
Practical approach lung health-global alliance against chronic respiratory diseases (PAL-GARD) initiative in Brazil
Introduction
Respiratory diseases account for about one-third of the workload in primary health care (PHC) facilities worldwide, and 20% of the 59 million annual deaths from all causes (1,2). Incorrect or delayed diagnosis and inappropriate prescribing for common acute and chronic respiratory diseases, including tuberculosis, are recognized as important modifiable factors that contribute to the high morbidity and mortality from these diseases (3). World Health Organization (WHO) has addressed this situation by implementing programs and strategies such as the practical approach to lung health (PAL) developed by the STOP TB Partnership, and the Package of Essential Non-communicable Diseases Interventions (PEN). The PAL strategy focuses on respiratory diseases-TB, pneumonia, acute respiratory infections (ARI), asthma and chronic obstructive pulmonary disease (COPD) (4,5) and has been recognized by the global alliance against chronic respiratory diseases (GARD) as an important tool for the improvement of the care of patients with these chronic diseases (6).
The WHO Deployment Manual (7), PAL recommended each country to adapt the guidelines to the local particularities. Based on that, a specific guide was developed for Brazil (available online: https://cdn.amegroups.cn/static/public/jtd-21-1345-1.pdf).
The current study aimed to evaluate the impact of training primary care physicians (PCPs) in the use of a clinical decision tool based on the PAL approach, namely the practical approach lung health-global alliance against chronic respiratory diseases (PAL-GARD) guide, developed for use in Brazil, on their diagnostic performance, by assessing changes in clinical diagnosis, before and after training, and comparing their diagnoses with that of a panel of senior pulmonologists. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1345/rc).
Methods
This real-life three-phase, controlled, parallel group study was carried out in public outpatient clinics in the metropolitan area of Belo Horizonte, in the Minas Gerais State, the fourth largest and the second most populous state, located in the Southeast Region of Brazil. The study comprised a baseline, an intervention and a post-intervention phase. During the baseline phase, all PCPs who consented to take part, were instructed on how to complete a standardized form regarding their patients’ respiratory symptoms and to record their primary clinical diagnosis for each patient’s first visit. More than one diagnosis could be recorded. PCPs who had recorded diagnoses during the baseline phase participated in the intervention phase. In this phase, PCPs were consecutively allocated to one of two groups, an intervention and a control group. The former received the PAL-GARD guide and were trained to use it during their subsequent medical consultations with patients with respiratory symptoms. PCPs in the control group received no training and provided their usual care to patients. As in the baseline phase, the PCPs of both groups attended patients and recorded their diagnoses. In the third phase, three senior pulmonologists, each with more than 15 years of clinical experience, reviewed independently the PCPs forms and recorded their own diagnosis on a separate form. They were blinded to the diagnosis recorded by the PCPs.
Setting
The study was carried out in PHC facilities of municipalities within the metropolitan region of Belo Horizonte, Brazil between the years 2011 and 2012. These are government-funded public health units that serve the local population on weekdays in an open-door routine.
Standardized questionnaire and baseline diagnostic performance of participants (available online: https://cdn.amegroups.cn/static/public/jtd-21-1345-2.pdf)
The questionnaire included clinical features suggestive of acute and chronic respiratory diseases such as common cold, influenza-like illness, acute otitis media, rhinosinusitis, tonsillitis, and pneumonia (grouped together as ARI) and suspected pleural or pulmonary tuberculosis, asthma and COPD (grouped as chronic respiratory diseases). After recording the symptoms PCPs had to write down their diagnostic hypothesis based only on symptoms and physical findings at the patients’ first visit. Descriptors of these different diagnosis were based on updated international guideline definitions.
Intervention
The PAL-GARD guide (available online: https://cdn.amegroups.cn/static/public/jtd-21-1345-1.pdf)
The guide was adapted from PAL-GARD handbooks and developed by the authors based on the WHO Deployment Manual (7) and modelled on the Practical Approach to Lung Health and HIV/AIDS in South Africa (PALSA PLUS) document (8). Briefly, the PAL-GARD handbook provides a template for the development of a symptom-based clinical decision tool that has been adapted to several epidemiological and health care scenarios aiming to support PCPs in the diagnoses and management of previously-mentioned respiratory disorders.
