
Diagnosis and prognosis of acute respiratory distress syndrome related to diffuse pneumonic-type adenocarcinoma: a single-center case series study
Introduction
Acute respiratory failure (ARF) is the leading cause of intensive care unit (ICU) admission in cancer patients (1). Despite recent advances in diagnostic investigations, the cause of ARF remains undetermined in up to 20% of cancer patients with ARF (2,3), even in patients meeting acute respiratory distress syndrome (ARDS) criteria (4). In these patients, failure to identify the cause of ARF is independently associated with increased mortality (3,5) and a delayed diagnosis and subsequent delayed treatment may also have unfavorable impact on prognosis (6).
Malignant lung involvement represents recognized causes of ARDS mimickers (7,8), accounting for 20% of ARDS without common risk factors (8) and up to 30% of unexplained pulmonary infiltrates in cancer patients (9). Likely to occur at the time of the malignancy diagnosis (1), the severity of malignant lung infiltration may range from scarce infiltrate to life-threatening ARDS especially in case delayed diagnosis (10).
Pneumonic-type adenocarcinoma (P-ADC) encompasses heterogeneous mechanisms of cancer-related lung injury that may also progress to malignant ARDS, especially in case of tumor spread through air spaces (diffuse P-ADC) (11). Its association with hypoxemia, chest pain, and sometimes fever makes the diagnosis challenging, mimicking infectious pneumonia (12), and may induce delayed diagnosis and management. Moreover, its treatment and prognosis may be substantially different from those of ARDS with common risk factors. Indeed, in comparison with ARDS of common causes, malignant ARDS has been demonstrated with high risk of ICU mortality (up to 96%) (8) and diffuse P-ADC may benefit from early administration of anti-cancer treatments. Thus, a better understanding of the diagnostic features and the determinants of the outcome of P-ADC patients presenting with ARDS are of major clinical importance, since timely diagnosis and appropriate management may improve the prognosis (13). However, no data are available regarding this type of malignant ARDS when ICU admission is required.
Here, we sought to describe the profile and prognosis of patients admitted to the ICU with diffuse P-ADC related ARDS. The primary objective was to provide the diagnostic clues from the clinical suspicion to the pathological confirmation, based on our clinical experience. The secondary objective was to assess the determinants of ICU and in-hospital mortality. We hypothesized that the diagnosis was delayed in most patients, which was associated with a worse prognosis. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-12/rc).
Methods
Study design and setting
This observational case series study was conducted from January 1998 to January 2018 in a 20-bed French medical ICU, part of the thoracic oncology department of Tenon University Hospital, Paris, France, a medical and surgical reference center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the French Intensive Care Society Institutional Review Board (CE SRLF 21-23) and informed consent was taken from the patients or their relatives.
Patient selection
Patients were included if they met the three following criteria: (I) admission to the ICU during the study period; (II) histologically or cytologically proven adenocarcinoma of the lung according to the 2011 IASLC/ATS/ERS classification of lung adenocarcinoma (14) and the 2015 WHO classification of lung tumors (11); and (III) presenting with ARDS on ICU admission. The ARDS was defined by (I) a new or worsening respiratory symptoms over the last seven days; (II) bilateral pneumonia-like opacities on chest radiograph or computed tomography scan; (III) the absence of suspected cardiogenic pulmonary edema and of common causes of ARDS; and (IV) a PaO2/FiO2 ratio ≤300 mmHg (15). A positive end-expiratory pressure level of at least 5 cmH2O was not necessary for inclusion.
Patients with a previous history of other thoracic or extra thoracic adenocarcinoma, presenting with ARDS of common causes, or under the age of 18 years were excluded. Concomitant bacterial pneumonia was not an exclusion criterion.
Data collection
Characteristics of the patients
Age, gender, performance status (PS) during the week preceding ICU admission, clinically important weight loss, defined by a >5% loss of usual body weight over the last six months, smoking history and main comorbidities using the Charlson Comorbidity Index were collected for each patient. Symptoms and physical signs on respiratory examination (e.g., dyspnea, cough, bronchorrhea, chest pain) were collected. Physiological variables such as body temperature, respiratory rate, heart rate, systolic arterial blood pressure and Glasgow coma scale were also recorded, as well as main laboratory variables (e.g., arterial blood gas, leukocyte count, C-reactive protein, serum creatinine). Severity on admission was assessed by the Simplified Acute Physiology Score (SAPS) II and the Sequential Organ Failure Assessment (SOFA). Advanced life support measures administered during the ICU stay such as mechanical ventilation (MV) either invasive or non-invasive (NIV), High Flow Oxygen Therapy, vasopressors and renal replacement therapy were also collected. Finally, we recorded ICU- and hospital mortality.
