
Minimally invasive repair of pectus carinatum with a new steel bar
Introduction
Pectus carinatum is the most common chest deformity, second only to pectus excavatum, it is characterized by an anterior protrusion of the sternum and adjacent ribs (1,2). This condition can be congenital or appear during a child’s growth. Most patients with pectus carinatum have no clinical symptoms and only go to the hospital because of cosmetic deformities (2). They often experience feelings of shame and embarrassment as well as low self-confidence, and like to hide their chests with clothing or posture adjustments; some patients appear kyphotic in appearance, and severe physiological deformities may also affect physical, social, and mental health (2,3). Many types of pectus carinatum have been identified, and the following three types are most common in the clinic according to the shape of the sternum and chest deformity (3,4):
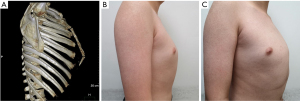
- Type A (typical pectus carinatum): the sternum protrudes forward in a straight line and forms an angle with the xiphoid. In this type, the maximum prominence is at the stern-xiphoid junction, and it is always accompanied by depression of the lateral ribs (Figure 1).
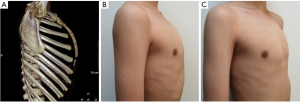
- Type B: the sternum and xiphoid form an arc shape, the xyphoid remains in a straight continuation of the sternal axis, and the maximum prominence is at the highest point of the arc, not the stern-xiphoid junction (Figure 2).
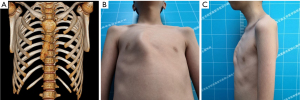
- Type C: this is the asymmetrical type, with a unilateral prominence of the elongated costal cartilage and concomitant tilting of the sternum towards the opposite side at various angles (Figure 3).
Minimally invasive repair of pectus carinatum (MIRPC) has become increasingly popular in recent years, and many different methods with Nuss steel bars or other modified bars have been reported (5-8). We have been treating pectus carinatum with Nuss steel bars for several years and achieved favorable results prior to 2018. However, the installation and removal of Nuss steel bars is sometimes difficult, time-consuming and traumatic. To further simplify the procedure, we designed a new steel bar to facilitate minimally invasive surgical correction of pectus carinatum and have treated more than 100 patients with good results and few complications. In this study, we examine our institutional experience with pectus carinatum repair and describe the efficacy of our minimally invasive approach. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-189/rc).
Methods
This is a single-centre clinical study based on the retrospective analysis of prospectively collected data on patients with pectus carinatum who underwent surgical correction between January 2018 and July 2021 in our centre. A total of 123 pectus carinatum patients were gathered from the database. Eleven patients were excluded: five patients were excluded because they were treated with a Lorenz bar, three patients underwent open surgery, two patients had pectus excavatum, and one had bullae of lung that required a simultaneous operation. The remaining 112 patients were included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients or their legal guardians, depending on the patients’ age. The study was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2021-171). The primary end point was Haller index change after operation. The secondary end points included length of stay after operation, short-term and long-term complications.
Every pectus carinatum patient underwent a preoperative “compression test” to determine the flexibility of the sternum and estimate the thoracic shape after minimally invasive surgery. While the patient stood with his or her back against a wall, one operator pressed the highest point of the patient’s front chest wall with a fist and felt the flexibility of the sternum, and the shape of the chest after pressing the sternum into the chest wall was maintained after the operation. The preoperative examination of the patients included routine blood analysis, electrolyte analysis, heart ultrasound, and electrocardiography. Pulmonary function tests only performed in patients older than 18 years. All patients underwent chest computed tomography (CT) imaging, the Haller index was measured, and three-dimensional reconstruction was performed to observe the morphology of the sternum, ribs and spine. Two experienced surgeons classified the type of pectus carinatum according to the shape of the sternum and chest deformity. Patient satisfaction was evaluated using questionnaires and was divided into four levels as follows:
- Excellent: the postoperative thoracic shape completely returned to normal, and the patient was very satisfied with the therapeutic effect.
- Good: the postoperative thoracic shape was significantly improved, and the patient was satisfied with the therapeutic effect.
- Fair: the postoperative thoracic shape was improved, but the patient was still not satisfied with the therapeutic effect.
- Poor: the postoperative thoracic shape was not improved, or even worse, and the patient was very dissatisfied with the therapeutic effect.