Training
Training included a theoretical course and a practical component. The course comprised four 4-hour sessions conducted on different days over one week period. The second component took place in the outpatient clinic of the Department of Pulmonology and Thoracic Surgery at Hospital das Clínicas, Federal University of de Minas Gerais in Belo Horizonte, Brazil and at secondary level health care centers of the participating municipalities. This part comprised three 3-hour sessions over a period of three days.
Pulmonologist panel
A panel consisting of three senior pulmonologists reviewed independently the PCPs forms and recorded their suggested diagnosis on a separate form. Subsequently, the pulmonologists had a session when they discussed and reached consensus on the diagnosis of each patient.
Patients
In both the baseline and intervention phase of the study, consecutive patients aged 15 years or older who presented with at least one of three respiratory symptoms-cough, dyspnea and/or wheezing, and regardless of the duration of their symptoms and previous diagnosis of respiratory diseases were invited to participate.
PCPs
All PCPs employed at the PHC of the three municipalities were invited to take part in the study. Those who volunteered, provided written consent and received training on how to complete the study questionnaire. They were allocated consecutively and alternatively to either the PAL-GARD or control group. Only those that completed at least five questionnaires during both the baseline and intervention phase were included in the analysis.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Research Ethics Committee of the Federal University of Minas Gerais (CAAE 0069.0.203.00.10). Informed consent was taken from all individual participants.
Statistical analysis
The primary assessment was the level of agreement between PCPs and senior pulmonologists for each diagnosis during the intervention phase measured using the kappa inter-rater method. Secondly, we compared changes in the proportion of correct diagnoses between PCPs groups before and after the training.
For the primary endpoint, we estimate the minimum number of patients to be included in the intervention phase as 529, considering an alpha and beta error of 0.05 and 0.20, respectively, a null hypothesis of non-agreement between the groups, an alternative hypothesis of kappa >0 and a difference ≥0.20 (meaning a change of at least one kappa level after the training in the PAL-GARD group). The agreement was classified as low, reasonable, moderate, good and very good, for if <0.20, between 0.21 and 0.40, between 0.41 and 0.60, between 0.61 and 0.80, and between 0.81 and 1.00, respectively (9).
Data are presented as proportions and mean (± SD). The study variables were compared between PAL-GARD and control groups using chi-squared test for categorical variables and the Mann-Whitney for the continuous ones.
The Statistical Package for the Social Sciences, version 17 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Out of a total of 195 PCPs that were invited, 132 (67.7%) agreed to participate, but only 30 (22.7%) fulfilled the predefined criterion of assessing at least 5 patients in both the baseline and intervention phases. This provided 17 PCPs in the PAL-GARD and 13 in the control group.
The PCPs thus selected enrolled 536 patients, 358 (66.8%) by the PAL-GARD PCPs and 178 (33.2%) by the PCPs control group (Figure 1).
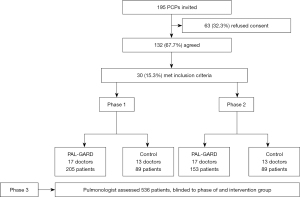
The characteristics of PCPs in the PAL-GARD and control groups are shown in Table 1.
Table 1
| Variable | Study group | P value | |
|---|---|---|---|
| PAL-GARD | Control | ||
| Total, n | 17 | 13 | |
| Sex, n (%) | 0.127* | ||
| Female | 10 (58.8) | 4 (30.8) | |
| Male | 7 (41.2) | 9 (69.2) | |
| Age range (years), n (%) | 0.127* | ||
| 25–30 | 11 (64.8) | 4 (30.8) | |
| 31–45 | 3 (17.6) | 7 (53.8) | |
| >45 | 3 (17.6) | 2 (15.4) | |
| Years working in primary health care, mean ± SD | 5.4±3.3 | 3.3±2.5 | 0.123*** |
| Residency/specialisation in family medicine, n (%) | 0.672** | ||
| Yes | 4 (23.5) | 2 (15.4) | |
| No | 13 (76.5) | 11 (84.6) | |
| 0.491*Residency/specialisation in any other medical field, n (%) | 0.672** | ||
| Yes | 10 (58.8) | 6 (46.2) | |
| No | 7 (41.2) | 7 (53.8) | |
The probability of significance (P) refers to the Chi-square test (*), Fisher’s exact test (**), and the Mann Whitney test (***). PCP, primary care physician; PAL-GARD, practical approach lung health-global alliance against chronic respiratory diseases; SD, standard deviation.
