001). However, after excluding patients who died within 90 days after surgery, Cox regression did not show a statistically significant difference anymore (P=0.393).
In a non-adjusted analysis, patients with a more severe stroke (global or both hemispheres) had a higher 30-day (8.0% vs. 43.5%, P=0.001) and 1-year mortality (31.3% vs. 76.6%, P=0.001) than patients with a focal and one-sided stroke.
In patients with postoperative stroke, we evaluated the mRS score before discharge and 1 year after the operation in our aortic outpatient clinic. Unfortunately, mRS score data after 1 year is only available of 16 out of a total of 22 stroke patients surviving the first year after surgery. At discharge, 79.4% (n=50) of stroke patients had a mRS score higher than 3. One year after surgery, this rate decreased to 68.8% (n=11) of patients with a score higher than 3.
Discussion
The rate of postoperative ND after thoracic aortic surgery in our cohort was 7.0%. Adjusted logistic regression analysis revealed preoperative factors, such as advanced age and ATAD, as well as surgical parameters, such as arch replacement, concomitant CABG, and high cardiopulmonary bypass time as independent risk factors for all types of strokes after aortic surgery.
Survival
This study showed an association of a postoperative stroke after thoracic aortic surgery with an impaired early and long-term survival, as demonstrated by Kaplan-Meier curves and Cox regression analyses (Figure 2). Stroke was also associated with further complications such as pneumonia and prolonged intensive care unit stay. A landmark analysis after 90 days, however, did not show a significant difference in the survival between the groups. This demonstrates that perioperative strokes have a significant impact on early survival within the first 3 months. However, if the patient survived these first 3 months after surgery, perioperative neurological dysfunction is not significantly associated with impaired mid- and long-term survival. An impaired survival after stroke in patients undergoing aortic surgery has also been shown by other groups. Okada et al. reported that patients with stroke after aortic surgery had a significantly higher early mortality than patients without (15). In contrast to the present study, Okada et al. observed an impaired late survival after 5 years in the stroke group. Here, Okada et al. did not perform a landmark analysis and therefore included early perioperative deaths in their analysis, which may have triggered the impaired overall and long-term survival in the stroke group. Summarizing, we conclude, that postoperative stroke is indeed relevant for an impaired long-term outcome. The impaired long-term outcome is mainly caused by events within the first 90 days after surgery. We observed a low rate of recovery in the long-term follow-up. We cautiously conclude that only few patients recover from a severe postoperative stroke after aortic surgery if symptoms remained until hospital discharge.
High-risk patients
The present study showed that especially older patients over 70 years of age and patients presenting with ATAD have a higher stroke risk after thoracic aortic surgery.
Prior studies already identified ATAD as independent risk factor for stroke (2,16,17). In the literature, stroke rates after surgery for ATAD are reported from 3.4% to 26.5% (18,19). Accordingly, the subgroup of ATAD patients in our cohort showed a stroke rate of 15.8%. Especially patients with dissection affecting supra-aortic vessels are at high risk since CP might be impaired perioperatively and lead to insufficient oxygen supply to the brain (20,21). Stroke prevention strategies in this patient cohort are of utmost importance. This may include pressure controlled SACP during HCA, near-infrared spectroscopy (NIRS) monitoring to detect early perfusion impairment, or specific cannulation techniques to improve CP (22,23).
Age is a well-known risk factor for the occurrence of stroke in the general population (24). An analysis of Hagl et al. in 2001 already revealed age as a risk factor for stroke in patients undergoing aortic surgery in HCA with or without SACP (7). Analyses using data of the German Registry for Acute Aortic Dissection type A (GERAADA) did not find age to be an independent risk factor for stroke in patients with ATAD (25). In the present study, a more heterogeneous patient group undergoing both elective or emergency surgery for aortic aneurysm or dissection was analyzed. Here, advanced age was identified as independent risk factor. We conclude that advanced age may especially play a role as stroke risk factor in patients with other pathologies than ATAD undergoing aortic surgery. This cautious assumption needs to be evaluated in further studies.
Arch surgery as a high-risk procedure
The analysis of the overall cohort as well as the subgroup analyses identified aortic arch surgery, including hemi- and total arch replacement, as independent risk factor for strokes of hemodynamic as well as embolic origin. In each risk factor analysis, arch surgery showed the highest OR of all identified independent risk factors. This underlines the importance of neuroprotection and adequate perfusion strategy when performing arch surgery in HCA.