All patients were followed up for 3 months after the operation to observe wound healing and displacement of the steel bar via chest CT. Subsequently, patients were followed up for every 6 months, complications as wound infection, nickel allergy, screw loosening, wire breakdown, bar fraction, overcorrection and recurrence of pectus carinatum were recorded.
New steel bar configuration and accessories
The steel bar is composed of three parts: the central part, the deformable part and the connecting part (Figure 4A). The whole steel bar is 1.25 cm wide, but the thicknesses of the different parts vary. The central part is 2 mm thick and curved, similar to the physiological curvature of the front chest wall of a healthy person. The deformable part of the steel bar is 1 mm thick, is connected to both sides of the central part, and can be curved by hand or with simple tools into the desired shape. The connecting part is extended by the deformable parts on both sides, and it is designed to connect to the stabilizer. The thickness of this part is the same as that of the central part. The connecting part contains a longitudinal arrangement of four screw holes, allowing fixation to the stabilizer by screws, and we can adjust the pressure on the chest wall on both sides by connecting the stabilizer to different screw holes. In addition, 5 different specifications of the steel bar exist, and these are distinguished by different lengths vary from 28 to 36 cm, with 2 cm differences between each specification. We designed two generations of bars, as shown in Figure 4B,4C. In the first generation, the connection between the deformable part and the other two parts was too sharp, and two cases of bar fracture occurred early after surgery, and reoperation was needed to replace the bar; thus, we designed the second-generation bar, for which the joints between parts were smoother and not easily broken.
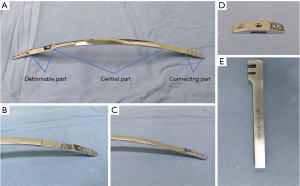
The related accessories are as follows: the stabilizer was designed with three holes and a groove in the middle so the stabilizer can attach to the side of the connecting part of the steel bar (Figure 4D). The stabilizer can be connected to the connecting part through screws in the groove, and the two side holes were designed to fix the bar with steel wire to the ribs. The steel bar orthotic contains two card slots that can be used for orthosis (Figure 4E). There were matching screws and screwdrivers to the screw holes designed for the steel bar.
Surgical technique
The patient was placed in the supine position with bilateral upper extremity abduction of 90 degrees after administering combined general anaesthesia and tracheal intubation. The highest point of the sternum was marked and pressed to correct the profile of the chest wall, and then we marked the sites for the bilateral incisions. Generally, 2–3 cm vertical incisions were made along the line of the highest point of the sternum at the level of the midaxillary line. Intercostal nerve block was performed on the two intercostal spaces next to the incisions. Routine disinfection and draping were performed.
The highest point was pressed to correct the chest wall, and the distance between the two incisions were measured to choose a suitable steel bar; then, the bar was bent by hand or the orthotic to the desired shape according to the shape of the chest. After the incisions were made, the subcutaneous tissue and muscle were exposed with an operative pencil, and submuscular pockets were made so that the stabilizer could be placed. Then, a submuscular tunnel was built connecting both incisions with oval forceps, and a long rope was placed in the tunnel in preparation for a later step. The ribs next to the incisions were sutured using steel wires (four steel wires on each side). The stabilizers were then placed into the submuscular pockets, and the wires on either side were placed in the two side holes of the stabilizer for later use. The selected bar was then introduced with the help of the rope placed through the submuscular tunnel, and the rope was then removed. One operator pressed the highest point of the sternum to optimize the chest wall shape; at the same time, another operator fixed the connecting part of the bar and stabilizer with a screw on one side and then fixed the other side in the same way. If the shape of the steel bar did not fit well with the thorax, the orthotic could be used again to adjust the bar to an ideal shape, and then the steel wires on both sides could be tightened to fix the stabilizers to the ribs firmly. If the shape of the thorax was not satisfactory, the degree of indentation of the chest wall could be adjusted through the lateral hole of the bar to achieve the best corrected shape. Then, the incisions were sutured layer-by-layer. A self-controlled analgesia infusion pump was used to alleviate postoperative pain for 2 days. All patients underwent chest X-ray on the day of the operation. All patients received antibiotics intravenously for 2 days after the operation. The bar was removed 2 years after placement.