The descriptive characteristics of the two studied groups were similar. PAL-GARD group recorded a diagnosis of asthma more frequently than the control group in both baseline and intervention phases (P=0.025 and <0.001, respectively). Whereas PAL-GARD PCPs increased their frequency of diagnosing COPD from phase 1 to phase 2 (15.1% to 25.5%; P=0.014), the control PCPs did not (18.0% to 12.4%; P=0.296). The rates of diagnosis of asthma, tuberculosis and ARIs at baseline and change between baseline and intervention phases were not statistically different in either group (see Table 2).
Table 2
| Diagnosis | Study group | P value | |
|---|---|---|---|
| PAL-GARD | Control | ||
| Asthma | |||
| Phase 1, n/N (%) | 60/205 (29.3%) | 15/89 (16.9%) | 0.025 |
| Phase 2, n/N (%) | 59/153 (38.6%) | 12/89 (13.5%) | <0.001 |
| P value | 0.065 | 0.531 | |
| COPD | |||
| Phase 1, n/N (%) | 31/205 (15.1%) | 16/89 (18.0%) | 0.539 |
| Phase 2, n/N (%) | 39/153 (25.5%) | 11/89 (12.4%) | 0.015 |
| P value | 0.014 | 0.296 | |
| Tuberculosis | |||
| Phase 1, n/N (%) | 20/205 (9.8%) | 13/89 (14.6%) | 0.226 |
| Phase 2, n/N (%) | 21/153 (13.7%) | 10/89 (11.2%) | 0.576 |
| P value | 0.243 | 0.503 | |
| ARI | |||
| Phase 1, n/N (%) | 90/205 (43.9%) | 39/89 (43.8%) | 0.990 |
| Phase 2, n/N (%) | 60/153 (39.2%) | 39/89 (43.8%) | 0.482 |
| P value | 0.374 | 1.000 | |
PCP, primary care physician; PAL-GARD, practical approach lung health-global alliance against chronic respiratory diseases; COPD, chronic obstructive pulmonary disease; ARI, acute respiratory infection.
For asthma diagnoses, there was an improvement in concordance from moderate to good (0.546; 95% CI: 0.423–0.670); (0.638; 95% CI: 0.515–0.761) in the PAL-GARD group, from phase 1 to phase 2 (Table 3). For tuberculosis, there was also an improvement in agreement, from reasonable to good (0.393; 95% CI: 0.170–0.617); (0.655; 95% CI: 0.461–0.849). Conversely, there was a decrease in the rate of concordance regarding COPD (from moderate in phase 1 to a reasonable in phase 2) (0.430; 95% CI: 0.250–0.611); (0.284; 95% CI: 0.111–0.457). For ARI’s, there was a numerical increase in the degree of concordance from phase 1 to phase 2, but within the same kappa category of agreement (0.577; 95% CI: 0.465–0.689); (0.584; 95% CI: 0.452–0.716).
Table 3
| Conditions | Baseline | Intervention | |||||
|---|---|---|---|---|---|---|---|
| PCPs PAL-GARD | Pulmonologists | Kappa (95% CI) | PCPs PAL-GARD | Pulmonologists | Kappa (95% CI) | ||
| Asthma, n (%) | 0.546 (0.423–0.670) | 0.638 (0.515–0.761) | |||||
| Yes | 60 (29.3) | 68 (33.2) | 59 (38.6) | 68 (44.4) | |||
| No | 145 (70.7) | 137 (66.8) | 94 (61.4) | 85 (55.6) | |||
| COPD, n (%) | 0.430 (0.250–0.611) | 0.284 (0.111–0.457) | |||||
| Yes | 31 (15.1) | 21 (10.2) | 39 (25.5) | 23 (15.0) | |||
| No | 174 (84.9) | 184 (89.8) | 114 (74.5) | 130 (85.0) | |||
| Tuberculosis, n (%) | 0.393 (0.170–0.617) | 0.655 (0.461–0.849) | |||||
| Yes | 20 (9.8) | 12 (5.9) | 21 (13.7) | 11 (7.2) | |||
| No | 185 (90.2) | 193 (94.1) | 132 (86.3) | 142 (92.8) | |||
| ARI, n (%) | 0.577 (0.465–0.689) | 0.584 (0.452–0.716) | |||||
| Yes | 90 (43.9) | 95 (46.3) | 60 (39.2) | 45 (29.4) | |||
| No | 115 (56.1) | 110 (53.7) | 93 (60.8) | 108 (70.6) | |||
PCP, primary care physician; PAL-GARD, practical approach lung health-global alliance against chronic respiratory diseases; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ARI, acute respiratory infection.