Due to the manipulation of calcified head vessels and the complexity of arch procedures using HCA in combination with CP strategies as well as increased ECC times due to prolonged cooling and rewarming times, aortic arch surgery is well known to impose a relevant stroke risk (26). Therefore, preoperatively, brain-supplying vessels should be evaluated thoroughly using doppler sonography. In patients with history of TIA or stroke and in patients with relevant stenoses or dissections further imaging is necessary. CT angiography with imaging of extra- and intracranial brain-supplying arteries should be performed to evaluate the degree of known stenoses and to rule out an incomplete circle of Willis.
Intraoperatively, neuroprotective measures include monitoring of cerebral oxygen saturation via NIRS, the individual adjustment of the arterial blood pressure as well as an adapted CP flow based on the patient’s individual range of cerebral autoregulation and monitored brain oxygen saturation.
It is an ongoing debate which CP strategy provides the best neuroprotection. A current meta-analysis by Hameed et al. including 26,968 patients of 68 studies, showed antegrade or retrograde CP each leading to a better neurological outcome than deep HCA alone. Despite the wide consensus about the use of SACP, Hameed and colleagues did not observe a difference in outcome between antegrade and retrograde CP (27). Accordingly, a meta-analysis by Takagi et al. comparing antegrade and retrograde CP did not find a difference in postoperative ND either (28).
Nevertheless, especially in procedures with presumed circulatory arrest times beyond 30 to 40 minutes, bilateral CP should be used to reduce stroke risk (29). At our center and in most European centers, SACP has been used as the preferred method for cerebral protection in the last decade (30). Surgical techniques continuously need to be facilitated and improved to lower the risk of early mortality and perioperative stroke in arch surgery. Accordingly, the simplified FET technique with the distal anastomosis in landing zone 2 and an extra-anatomic bypass to the distal left subclavian artery has been shown to reduce stroke risk as previously reported by our group (31). In addition, the use of endovascular techniques to accelerate the supra-aortic vessel anastomoses and to reduce circulatory arrest times by simplifying the surgical procedure seems to be a valid option (32).
Secondary endpoints
Additional analyses of the present study revealed different risk factors for the secondary endpoints hemodynamic and embolic strokes.
Hemodynamic stroke origin
Advanced age, any atherosclerotic entity, ATAD, and aortic arch surgery were identified as independent risk factors for the occurrence of a hemodynamic stroke. The spectrum of hemodynamic stroke ranges from borderline infarcts due to impaired cerebral microcirculation to massive hypoxic encephalopathy caused by hypoperfusion.
In older patients and patients with atherosclerosis microcirculation in the brain is diminished. In elderly, blood pressure and flow might not be adapted by autoregulation as quickly to changes that occur perioperatively. Already minor decreases in cerebral blood flow or pressure changes may negatively affect oxygen supply. This leads to insufficient oxygen saturation especially in distal brain areas and watershed strokes may occur. In patients with atherosclerosis this effect might be further enhanced due to relevant stenoses of brain-supplying arteries. In cardiac surgery, intraoperative drops in blood pressure were already found to be a risk factor for watershed stroke (33).
Embolic stroke origin
Apart from arch surgery, subgroup analysis identified concomitant CABG and preoperative cerebral malperfusion as independent risk factors for embolic stroke.
The role of atherosclerosis in the occurrence of embolic strokes during aortic surgery appears complex. In our study, CABG was identified as independent risk factor for the development of an embolic stroke. In these patients, atherosclerosis may not be limited to the coronary arteries but might also affect the aorta as well as the supraaortic and intracranial brain-supplying vessels. Here, especially thrombi formed on ruptured atherosclerotic plaques are a well-known disease mechanism (34). Whether atherosclerotic plaques on their own might be mobilized during aortic surgery and thereby cause embolic strokes is not well investigated so far. Nevertheless, the presence of atherosclerotic plaques in the ascending aorta and the aortic arch is known to be associated with stroke of unknown cause (35).
However, atherosclerosis itself was not found to be an independent risk factor for occurrence of embolic strokes in our analysis. This leads to the assumption, that atherosclerosis during aortic surgery does not mainly increase the stroke risk by causing emboli, but by impairing cerebral microcirculation. In the general population, stroke often is caused by a combination of both atherosclerotic stenotic vessels and embolism of diverse origins (36).
Moreover, preoperative cerebral malperfusion was identified as risk factor for embolic, but not for hemodynamic stroke. Cerebral malperfusion may induce thrombus formation in the false and true lumen preoperatively that not yet cause stroke. Due to hemodynamic changes and/or SACP, these thrombi may be dislocated during surgery resulting in postoperative embolic stroke. However, this field is scarcely investigated, and more studies are needed to further examine underlying mechanisms.