Oncological evaluation
All histological (trans-bronchial biopsy, open-lung biopsy and autopsy) and cytological [sputum examination, bronchial aspirate, broncho-alveolar lavage (BAL)] samples were reviewed by experienced lung pathologist (M Antoine) and cytologist (A Fajac) and histological samples of patients admitted before 2011 were re-classified according to current classifications (11,14). For the BAL procedures we used 50 mL of room temperature, sterile 0.9% saline injected via handheld 50 mL syringe, this repeated 4 times to reach a total of 200 mL instilled in the lungs. The cancer diagnosis could be confirmed based on cytological analysis (e.g., BAL), only if at least one agglomerate of neoplastic cells forming typical cytological features of P-ADC was identified. Details on pathological definitions are available in the Appendix 1. Patients were classified as already diagnosed or newly diagnosed P-ADC, depending on whether cancer had been diagnosed before ICU referral or during ICU stay. Molecular testing (i.e., cancer biomarkers) was also collected, when performed. Staging was recorded according to the current TNM Classification System for lung cancer (16). Finally, anticancer treatment (chemotherapy, high doses of corticosteroids) administered during the ICU stay was also collected.
Radiological evaluation
Radiologic characteristics were assessed by an independent radiologist expert (MF Carette). Main CT findings, including (I) normal attenuation, (II) ground-glass attenuation, (III) alveolar consolidation and (IV) crazy paving were quantitatively measured, using a CT-scan extent score (17). Briefly, each lung was divided in three zones, i.e., upper, middle, and lower. Then, the percentage of lung parenchymal surface represented by each pattern was estimated in each six zones (3 right, 3 left). Finally, the average score of the six lung zones was calculated (adding each zone score, divided by 6).
Statistical analysis
Continuous variables are expressed as median (0.25–0.75 interquartile range) and categorical variables are expressed as absolute and relative frequency (%). Each potential factor associated with ICU or hospital mortality was evaluated in a univariate model. Variables were compared with Mann-Whitney test for quantitative variables and chi-square test or Fisher exact test for qualitative variables. All tests were two-sided and P values <0.05 were considered statistically significant. Because of the small sample size, a maximum of three variables identified with a P value less than 0.20 in univariate analyses, and/or clinically relevant (including time between first symptoms and diagnosis—the tested hypothesis) were included in a multivariate logistic regression model. The final models were determined using a forward stepwise logistic regression. The Hosmer-Lemeshow Chi-square test was used to check the goodness-of-fit of the final model. Odds ratios (ORs) and their 95% confidence intervals (CI) were calculated for significant factors. Because SAPS II and SOFA scores are highly correlated, SOFA was not entered in the models. Missing data (less than 1%) were not imputed. Statistical analysis was performed with SPSS Base 21.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Results
The flowchart of the study is represented in the Figure 1. During the study period, 24 patients with P-ADC related ARDS were referred to our ICU and thus included. These admissions resulted in transfer from the respiratory wards (n=13; 54%), the emergency services (n=6; 25%) or other ICUs (n=5; 21%).
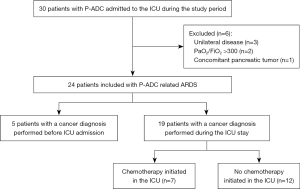
Patient’s characteristics
All the patients had a confirmed diagnosis of adenocarcinoma, all TNM staged M1a. The diagnosis was based on the examination of histological samples in 16 (67%) patients (13 trans-bronchial biopsies, 2 open-lung biopsies and 1 lung resection specimen) divided in 9 invasive mucinous adenocarcinoma (IMA) and 7 lepidic predominant adenocarcinoma (LPA). For the eight (33%) remaining patient without histological specimen, the diagnosis of adenocarcinoma was based on cytological analysis of BAL. More details on pathological findings are available in Table S1. The main characteristics of the 24 patients are displayed in Table 1 and Table S2. The diagnosis of cancer was established during the ICU stay in 19 (79%) patients, 1 (1 to 4) day after ICU admission. For the remaining 5 (21%) patients, the diagnosis was established prior to ICU admission, a median of 2 (0.5–4) months before admission.