The agreement between the PAL-GARD and control groups is shown in Table 4.
Table 4
| Conditions | Phase | Study group, Kappa (95% CI) | |
|---|---|---|---|
| PAL-GARD | Control | ||
| Asthma | 1 | 0.546 (0.423–0.670) | 0.516 (0.298–0.734) |
| 2 | 0.638 (0.515–0.761) | 0.372 (0.158–0.586) | |
| COPD | 1 | 0.430 (0.250–0.611) | 0.262 (0.010–0.518) |
| 2 | 0.284 (0.111–0.457) | 0.291 (0.001–0.595) | |
| Tuberculosis | 1 | 0.393 (0.170–0.617) | 0.253 (0.001–0.525) |
| 2 | 0.655 (0.461–0.849) | 0.549 (0.272–0.827) | |
| ARI | 1 | 0.577 (0.465–0.689) | 0.594 (0.427–0.761) |
| 2 | 0.584 (0.452–0.716) | 0.392 (0.202–0.582) | |
PCP, primary care physician; PAL-GARD, practical approach lung health-global alliance against chronic respiratory diseases; COPD, chronic obstructive pulmonary disease; ARI, acute respiratory infection.
Comparison of changes in concordance with between the PAL-GARD and control groups (Table 4).
PAL-GARD and control groups had similar kappa indices (reasonable, moderate or good) in the first phase of the study for asthma, tuberculosis and ARI. For COPD the agreement was moderate in the PAL-GARD group in phase 1 and reasonable in the control group.
Considering intra-group comparisons inside each group, the PAL-GARD group had an improvement in the agreement from moderate (phase 1) to good (phase 2). For asthma, in the control group, there was a worsening in the agreement, from moderate (phase 1) to reasonable (phase 2). For ARI, the degree of concordance remained moderate in the PAL-GARD group and fell from moderate to reasonable in the control group. As for tuberculosis, the PAL-GARD group went from reasonable to good agreement and the control group from reasonable to moderate. Regarding COPD, there was a decrement in concordance, from moderate in phase 1 to reasonable in phase 2 in the PAL-GARD group whereas it remained reasonable in the control group (see Table 4).
Discussion
The present study evaluated the effect of training PCPs in a PAL-GARD-based guide about symptoms and diagnosis of prevalent respiratory disorders in the PHC. This study has shown that using such kind of training for PCPs may improve their abilities to recognize these conditions at the first-visit of symptomatic patients in PHC facilities as compared to the hypothesis raised by a panel of senior pulmonologists. An improvement in the agreement between them and such panel was observed for asthma (0.546 to 0.638), tuberculosis (0.393 to 0.655) and ARIs (0.577 to 0.584) in the PAL-GARD group whereas a reduction was found in cases of COPD (0.430 to 0.284). A study conducted under the similar conditions has shown that PCPs without previous specific training in these protocols have a reasonable to moderate agreement with specialists (10).
Our results may give rise to some questions. Firstly, could those improvements be considered statistically significant? It has been shown that the kappa agreement method is more accurate than the simple ratio of concordance. However, it should be mentioned that many superimposable 95% CI: of some findings of the present study may limit their statistical significance. It is reasonable to assume that these results point out to some improvement in the degree of agreement after the training of PCPs in the PAL-GARD protocols which seems to be of some clinical relevance in this context.
An intriguing result was the observation of a reduction in the agreement between PCPs and pulmonologists in COPD-suspected cases (from 0.43 to 0.28). This finding occurred in parallel with a significant increase in the number COPD diagnoses as compared with the phase 1 in the PAL-GARD group. This could be explained by the known rate of under-diagnosis of COPD in primary care level (3) and therefore, at a first glance, that could be encouraging. However, this didn’t happen in the case of the panelists, that may suggest that the diagnostic algorithms in PAL-GARD that rely on syndromic recognition of COPD are faulty and need to be revised. Alternatively, the duration and/or quality of the provided training on this topic may have been insufficient. Unlike asthma, in which the history of symptoms plays a major role in the diagnosis, symptoms of COPD are more subtle, occur late in the course of the disease and have high false positive rates. Therefore, it would be necessary to add spirometry for definitive diagnosis.