Statistical analysis
The numerical data are expressed as the number of cases (n) and percentage (%). Normal distribution of the data was checked by Kolmogorov-Smirnov test. The continuous variables with normal distribution are expressed by mean ± standard deviation. The comparisons among normally distributed continuous variables were conducted via t-test. Comparisons between enumeration data were conducted by Chi-Square or Fisher exact method. Statistical analysis and data management were performed using Excel (from Microsoft version 16.32) and SPSS (IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA). Significance is indicated by P<0.05.
Results
A total of 112 patients (109 male, 3 female) underwent MIRPC with the new steel bar, including 62 cases of type A pectus carinatum, 31 cases of type B pectus carinatum and 19 cases of type C pectus carinatum. The mean patient age was 14.46±2.17 years (range, 10–23 years), with a mean Haller index of 1.96±0.22 (range, 1.44–2.48). The comorbidities included 10 cases of scoliosis, 22 cases of kyphosis, and 1 case of scoliosis and kyphosis. All patients were successfully treated with one bar. The mean operation duration was 67.74±17.56 minutes (range, 35–120 minutes). The mean hospital length of stay was 3.64±0.70 days (range, 3–6 days).
Twenty patients complained of chest tightness after the operation that was gradually relieved without intervention, three patients complained of noticeable chest pain that was relieved by oral analgesics, and the remaining patients tolerated the operation well. Postoperatively, pneumothorax was diagnosed in 12 patients, and pleural effusion was diagnosed in 5 patients, but these patients had only a small amount of pleural effusion or pneumothorax, and none needed a drainage tube. All patients underwent a CT examination in the third month after the operation, and the Haller index (postoperative Haller index) was measured. The results showed that the Haller index of the patients improved significantly after the operation (preoperative Haller index vs. postoperative Haller index, 1.96±0.22 vs. 2.78±0.35, t =−34.09, P=0.00).
All patients were followed up for 22.04±12.07 (range, 3–44) months, wound infections occurred in 3 patients; two patients recovered after debridement, and another patient needed flap transfer because of bar exposure caused by delayed wound healing. A nickel allergy was observed in 2 patients, and the incision complications were not successfully treated with prednisone and debridement, so we had to remove the bars earlier than planned. In one case, the bar was removed 3 months postoperatively, and in the other case, the bar was removed 6 months postoperatively.
Screw loosening resulted in the separation of the steel bar and stabilizer (Figure 5) in 3 patients. One case occurred at 6 months after the operation, and 1 case occurred at 8 months after the operation. In both cases, reoperation was needed to fix the bars and stabilizers. The remaining 1 case of screw loosening occurred close to bar removal, and the deformity was utterly corrected, so the screw was kept in place until bar removal.
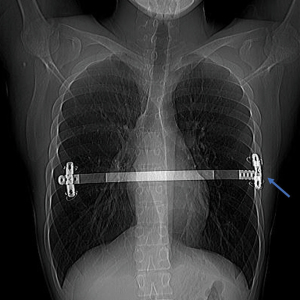
Wire breakdowns were observed in 9 patients. In one case, the broken steel wire poked out of the skin, while the other three steel wires of the patient were firmly fixed on the stabilizer. There was no displacement of the steel bar, so we removed the broken steel wire and did not replace it with a new wire. In the remaining 8 cases, although wire breakdowns occurred, no displacement of the steel bar and stabilizer was observed, so the broken wires remained in place without any intervention.
Bar fraction (Figure 6) was observed in two patients with first-generation steel bars. One case occurred 6 months after the operation, and the steel bar was replaced in a repeat MIRPC. One case of bar fracture occurred 8 months after the operation; in this case, the appearance of the chest was expected, and the patient refused to undergo placement of a new bar, so the fractured bar was removed.
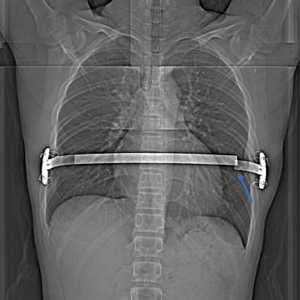
Excavatum deformities of varying degrees developed in 6 patients during follow-up because of overcorrection: 3 cases occurred in patients with type A pectus carinatum (3/62), 2 cases occurred in patients with type B pectus carinatum (2/31), and 1 case occurred in a patient with type C pectus carinatum (1/19). There was no statistical significance in the incidence of excavatum deformities among the three groups (χ²=0.10, P=0.95). Of these 6 patients, 3 patients with moderate pectus excavatum had to undergo bar removal earlier than the planned 24 months after the operation: two patients had to undergo bar removal 12 months postoperatively, and one patient had to undergo bar removal 18 months after the operation. Overcorrection regressed spontaneously during follow-up after bar removal without any intervention in 1 patient. One patient required placement of an excavatum bar, and the last patient was followed up under observation. Three patients with mild excavatum deformities did not undergo bar removal earlier than planned, two patients underwent bar removal 24 months postoperatively, and the remaining patient is still under observation.