Table 1
| Variables | All (n=24) | ICU mortality | P value | |
|---|---|---|---|---|
| Non-survivors (n=6) | Survivors (n=18) | |||
| Age (years) | 70 (61–75) | 71 (67–77) | 69 (60–75) | 0.42 |
| Gender (male), n (%) | 17 (71) | 4 (67) | 13 (72) | 1 |
| Performance status 3–4, n (%) | 9 (38) | 2 (33) | 7 (39) | 1 |
| Charlson comorbidity Index | 6 (6–7) | 6 (6–7) | 6 (6–7) | 0.99 |
| Time from first symptoms to diagnosis (days) | 210 (92–246) | 234 (199–413) | 155 (88–244) | 0.047 |
| Never received anticancer treatment*, n (%) | 8 (33) | 1 (17) | 7 (39) | 0.621 |
| Severity assessment on ICU admission | 0.04 | |||
| SAPS II | 41 (33–46) | 48 (41–56) | 36 (31–44) | 0.094 |
| SOFA score | 3 (2–4) | 4 (3–5) | 2 (3–4) | – |
| ARDS severity, n (%) | 0.06 | |||
| Mild | 6 (25) | 2 (33) | 4 (17) | |
| Moderate | 5 (21) | 2 (33) | 3 (13) | |
| Severe | 13 (54) | 2 (33 | 11 (45) | |
| Physiological variables on ICU admission | ||||
| Systolic blood pressure (mmHg) | 129 (105–138) | 125 (104–138) | 131 (98–140) | 0.86 |
| Respiratory rate (cycles/min) | 26 (24–30) | 44 (34–48) | 26 (23–28) | 0.004 |
| Heart rate (beats/min) | 95 (88–114) | 121 (111–129) | 93 (85–107) | 0.015 |
| Temperature (°C) | 37.5 (37.0–38.0) | 37.6 (35.0-38.5) | 37.5 (37.0–38.0) | 0.782 |
| Laboratory variables on ICU admission | ||||
| Leukocyte count (109/L) | 12.3 (8.3–18.9) | 12.5 (12.4–21.7) | 11.4 (7.7–16.5) | 0.121 |
| C-reactive protein (mg/L) (on 21 patients) | 34 (14–75) | 58 (21–93) | 32 (8–77) | 0.512 |
| Serum creatinine (μmol/L) | 73 (66–93) | 83 (63–147) | 68 (65–93) | 0.613 |
| pH on arterial blood gas | 7.43 (7.40–7.44) | 7.37 (7.33–7.41) | 7.44 (7.41–7.44) | 0.01 |
| Total BAL cell count (103/mL) | 520 (240–900) | 630 (160–840) | 480 (255–952) | 0.864 |
| BAL neutrophil count (103/mL) | 289 (79–614) | 100 (64–563) | 300 (54–782) | 0.522 |
| Radiological assessment on ICU admission | ||||
| Alveolar consolidation extent score | 18 (12–43) | 45 (13–58) | 17 (12–28) | 0.321 |
| Normal lung extent score | 48 (33–63) | 37 (32–58) | 52 (34–66) | 0.513 |
| Mediastinal lymphadenopathy, n (%) | 3 (13) | 2 (33) | 1 (6) | 0.133 |
| Life supporting interventions, n (%) | ||||
| Mechanical ventilation | 17 (71) | 6 (100) | 11 (61) | 0.134 |
| Non-invasive ventilation only | 6 (25) | 2 (33) | 4 (22) | 0.621 |
| Vasopressors | 4 (17) | 2 (33) | 2 (11) | 0.257 |
Data are expressed as number and percentage [n (%)] for categorical variables, and median (interquartile interval) for continuous variables. *, impossibility to dispense anticancer treatment at any time before, during or after ICU discharge. ICU, intensive care unit; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; ARDS, acute respiratory distress syndrome; BAL, broncho-alveolar lavage.
Bedside diagnostic reasoning process: from the clinic-radiological suspicion to the quick cytological examination
Main clinical, biological, radiological and cytological diagnostic features of the 24 patients are reported in Table 2. Eighteen (75%) had a smoking history (9 active smokers on ICU admission), with a cumulative consumption of 38 (15 to 48) pack-year.