There are some reports from WHO-sponsored studies about the usefulness of adopting PAL strategy in PHCs around the world despite using somewhat different study design and outcomes than those used in the present study (11-22). Regarding the design, in the studies available so far, there was no PCPs allocation criterion (11-20). In relation to outcomes, others have prioritized the reduction of overuse of antibiotics and symptomatic drugs (12-20). Despite this, there has some consensus that the use of PAL strategy may improve the quality of the health care provided to patients with respiratory diseases through the improvement of PCPs’ diagnostic skills (5).
For instance, there was a reduction in the prescription of antibiotics and symptomatic drugs in Algeria, Bolivia, El Salvador, Chile, Jordan, Morocco, Kyrgyzstan, South Africa, Tunisia, and Syria in parallel with an increase in the diagnosis of asthma and COPD (13,14,16,18,22) and an increase in the detection of TB in Tunisia, South Africa and Algeria (14,18,20).
Some limitations of the present study should be mentioned. One of them was the high rate of turnover of PCPs during the study—a usual occurrence in PHC facilities in Brazil—(23) which led to inclusion of new PCPs and partial loss of the of the paired allocation that was initially planned. This fact also led to differences in numbers between the PAL-GARD (17 PCPs) and control (13 PCPs) groups and, consequently, to differences in the total number of patients per group and phases (205/89 in the first and 153/89 in the second phase). However, in our viewpoint these circumstances did not affect the direction of the results. In the case of pairing, the main analysis of the impact of the training referred primarily to the PAL-GARD group itself, so that, even without perfect matching, the groups remained similar (Table 3) and didn’t alter the control group function. In relation to the sample size, the minimum required number of five patients per PCPs and per phase was reached. Another point to be mentioned is the increase in the duration of the study, from 12 to 18 months. Although 132 (67.7%) PCPs agreed to participate, only 30 (15.3%) attended the minimum of five patients in each phase. However, this rate of PCPs participation is similar to that previously reported by other authors in a study performed in France (15%) (24).
On the other hand, this study has several strengths. It was performed in real-life conditions involving healthcare professionals in their own workplace and in the setting of huge demand for services. This is the first study on the viability of the PAL strategy that used allocation methodologies and a control group. In relation to other studies on this topic (11-22), this is the only one that evaluated the diagnostic agreement of PCPs and pulmonologists, before and after training. Another limitation of the study had been related to updating PAL GARD Guide. In fact, it was prepared with guidelines prior to the year 2010. Therefore, the Guide did not contain the last version of GOLD (25), GINA (26), and NICE (27) guidelines for pneumonia. So, it must be updated for its current application.
In conclusion, the use of PAL-GARD tools in PHC may improve diagnostic abilities of PCPs as it was shown in the municipalities included in the current study. Some misclassification observed with asthma and COPD may suggest the need of better approach to these topics and also to the importance of the maintenance of programs of continuous medical education of PCPs attending in PHC. PAL-GARD guide and needs updating. Training in the GARD-PAL guide proved demonstrated to be very efficient for PCP and it should continue with updated further versions.
Acknowledgments
The University Federal of Minas Gerais supported this work.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by Dr. Yousser Mohammad and Dr. Alvaro A. Cruz for the series “GARD Section” published in the Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1345/rc
Data Sharing Statement: https://jtd.amegroups.com/article/view/10.21037/jtd-21-1345/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1345/coif). The series GARD Section was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ottmani S, Scherpbier R, Chaulet P, et al. Respiratory care in primary care services. A survey in 9 countries. WHO: Geneva, Switzerland. Document WHO/HTM/TB/2004; 333.
- The global burden of disease: 2004 update. WHO Library Cataloguing-in-Publication Data. [Cited 2012 Dec. 10]. Available online: http://www.who.int/healthinfo/global_burden_disease/GBD
- José BP, Camargos PA, Cruz Filho ÁA, et al. Diagnostic accuracy of respiratory diseases in primary health units. Rev Assoc Med Bras (1992) 2014;60:599-612. [Crossref] [PubMed]
- STOP TB Partnership. Stop TB Planning Tools for Global Fund Round 10 TB proposal preparation. Geneva: World Health Organization; 2010. [Cited 2012 Dec. 10]. Available online: www.who.int/tb/strategy/en
- Hamzaoui A, Ottmani S. Practical approach to lung health: lung health for everyone? Eur Respir Rev 2012;21:186-95. [Crossref] [PubMed]
- Bousquet J, Khaltaev N. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. World Health Organization; 2007.