Limitations
The main limitation of our study is its single-center and retrospective nature. Multicenter analyses are needed. Nevertheless, our study included a high number of patients over a significant time period of 10 years. Our cohort is very heterogenic including a broad range of patients presenting with different pathologies and undergoing the whole variety of surgical procedures of the thoracic aorta. However, this could also be seen as a strength as the presented cohort and study results may well represent clinical practice and may substantially improve preoperative risk evaluation of aortic patients. Moreover, our data represent the outcome of a high-volume aortic center. It is not certain if these results apply to lower volume centers as well.
When defining stroke for our analyses, we did not distinguish between different grades of severity and excluded strokes resolving within 7 days. Also, we excluded patients who died intraoperatively, since it is not possible to evaluate whether these patients suffered from a stroke or not. This might influence our results in either one or the other direction and leading to an over- or underestimation of the stroke risk.
Another limitation regarding the statistical analysis is that ECC time was the only procedural time which correlated with stroke. This might be caused by statistical multicollinearity, as the length of the ECC time correlates with the length of other surgical times (e.g., aortic clamp time or CP time).
Conclusions
Stroke remains a major complication after thoracic aortic surgery. To lower the risk of postoperative ND, attention to pre- and intraoperative factors should be paid. Especially in elderly patients and in patients with ATAD, the least complex surgical technique possible should be used to improve neurological outcome. In these patients, ECC time should be kept short and the indication for arch replacement should only be given cautiously. As concomitant CABG was found to be a risk factor for stroke, percutaneous coronary intervention (PCI) might be a more favorable option in elderly patients who are suffering from coronary artery disease and undergo thoracic aortic surgery. In the case of prior PCI, the risk of bleeding might be increased due to postinterventional dual antiplatelet therapy (DAPT). Therefore, the individual stroke and bleeding risk needs to be evaluated in these patients to decide between concomitant CABG and PCI. Postponing aortic surgery in elective cases with low risk for spontaneous complications (dissection or rupture) until DAPT after PCI may be discontinued might be an option in selected cases. Especially in arch surgery, a particular focus should be placed upon the whole spectrum of neuroprotective measures and simplified surgical techniques.
Acknowledgments
Funding: This work was supported by the clinician scientist program of the German Center for Cardiovascular Research (DZHK; FKZ 8IX3710109 to Till J. Demal).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1591/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1591/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1591/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1591/coif). CD is a proctor for Terumo Aortic. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data acquisition was performed anonymized and retrospectively. Therefore, in accordance with German law, no ethical approval is needed and informed patient consent was waived (§12 HmbKHG, hospital law of Hamburg, and §15 Medical Association’s professional code of conduct).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ghoreishi M, Sundt TM, Cameron DE, et al. Factors associated with acute stroke after type A aortic dissection repair: An analysis of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. J Thorac Cardiovasc Surg 2020;159:2143-54.e3. [Crossref] [PubMed]
- Chung J, Stevens LM, Ouzounian M, et al. Sex-Related Differences in Patients Undergoing Thoracic Aortic Surgery. Circulation 2019;139:1177-84. [Crossref] [PubMed]
- Goldstein LJ, Davies RR, Rizzo JA, et al. Stroke in surgery of the thoracic aorta: incidence, impact, etiology, and prevention. J Thorac Cardiovasc Surg 2001;122:935-45. [Crossref] [PubMed]
- Conzelmann LO, Hoffmann I, Blettner M, et al. Analysis of risk factors for neurological dysfunction in patients with acute aortic dissection type A: data from the German Registry for Acute Aortic Dissection type A (GERAADA). Eur J Cardiothorac Surg 2012;42:557-65. [Crossref] [PubMed]
- Dumfarth J, Kofler M, Stastny L, et al. Stroke after emergent surgery for acute type A aortic dissection: predictors, outcome and neurological recovery. Eur J Cardiothorac Surg 2018;53:1013-20. [Crossref] [PubMed]
- Pacini D, Leone A, Di Marco L, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg 2007;31:618-22. [Crossref] [PubMed]