Table 2
| Variables | Values |
|---|---|
| Physical examination features, n (%) | |
| Dyspnea | 24 (100) |
| Cough | 20 (83) |
| Bronchorrhea | 20 (83) |
| Salty expectoration on 13 patients | 9 (69) |
| Crackles on auscultation | 12 (50) |
| Significant weight loss | 10 (42) |
| Fever | 6 (25) |
| Chest pain | 2 (8) |
| Hemoptysis | 1 (4) |
| Clubbing | 1 (4) |
| Biological features | |
| Leukocyte count (109/L) | 12.3 (8.3–18.9) |
| C-reactive protein (mg/L) on 21 patients | 34 (19–75) |
| Procalcitonin (ng/mL) on 15 patients | 0.11 (0.09–0.94) |
| Serum lactate dehydrogenase (IU/L) on 21 patients | 412 (285–645) |
| Arterial lactate (mmol/L) | 1.2 (0.9–1.5) |
| Serum creatinine (μmol/L) | 72 (65–92) |
| CT-scan radiological features (on 22 patients), n (%)/lung extent score (%) | |
| Alveolar consolidation | 20 (95)/18 (12–43) |
| Ground-glass attenuation | 19 (90)/10 (5–23) |
| Crazy paving | 6 (29)/0 (0–2) |
| Bronchogram within consolidation | 19 (90) |
| Fissural bulging | 12 (57) |
| Compressed bronchus and vessel | 9 (43) |
| Nodules/micronodules | 12 (57) |
| <10 | 4 (33) |
| 10–30 | 5 (42) |
| >30 | 3 (2) |
| Cyst/cavitation | 8 (38) |
| Broncho-alveolar lavage features (on 22 patients), cell count (103/mL)/cell proportion (%) | |
| Total cell count | 520 (240–900) |
| Neutrophil | 289 (79–614)/64 (41–85) |
| Macrophage | 141 (35–272)/20 (11–53) |
| Lymphocyte | 11 (2–39)/4 (2–5) |
| Eosinophil | 0 (0–11)/0 (0–2) |
Data are expressed as number and percentage (n, %) for categorical variables, and median (interquartile interval) for continuous variables. P-ADC, pneumonic-type adenocarcinoma; CT, computed tomography.
Clinical and biological features
At the time of diagnosis, all except one patient, presented with a clinical picture of a non-resolving pneumonia for which they had received 3 (2 to 3) antibiotic lines. Most patients presented with isolated respiratory failure. No patients had consciousness disorders. Vasopressors were required in 4 patients (all received also mechanical ventilation). Laboratory tests revealed a mild biological inflammatory syndrome.
Radiological features
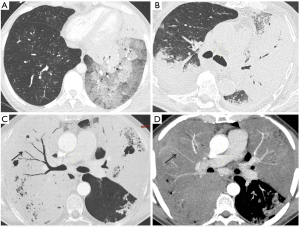
Alveolar consolidation and ground glass opacities were the two most frequent and extended radiological patterns (Figure 2), with several patterns coexisting in some patients. When present, fissural bulging was associated in 75% of cases (9 of 12 patients) with a particular aspect of compressed bronchi and vessels (Figure 2). Mediastinal lymphadenopathy, pleural effusion, atelectasis, pulmonary embolism, interlobular thickening were less frequent and encountered in 3 (13%), 5 (24%), 4 (19%), 2 (10%) and 5 (24%) patients, respectively. Right lower lobe was the most affected lobe in 9 (43%) cases, followed by the left lower lobe in 7 (33%) cases. All these lesions resulted in a remaining lung speared area extent of 48% (33–63%). Repeated CT-scans over time were available in three patients, providing information on natural dynamic expansion of the disease (Figure S1).
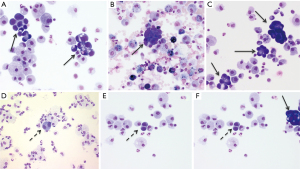
Cytological diagnostic challenge and expertise
Fiberoptic bronchoscopy found abundant clear secretions, mucosa inflammation and infiltration in 96%, 36% and 22% of cases, respectively. BAL showed a marked hyper cellularity with neutrophil predominance, and confirmed the diagnosis in 17 of 23 patients (74% diagnostic yield), exhibiting agglomerated neoplastic cells (Figure 3A-3C). Interestingly, among the 3 patients transferred from other ICUs who underwent a BAL before being referred to the ICU, local cytologist reported the presence of desquamated type II pneumocytes in 2 cases, and wrongly concluded to the diagnostic of diffuse alveolar damage (DAD). Mere sputum cytological examination was performed in seven patients with bronchorrhea, and provided the diagnosis in 5 (71%) patients. More details on diagnostic procedure yields are available in Table S3.