Overall, 72 of 112 patients (64.3%) underwent bar removal, and 63 patients (87.5%) achieved excellent or good results, 9 patients achieved fair results, and no patients had poor results. The carinatum deformity recurred in 2 of 72 patients (2.8%), and both patients were who had to undergo bar removal earlier than planned because of a nickel allergy. The Haller index and complications were listed in Table 1.
Table 1
| Variables | Patients (n=112) |
|---|---|
| Preoperative Haller index (range) | 1.96±0.22 (1.44 to 2.48) |
| Postoperative Haller index (range) | 2.78±0.35 (1.92 to 3.71) |
| Complications during hospitalization | |
| Chest tightness | 20 |
| Chest pain | 3 |
| Pneumothorax | 12 |
| Pleural effusion | 5 |
| Complications after discharge | |
| Wound infection | 3 |
| Nickel allergy | 2 |
| Screw loosening | 3 |
| Wire breakdown | 9 |
| Bar fraction | 2 |
| Overcorrection | 6 |
| Recurrence of pectus carinatum | 2 |
Data is presented as mean ± standard deviation.
Discussion
For a long time, the Ravitch procedure or its modified versions were considered classic correction procedures for pectus carinatum. The procedure involves resection of the deformed costal cartilage, xiphoid division from the sternum, and transverse sternal osteotomy to displace the sternum anteriorly (9,10). Although the method has achieved good results, it has disadvantages, including a long operation duration, a long hospitalization period, a large amount of blood loss, and scarring of the anterior chest wall. In 1987, Dr. Donald Nuss designed a new steel bar for the correction of pectus excavatum, and after his publication in 1998, some used the bar to correct pectus carinatum as well (11-13). In the last 10 years, we also used the Nuss method to correct more than 200 cases of pectus carinatum (14). Although good results were achieved, quite a few disadvantages were revealed during the operations. For example, the steel bar has to be plasticized before operation, there were difficulties in placing or removing the steel bar through the tissue in front of the sternum, and there were long operation times for both the implant procedure and removal procedure. Based on the disadvantages above, we designed a new steel bar that could be placed through a modified procedure. The minimally invasive technique has overcome the disadvantages of the Nuss procedure, resulting in satisfying aesthetic outcomes with few complications.
The bar we designed are different from the configuration reported by Nuss and others (5,6,11,15,16). The thickness of the steel bar reported before is the same for the whole length of the steel bar, and the bar is scleroid and hard to bend. Before the operation, a template model needs to be made according to the shape of the thorax, and then the bar needs to be bent into a convex configuration to match the template model; this is time-consuming, and the curved bar according to the measured mould may not be optimal for placement into the patient’s body. We observed that the only part of the steel bar that needs to be bent is the boundary of the lateral and anterior chest wall, so we made that part thinner and easier to plasticize, even in the body of patients. Therefore, we do not need a model, and the steel bar only needs approximate bending before placement. After the bar is placed in the body, it can be further corrected to ensure a closer fit with the chest wall. This design also makes it easier to remove the bar because we can easily straighten the steel bar and pull it out.
The connection part we designed has four vertical screw holes that could be connected to the stabilizer, and we could adjust the pressure on the chest wall by connecting the stabilizer through different screw holes, which can yield better correction results for asymmetric pectus carinatum. There were 19 patients with asymmetric pectus carinatum in this group, and they all achieved excellent results (Figure 7).
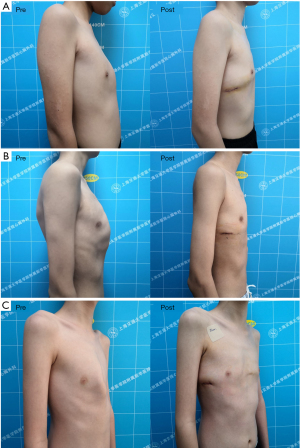
The design of the stabilizer is different from that of Abramson’s steel bar configuration (6). We placed the stabilizer on the outside rather than inside of the steel bar so that the steel plate can fit the chest wall better and cannot not easily move after we fix the stabilizers to ribs. The stabilizer is also different from that of four generation reported by Yuksel and colleagues (5). The stabilizer we designed with three holes, the bar and stabilizer can be easily connected through the middle hole with screw, and the stabilizer can be firmly fixed to ribs through side holes with multiple wires. This bar and stabilizer configuration provides the best configuration and simplifies the surgical procedure to achieve successful correction rates and avoid complications.