- Hagl C, Ergin MA, Galla JD, et al. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg 2001;121:1107-21. [Crossref] [PubMed]
- Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg 2008;135:908-14. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg 2012;42:S45-60. [Crossref] [PubMed]
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Zhou T, Li J, Sun Y, et al. Surgical and early outcomes for Type A aortic dissection with preoperative renal dysfunction stratified by estimated glomerular filtration rate. Eur J Cardiothorac Surg 2018;54:940-5. [Crossref] [PubMed]
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Higgins J, Lee MK, Co C, et al. Long-term outcomes after thoracic aortic surgery: a population-based study. J Thorac Cardiovasc Surg 2014;148:47-52. [Crossref] [PubMed]
- Okada N, Oshima H, Narita Y, et al. Impact of Surgical Stroke on the Early and Late Outcomes After Thoracic Aortic Operations. Ann Thorac Surg 2015;99:2017-23. [Crossref] [PubMed]
- Preventza O, Coselli JS, Akvan S, et al. The impact of temperature in aortic arch surgery patients receiving antegrade cerebral perfusion for >30 minutes: How relevant is it really? J Thorac Cardiovasc Surg 2017;153:767-76. [Crossref] [PubMed]
- Di Eusanio M, Wesselink RM, Morshuis WJ, et al. Deep hypothermic circulatory arrest and antegrade selective cerebral perfusion during ascending aorta-hemiarch replacement: a retrospective comparative study. J Thorac Cardiovasc Surg 2003;125:849-54. [Crossref] [PubMed]
- Trivedi D, Navid F, Balzer JR, et al. Aggressive Aortic Arch and Carotid Replacement Strategy for Type A Aortic Dissection Improves Neurologic Outcomes. Ann Thorac Surg 2016;101:896-903; Discussion 903-5. [Crossref] [PubMed]
- Rahimi-Barfeh A, Grothusen C, Haneya A, et al. Transatrial Cannulation of the Left Ventricle for Acute Type A Aortic Dissection: A 5-Year Experience. Ann Thorac Surg 2016;101:1753-8. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol 2015;65:2628-35. [Crossref] [PubMed]
- Rylski B, Hoffmann I, Beyersdorf F, et al. Acute aortic dissection type A: age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann Surg 2014;259:598-604. [Crossref] [PubMed]
- Hage A, Stevens LM, Ouzounian M, et al. Impact of brain protection strategies on mortality and stroke in patients undergoing aortic arch repair with hypothermic circulatory arrest: evidence from the Canadian Thoracic Aortic Collaborative. Eur J Cardiothorac Surg 2020;58:95-103. [Crossref] [PubMed]
- Qu JZ, Kao LW, Smith JE, et al. Brain Protection in Aortic Arch Surgery: An Evolving Field. J Cardiothorac Vasc Anesth 2021;35:1176-88. [Crossref] [PubMed]
- Boehme AK, Esenwa C, Elkind MS. Stroke Risk Factors, Genetics, and Prevention. Circ Res 2017;120:472-95. [Crossref] [PubMed]
- Boening A, Karck M, Conzelmann LO, et al. German Registry for Acute Aortic Dissection Type A: Structure, Results, and Future Perspectives. Thorac Cardiovasc Surg 2017;65:77-84. [PubMed]
- Itagaki S, Chikwe J, Sun E, et al. Impact of Cerebral Perfusion on Outcomes of Aortic Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann Thorac Surg 2020;109:428-35. [Crossref] [PubMed]
- Hameed I, Rahouma M, Khan FM, et al. Cerebral protection strategies in aortic arch surgery: A network meta-analysis. J Thorac Cardiovasc Surg 2019; Epub ahead of print. [Crossref] [PubMed]
- Takagi H, Mitta S, Ando T. A Contemporary Meta-Analysis of Antegrade versus Retrograde Cerebral Perfusion for Thoracic Aortic Surgery. Thorac Cardiovasc Surg 2019;67:351-62. [Crossref] [PubMed]
- Angeloni E, Melina G, Refice SK, et al. Unilateral Versus Bilateral Antegrade Cerebral Protection During Aortic Surgery: An Updated Meta-Analysis. Ann Thorac Surg 2015;99:2024-31. [Crossref] [PubMed]
- De Paulis R, Czerny M, Weltert L, et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch in Europe. Eur J Cardiothorac Surg 2015;47:917-23. [Crossref] [PubMed]
- Detter C, Demal TJ, Bax L, et al. Simplified frozen elephant trunk technique for combined open and endovascular treatment of extensive aortic diseases. Eur J Cardiothorac Surg 2019;56:738-45. [Crossref] [PubMed]
- Detter C, Brickwedel J. The goal of simplifying complex aortic arch surgery. Eur J Cardiothorac Surg 2021;59:1254-5. [Crossref] [PubMed]
- Gottesman RF, Sherman PM, Grega MA, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke 2006;37:2306-11. [Crossref] [PubMed]
- Viedma-Guiard E, Guidoux C, Amarenco P, et al. Aortic Sources of Embolism. Front Neurol 2020;11:606663. [Crossref] [PubMed]
- Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med 1994;331:1474-9. [Crossref] [PubMed]
- Knight-Greenfield A, Nario JJQ, Gupta A. Causes of Acute Stroke: A Patterned Approach. Radiol Clin North Am 2019;57:1093-108. [Crossref] [PubMed]