ICU and in-hospital mortality
ICU and in-hospital mortality rates were 25% and 63%, respectively. Lengths of ICU and hospital stays were 9 (5 to 15) and 20 (9 to 39) days, respectively. Factors associated with ICU and hospital mortality, identified in univariate analysis, are shown Table 1 and Table S4 respectively.
Multivariate analysis of factors associated with ICU and hospital mortality are reported in Table 3. More details about the variables selected, and the goodness-of-fit of the models are available in Appendix 2. Neither the type of P-ADC (IMA or LPA) nor the mucinous feature was associated with ICU (P=0.68 and P=0.46 respectively) or in-hospital (P=0.68 and P=0.46 respectively) mortality.
Table 3
| Variables | Prediction model of ICU mortality | Prediction model of hospital mortality | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Time between first symptoms and diagnosis (per day) | 1.02 (1.00–1.03) | 0.046 | – | ns | |
| SAPS II (per point) | 1.16 (1.01–1.33) | 0.040 | – | – | |
| Need for mechanical ventilation | – | ns | – | – | |
| Heart rate (per point) | – | – | 1.07 (1.00–1.15) | 0.041 | |
| Impossibility to dispense chemotherapy at any time after diagnosis of the cancer | – | – | 17.57 (1.19–254.48) | 0.041 | |
Dashes signifies that the variable has been proposed but excluded from the stepwise procedure. ICU, intensive care unit; OR, odds ratio; CI, confidence interval; SAPS, Simplified Acute Physiology Score; ns, no statistical significance.
Sub-group of newly diagnosed patients
Among the 19 newly diagnosed patients with P-ADC, 7 (37%) received chemotherapy in the ICU in combination with high-dose steroid therapy (Table S2). The patients presenting with fever or biological inflammatory syndrome were more likely to not receive chemotherapy during their ICU stay (Table 4). The initiation of chemotherapy in the ICU was not associated with better ICU (P=1.0) or in-hospital (P=0.382) survival.
Table 4
| Variables | Chemotherapy (n=7) | No chemotherapy (n=12) | P value |
|---|---|---|---|
| Gender (male), n (%) | 4 (57) | 10 (83) | 0.352 |
| Age (years) | 72 (61–74) | 72 (65–76) | 0.316 |
| Performance status 3–4, n (%) | 1 (14) | 6 (50) | 0.171 |
| Clinical, laboratory and radiological variables | |||
| Significant weight loss, n (%) | 1 (14) | 6 (50) | 0.174 |
| Temperature (°C) | 36.6 (35.9–37.0) | 37.5 (37.4–38.4) | 0.042 |
| Presence of molecular alterations, n (%) | 2 (25) | 3 (38) | 0.675 |
| Normal lung extent score (%) | 52 (37–53) | 63 (2–68) | 0.492 |
| Serum level of C-reactive protein (mg/L) | 8 (6–16) | 39 (33–70) | 0.007 |
| Presence of bacteria in LRT sample* | 1 (14) | 4 (33) | 0.604 |
| Severity assessment | |||
| SAPS II | 36 (34–44) | 39 (32–48) | 1 |
| SOFA score | 4 (2–5) | 3 (2–3) | 0.445 |
| Severity of the ARDS, n (%) | 0.427 | ||
| Mild | 5 (71) | 6 (50) | |
| Moderate | 0 (0) | 4 (33) | |
| Severe | 2 (29) | 2 (17) | |
| Life supporting interventions, n (%) | |||
| Mechanical ventilation | 5 (71) | 7 (58) | 0.662 |
| Vasopressors | 2 (29) | 1 (8) | 0.526 |
| ICU mortality | 1 (14) | 2 (17) | 1 |
| Hospital mortality | 3 (43) | 8 (67) | 0.381 |
Data are expressed as number and percentage (n, %) for categorical variables, and median (interquartile interval) for continuous variables. *, at significant threshold: >104 cfu/mL for broncho-alveolar lavage and >103 cfu/mL for plugged telescopic catheter. P-ADC, pneumonic-type adenocarcinoma; ARDS, acute respiratory distress syndrome; LRT, lower respiratory tract; ICU, intensive care unit; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; Cfu, colony forming unit.