Our surgical technique differs from the surgical technique of Ping and Kálmán and their colleagues (8,16). In their technique, the upper and lower intercostal spaces were divided bilaterally at the anterior axillary line, the bar was inserted from the lower intercostal space to the upper intercostal space of the rib in one side, after the bar was pulled out from one side to another side, the bar was pulled out from the upper intercostal space to the lower intercostal space. This technique involves entry to both thoracic cavities, passing the thoracic wall 4 times, the method is relatively complex and time-consuming. In our technique, there is no need to divide the intercostal space, the bar was placed outside the ribs without passing the thoracic cavity, and the bar can be firmly fixed to the ribs with multiple wires easily, these may simplify the operation and reduce the risk of entering the thoracic cavity.
The complications during hospitalization included pneumothorax and pleural effusion, but no drainage tubes needed to be placed. The complications after discharge included wound infection, nickel allergy, screw loosening, wire breakdown, bar fraction, and overcorrection leading to excavatum. Incision disunion caused by nickel allergy and incision infection are very difficult to treat, especially in patients with nickel allergy. In this group, the incision disunion of 2 patients caused by nickel allergy could not heal after debridement and ultimately the steel bar had to be removed earlier than planned, and both patients had pectus carinatum recurrence during the follow-up.
Steel wire breakdown is not rare and may lead to failed MIRPC, and we addressed this problem well through the use of multiple steel wires for fixation of the bar and ribs (17). Although the incidence of steel wire breakdown in this group was relatively high (9/112), the broken steel wire needed to be removed in only 1 case, and no patients needed to undergo a repeat MIRPC. We believe that multiple steel wire fixation can well avoid the displacement of the steel bar. Even if some wire breakdowns occurred, the remaining steel wires were enough to keep the steel bar in place.
Screw loosening may lead to bar displacement and pectus carinatum recurrence. There were 3 cases of screw loosening in this group, among which two patients with pectus carinatum recurrence needed reoperation, but the reason why the screws would loosen has not been clear until now. Perhaps two screws are necessary to fix the bar and stabilizer. Two cases of bar fracture occurred in patients treated with the first-generation steel bar (2/36), both at the junction of the main part and deformable part. The problem was solved after we improved the steel bar, and no second-generation bar fracture occurred in the last 76 patients.
Six cases of pectus excavatum occurred in this group and have no obvious correlation with the type of pectus carinatum. The appropriate time for bar removal in these patients is difficult to gauge. The overcorrection may regress spontaneously if the bar is removed earlier, but early removal may also lead to pectus carinatum recurrence. In the study of Yuksel et al., 10 patients with overcorrection underwent bar removal 12–13 months after the operation, and 9 patients regressed spontaneously without any intervention, but the carinatum deformity recurred in 4 patients (5). We only removed the steel bar early for patients with moderate pectus excavatum, while 3 patients with mild pectus excavatum chose to undergo bar removal at the planned 24 months after the operation, and no further progression of the pectus excavatum or recurrence of pectus carinatum was observed.
Earlier bar removal may cause recurrence of the pectus carinatum (17). In the majority of studies, bar removal was planned after at least 24 to 36 months (12,18-20). We aim to keep the bar in place for 24 months, and no recurrence occurred in 66 patients removed the bar in plan. There were 6 patients had the bar removal prematurely in our study, including 2 patients with nickel allergy, 1 patient with bar fraction and 3 patients with overcorrection, and recurrence occurred in the 2 patients with nickel allergy. The remaining 40 patients retaining the steel bar are still under observation, and no recurrence of pectus carinatum is observed.
In short, the modified procedure with a new steel bar is effective in the treatment of pectus carinatum. The newly designed bar makes the placement and removal procedures more convenient and efficient. The newly designed stabilizer and use of multiple wires for fixation can better maintain the stability of the steel bar and avoid bar displacement, and the multiple screw hole design of the steel bar makes it to adjust the pressure on both chest walls, which is effective for repairing both symmetric and asymmetric carinatum deformities. However, there are still some limitations in this study. Firstly, this is a retrospective single-center study without control group. Secondly, the application time of the new bar is relatively short, the number of patients with bar removal is small, we need a long-time follow-up to further drawn our conclusion.