- Practical approach to lung health. Manual on initiating PAL implementation. Geneva: World Health Organization. WHO/HTM/TB/2008.410. [Cited 2013 Jan 23]. Available online: http://whqlibdoc.who.int/hq/2008/WHO_HTM_TB_2008.410_eng.pdf
- PALSA PLUS. Practical Approach to Lung Health And Hiv/Aids In South Africa. Ministério da Saúde da África do Sul. [Cited 2016 July 23]. Available online: http://knowledgetranslation.co.za/programmes/palsa-plus/
- Altman DG. Practical statistics for medical research. London: Chapman & Hall, 1991:611.
- São José BP, Camargos PAM, Bateman ED, et al. Primary care physicians’ ability to diagnose the most prevalent respiratory diseases. Int J Tuberc Lung Dis 2016;20:1392-98. [Crossref] [PubMed]
- Samir KC. Lung health in rural Nepal. Multi-state modeling of health status and economic evaluation of integrated respiratory care guidelines. Laxenburg, International Institute for Applied Systems Analysis; 2009.
- Camacho M, Nogales M, Manjon R, et al. Results of PAL feasibility test in primary care facilities in four regions of Bolivia. Int J Tuberc Lung Dis 2007;11:1246-52. [PubMed]
- Me'emary F, Ottmani SE, Pio A, et al. Results of the feasibility test of the Practical Approach to Lung Health in the Syrian Arab Republic. East Mediterr Health J 2009;15:504-15. [Crossref] [PubMed]
- Zidouni N, Baough L, Laid Y, et al. Practical approach to lung health strategy in Algeria. Int J Tuberc Lung Dis 2009;13:1029-37. [PubMed]
- Abu Rumman K, Ottmani S, Abu Sabra N, et al. Training on the Practical Approach to Lung Health: effect on drug prescribing in PHC settings in Jordan. East Mediterr Health J 2009;15:111-21. [Crossref] [PubMed]
- Brimkulov N, Ottmani SE, Pio A, et al. Feasibility test results of the Practical Approach to Lung Health in Bishkek, Kyrgyzstan. Int J Tuberc Lung Dis 2009;13:533-9. [PubMed]
- Seung KJ, Rigodon J, Finch M, et al. Distribution of adult respiratory illnesses at a primary health centre in Lesotho. Int J Tuberc Lung Dis 2012;16:418-22. [Crossref] [PubMed]
- English RG, Bachmann MO, Bateman ED, et al. Diagnostic accuracy of an integrated respiratory guideline in identifying patients with respiratory symptoms requiring screening for pulmonary tuberculosis: a cross-sectional study. BMC Pulm Med 2006;6:22. [Crossref] [PubMed]
- Erhola ML, Brimkulov N, Chubakov T, et al. Development process of the Practical Approach to Lung Health in Kyrgyzstan. Int J Tuberc Lung Dis 2009;13:540-4. [PubMed]
- Shrestha N, Samir KC, Baltussen R, et al. Practical approach to lung health in Nepal: better prescribing and reduction of cost. Trop Med Int Health 2006;11:765-72. [Crossref] [PubMed]
- ten Asbroek AH, Delnoij DM, Niessen LW, et al. Implementing global knowledge in local practice: a WHO lung health initiative in Nepal. Health Policy Plan 2005;20:290-301. [Crossref] [PubMed]
- World Health Organization. Evaluation of the practical approach to lung health. Report of meeting held on 18–19 June 2007. WHO/ HTM/TB/2008.396. Geneva, WHO, 2007.
- Pierantoni CR, Vianna CMM, França T, et al. Rotatividade da força de trabalho médica no Brasil. Saúde Debate 2015;39:637-47. [Crossref]
- Pulcini C, Pauvif L, Paraponaris A, et al. Perceptions and attitudes of French general practitioners towards rapid antigen diagnostic tests in acute pharyngitis using a randomized case vignette study. J Antimicrob Chemother 2012;67:1540-6. [Crossref] [PubMed]
- Global strategy for asthma management and prevention. NIH publication. Available online: http://www.ginasthma.org (Cited: 30 out. 2021).
- Global strategy for the diagnosis, management, and prevention of COPD. Available online: http://www.goldcopd.org/ (Cited: 30 out. 2021).
- 2018 surveillance of pneumonia in adults: diagnosis and management (NICE guideline CG191). [No authors listed]London: National Institute for Health and Care Excellence (UK); 2018 Oct 31. Cited: 30 out. 2021.