Discussion
To the best of our knowledge, this is the first series of P-ADC related ARDS patients admitted to the ICU.
Main results can be summarized as follows: in patients admitted to the ICU with an ARDS related to diffuse P-ADC, (I) the clinical diagnosis was suspected by specific clinical, biological and radiological features after several lines of antimicrobial therapies and a prolonged care-pathway, (II) the diagnosis was finally confirmed by mere cytological examination of sputum or BAL in 75% of the cases, (III) the diagnosis was markedly delayed in most of cases, and (IV) this delay was independently associated with ICU mortality.
Clinical suspicion of P-ADC related ARDS: synthesis and comparison with existing data
Faced with a clinical situation of ARDS mimickers or non-resolving pneumonia, the present study provides various elements suggestive of the diagnosis of P-ADC-related ARDS. Firstly, the high incidence of bronchorrhea observed in P-ADC with ARDS, contrasting with the 5–10% incidence observed in P-ADC without ARDS presentation (19), is in line with the fact that bronchorrhea is a late manifestation more likely to be observed in advanced or delayed diagnosed diffuse disease (20). Secondly, the salty taste of the bronchorrhea has been previously reported (21), and is highly specific to P-ADC related bronchorrhea. It is explained by an increased trans-epithelial chloride secretion (22,23), and an excessive transudation of plasma products into the airways (21,23) resulting in a broncho-alveolar mucus osmolality similar to that of plasma (20). Thirdly, nodules, fissural bulging and narrowed bronchus within consolidation at CT-scan were particularly frequent. These three signs have been demonstrated as helpful in differentiating P-ADC from infectious pneumonia (24). The proportion of pseudocavitation observed in our study is also similar to that reported in P-ADC (25). Fourthly, the mild biological inflammatory syndrome observed in our study is in contrast with that expected in patients with infectious pneumonia and should also evoke P-ADC. Finally, the high diagnostic yield of cytological examination (sputum, bronchial aspirate or BAL,) in P-ADC has been reported in different series (26-28), and relies on the identification of agglomerated neoplastic cells (type II pneumocytes or Clara cells) with specific cytological features (18).
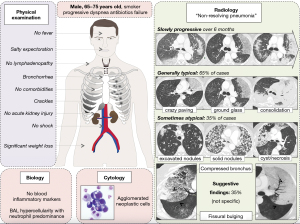
However, the identification of such agglomerated neoplastic cells conceals an important diagnostic pitfall. Indeed, diffuse alveolar damage, the pathological hallmark of ARDS, is also characterized by type II pneumocytes proliferation (29), that has been qualified as reactive (30), atypical (31) hyperplasic (32) or desquamated (33) type II pneumocytes. In some cases, and as illustrated in the Figure 3D-3F, these cells shed in agglomerates (30-32) with an increased nuclear-cytoplasmic ratio, nuclear membrane irregularities, and prominent nucleoli, thus resembling the cells of adenocarcinoma (32,34). For instance, several of our diffuse P-ADC patients had been misdiagnosed as “common” ARDS before ICU referral, highlighting the crucial cooperation between clinicians and cytologists in the diagnosis process. The Figure 4 provides a pictured summary of these main diagnostic features that intensivists should know about diffuse P-ADC mimicking ARDS.
Outcomes: comparison with existing data
The 63% hospital mortality observed in our study seemed substantially higher than the 36% hospital mortality observed in a cohort of 446 lung cancer patients requiring ICU admission for mixed medical and surgical reasons (35). However, this mortality bordered on the 54% hospital mortality of patients with lung cancer admitted for medical reasons (mostly acute respiratory failure) (36) and reached that observed in 1,004 cancer patients with ARDS criteria (64%) (4). In line with previous reports on cancers patients presenting with ARF (3,5,6,10) our results showed that the time between first symptoms and diagnostic was independently associated with ICU mortality even after adjustment on severity. Pragmatically, a subsequent timely initiated chemotherapy may explain this association. This is striking information for clinical practice regarding the possibility of reducing this delay with a better recognition of the key disease features. The positive influence of chemotherapy maintenance after ICU discharge on survival observed in our study, and reported by others (35,36), may supports a substantial efficacy of chemotherapy in these patients. Thus in the area of promising new therapies (targeted therapy, immunotherapy) (26,37) and the high prevalence of genomic molecular alteration in these patients (26,27,38), especially Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation (39) (33% of KRAS mutation in our study), delay the initiation of chemotherapy seems particularly unsuitable for these patients. Interestingly, the decision to not continue or initiate chemotherapy during the ICU stay was certainly influenced by the suspicion of infection in a patient (higher body temperature and C-reactive protein plasma levels in patients without chemotherapy during the ICU stay).