Acknowledgments
Funding: This study was funded by Shanghai Collaborative Innovation Center for Translational Medicine (No. TM201709).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-189/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-189/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-189/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-189/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2021-171). Written informed consent was obtained from the patients or their legal guardians, depending on the patients’ age.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cobben JM, Oostra RJ, van Dijk FS. Pectus excavatum and carinatum. Eur J Med Genet 2014;57:414-7. [Crossref] [PubMed]
- Desmarais TJ, Keller MS. Pectus carinatum. Curr Opin Pediatr 2013;25:375-81. [Crossref] [PubMed]
- Robicsek F, Watts LT. Pectus carinatum. Thorac Surg Clin 2010;20:563-74. [Crossref] [PubMed]
- Fokin AA, Steuerwald NM, Ahrens WA, et al. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 2009;21:44-57. [Crossref] [PubMed]
- Yuksel M, Lacin T, Ermerak NO, et al. Minimally Invasive Repair of Pectus Carinatum. Ann Thorac Surg 2018;105:915-23. [Crossref] [PubMed]
- Abramson H, Aragone X, Blanco JB, et al. Minimally invasive repair of pectus carinatum and how to deal with complications. J Vis Surg 2016;2:64. [Crossref] [PubMed]
- Bell R, Idowu O, Kim S. Minimally invasive repair of symmetric pectus carinatum: bilateral thoracoscopic chondrotomies and suprasternal compression bar placement. J Laparoendosc Adv Surg Tech A 2012;22:921-4. [Crossref] [PubMed]
- Ping W, Fu S, Li Y, et al. A new minimally invasive technique for correction of pectus carinatum. J Cardiothorac Surg 2021;16:280. [Crossref] [PubMed]
- Ravitch MM. The operative correction of pectus carinatum. Bull Soc Int Chir 1975;34:117-20. [PubMed]
- Scarci M, Bertolaccini L, Panagiotopoulos N, et al. Open repair of pectus carinatum. J Vis Surg 2016;2:50. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Schaarschmidt K, Lempe-Sellin M, Schlesinger F, et al. New Berlin-Buch "reversed Nuss," endoscopic pectus carinatum repair using eight-hole stabilizers, submuscular CO2, and presternal Nuss bar compression: first results in 35 patients. J Laparoendosc Adv Surg Tech A 2011;21:283-6. [Crossref] [PubMed]
- Poullis M. Modified Nuss repair for pectus carinatum. Interact Cardiovasc Thorac Surg 2010;11:221-2. [Crossref] [PubMed]
- Wang L, Liu J, Shen S, et al. Comparison of Outcomes Between Anti-Nuss Operation and Modified Anti-Nuss Operation Using a Flexible Plate for Correcting Pectus Carinatum: A Retrospective Study. Front Surg 2020;7:600755. [Crossref] [PubMed]
- Özkaya M, Bilgin M. Minimally invasive repair of pectus carinatum by modification of the Abramson technique. Wideochir Inne Tech Maloinwazyjne 2018;13:383-7. [Crossref] [PubMed]
- Kálmán A. Initial results with minimally invasive repair of pectus carinatum. J Thorac Cardiovasc Surg 2009;138:434-8. [Crossref] [PubMed]
- Geraedts TCM, Daemen JHT, Vissers YLJ, et al. Minimally invasive repair of pectus carinatum by the Abramson method: A systematic review. J Pediatr Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Katrancioglu O, Akkas Y, Karadayi S, et al. Is the Abramson technique effective in pectus carinatum repair? Asian J Surg 2018;41:73-6. [Crossref] [PubMed]
- Özkaya M, Bilgin M. Minimally invasive repair of pectus carinatum: a retrospective analysis based on a single surgeon's 10 years of experience. Gen Thorac Cardiovasc Surg 2018;66:653-7. [Crossref] [PubMed]
- Suh JW, Joo S, Lee GD, et al. Minimally Invasive Repair of Pectus Carinatum in Patients Unsuited to Bracing Therapy. Korean J Thorac Cardiovasc Surg 2016;49:92-8. [Crossref] [PubMed]