Limitations
First, this was a retrospective study, which involves a potential bias in patients’ selection or data collection, and the small number of subjects limited the performance (discrimination) and thus the interpretation of the multivariate analyses. However, the rarity of the disease remains a major obstacle to prospective or large sample-size studies, even with a multicenter design. Second, we did not compare P-ADC related ARDS patients with other type of ARDS with alveolar consolidation such as community-acquired pneumonia, since clinical, biological and radiological patterns of community acquired pneumonia are well documented. Third, we only considered patients admitted to the ICU. Patients who were not considered for ICU admission for any reason, such as an estimated poor prognosis or a poor performance status, were therefore not included in this analysis.
Conclusions
A rigorous physical, biological, radiological examination should raise a strong suspicion of P-ADC in patients presenting with atypical ARDS. Close collaboration with cytologist is the cornerstone of the diagnosis confirmation. Besides improvement in timely diagnostic recognition, further studies are warranted to test the benefits of high dose corticosteroids and specific anticancer therapy in these patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-12/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-12/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-12/coif). Maxens Decavèle reports non-financial support from ISIS medical. Michaël Duruisseaux declares grants, personal fees and non-financial support from Roche, Novartis, Pfizer, Takeda, Abbvie, BMS, MSD, ASTRAZENECA, Amgen, Boerhinger Ingelheim for participation to boards of experts, lectures, or congress. Marie Wislez reports grants from ASTRAZENECA, Lilly, Merck KgA, MERUS, GSK, AMGEN, Novartis, MSD, and personal fees from BMS, MSD, Boeringher, Roche, ASTRAZENECA and Novartis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the French Intensive Care Society Institutional Review Board (CE SRLF 21-23). Informed consent was taken from the patients or their relatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Azoulay E, Mokart D, Kouatchet A, et al. Acute respiratory failure in immunocompromised adults. Lancet Respir Med 2019;7:173-86. [Crossref] [PubMed]
- Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med 2010;182:1038-46. [Crossref] [PubMed]
- Contejean A, Lemiale V, Resche-Rigon M, et al. Increased mortality in hematological malignancy patients with acute respiratory failure from undetermined etiology: a Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologique (Grrr-OH) study. Ann Intensive Care 2016;6:102. [Crossref] [PubMed]
- Azoulay E, Lemiale V, Mokart D, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med 2014;40:1106-14. [Crossref] [PubMed]
- Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100-7. [Crossref] [PubMed]
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112:S92-107. [Crossref] [PubMed]
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:562-72. [Crossref] [PubMed]
- Gibelin A, Parrot A, Maitre B, et al. Acute respiratory distress syndrome mimickers lacking common risk factors of the Berlin definition. Intensive Care Med 2016;42:164-72. [Crossref] [PubMed]
- Zihlif M, Khanchandani G, Ahmed HP, et al. Surgical lung biopsy in patients with hematological malignancy or hematopoietic stem cell transplantation and unexplained pulmonary infiltrates: improved outcome with specific diagnosis. Am J Hematol 2005;78:94-9. [Crossref] [PubMed]
- Mokart D, Lambert J, Schnell D, et al. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma 2013;54:1724-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Li J, Yen A, Lin GY. Recurrent pneumonia, persistent cough, and dyspnea in a 41-year-old man. Chest 2012;142:1338-42. [Crossref] [PubMed]
- Ebright MI, Zakowski MF, Martin J, et al. Clinical pattern and pathologic stage but not histologic features predict outcome for bronchioloalveolar carcinoma. Ann Thorac Surg 2002;74:1640-6; discussion 1646-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 2008;177:433-9. [Crossref] [PubMed]
- Ohori NP, Santa Maria EL. Cytopathologic diagnosis of bronchioloalveolar carcinoma: does it correlate with the 1999 World Health Organization definition? Am J Clin Pathol 2004;122:44-50. [Crossref] [PubMed]
- Edgerton F, Rao U, Takita H, et al. Bronchio-alveolar carcinoma. A clinical overview and bibliography. Oncology 1981;38:269-73. [Crossref] [PubMed]
- Spiro SG, Lopez-Vidriero M-T, Charman J, et al. Bronchorrhoea in a case of alveolar cell carcinoma. J Clin Pathol 1975;28:60-5. [Crossref] [PubMed]
- Dwek JH, Charytan C, Stachura I, et al. Salt-wasting bronchorrhea and its mechanisms. Arch Intern Med 1977;137:791-4. [Crossref] [PubMed]
- Popat N, Raghavan N, McIvor RA. Severe bronchorrhea in a patient with bronchioloalveolar carcinoma. Chest 2012;141:513-4. [Crossref] [PubMed]
- Homma H, Kira S, Takahashi Y, et al. A case of alveolar cell carcinoma accompanied by fluid and electrolyte depletion through production of voluminous amounts of lung liquids. Am Rev Respir Dis 1975;111:857-62. [PubMed]
- Aquino SL, Chiles C, Halford P. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: do CT criteria work? AJR Am J Roentgenol 1998;171:359-63. [Crossref] [PubMed]
- Jung JI, Kim H, Park SH, et al. CT differentiation of pneumonic-type bronchioloalveolar cell carcinoma and infectious pneumonia. Br J Radiol 2001;74:490-4. [Crossref] [PubMed]
- Wei J, Tang D, Nie Y, et al. Clinical characteristics and prognosis of nonsurgically treated patients with pneumonic-type adenocarcinoma. Medicine (Baltimore) 2019;98:e15420. [Crossref] [PubMed]
- Zong Q, Zhu F, Wu S, et al. Advanced pneumonic type of lung adenocarcinoma: survival predictors and treatment efficacy of the tumor. Tumori 2021;107:216-25. [PubMed]
- Wislez M, Massiani MA, Milleron B, et al. Clinical characteristics of pneumonic-type adenocarcinoma of the lung. Chest 2003;123:1868-77. [Crossref] [PubMed]
- Poletti V, Casoni GL, Cancellieri A, et al. Diffuse alveolar damage. Pathologica 2010;102:453-63. [PubMed]
- Linssen KC, Jacobs JA, Poletti VE, et al. Reactive type II pneumocytes in bronchoalveolar lavage fluid. Acta Cytol 2004;48:497-504. [Crossref] [PubMed]
- Bonaccorsi A, Cancellieri A, Chilosi M, et al. Acute interstitial pneumonia: report of a series. Eur Respir J 2003;21:187-91. [Crossref] [PubMed]
- Stanley MW, Henry-Stanley MJ, Gajl-Peczalska KJ, et al. Hyperplasia of type II pneumocytes in acute lung injury. Cytologic findings of sequential bronchoalveolar lavage. Am J Clin Pathol 1992;97:669-77. [Crossref] [PubMed]
- Grigoriu B, Jacobs F, Beuzen F, et al. Bronchoalveolar lavage cytological alveolar damage in patients with severe pneumonia. Crit Care 2006;10:R2. [Crossref] [PubMed]
- Grotte D, Stanley MW, Swanson PE, et al. Reactive type II pneumocytes in bronchoalveolar lavage fluid from adult respiratory distress syndrome can be mistaken for cells of adenocarcinoma. Diagn Cytopathol 1990;6:317-22. [Crossref] [PubMed]
- Soares M, Toffart AC, Timsit JF, et al. Intensive care in patients with lung cancer: a multinational study. Ann Oncol 2014;25:1829-35. [Crossref] [PubMed]
- Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med 2009;35:2044-50. [Crossref] [PubMed]
- Hallin J, Engstrom LD, Hargis L, et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov 2020;10:54-71. [Crossref] [PubMed]
- Liu J, Shen J, Yang C, et al. High incidence of EGFR mutations in pneumonic-type non-small cell lung cancer. Medicine (Baltimore) 2015;94:e540. [Crossref] [PubMed]
- Chang JC, Offin M, Falcon C, et al. Comprehensive Molecular and Clinicopathologic Analysis of 200 Pulmonary Invasive Mucinous Adenocarcinomas Identifies Distinct Characteristics of Molecular Subtypes. Clin Cancer Res 2021;27:4066-76. [Crossref] [PubMed]


