
EGFR mutation types and abundance were associated with the overall survival of advanced lung adenocarcinoma patients receiving first-line tyrosine kinase inhibitors
Introduction
Epidermal growth factor receptor (EGFR) is a transmembrane receptor tyrosine kinase frequently over-expressed in non-small cell lung cancer (NSCLC) (1). Exon 19 deletions (19DEL) and exon 21 point mutation (L858R), accounting for up to 85–90% of all mutations (2-6), are hypersensitive to small molecule EGFR tyrosine kinase inhibitors (EGFR-TKIs) (7-10), leading to molecularly stratified therapy of NSCLC, including treatment with EGFR-TKIs (11).
EGFR-TKIs, which are anticancer agents that block overactive EGFR signaling in cancer cells (12), are currently recognized as the standard first-line treatment for advanced NSCLC patients with EGFR mutations (13). Several prospective trials have demonstrated remarkable efficacy of treatment with EGFR-TKIs, resulting in higher objective response rates (ORRs), longer progression-free survival (PFS), and few adverse effects when compared to chemotherapy in NSCLC patients harboring sensitive EGFR mutations (14-21). However, 2 exon 20 mutations including exon 20 point mutation (T790M) (22,23) and E20 insertions (24) confer primary resistance (T790M accounts for about 70%) (25) to the action of EGFR-TKIs on activating mutations by inducing a conformation change that re-activates the tyrosine kinase domain. The frequency of doublets overall is 6%, and T790M mutation is frequent in doublets but uncommon in singlets (26). With respect to echinoderm microtubule-associated protein-like 4 (EML4) anaplastic lymphoma kinase (ALK) translocation/EGFR co-alterations, Ulivi et al. conducted a study of 28 patients with EGFR mutation who underwent first-line EGFR-TKI treatment and demonstrated a clinical benefit on the disease control rate (DCR) in 81.8% of patients with EGFR mutations only, which was higher than the 67% of the 6 co-altered patients who received an EGFR-TKI (27). Recent studies (28,29) have demonstrated spatial heterogeneity in NSCLC, as on average, spatially heterogeneous mutations are present in 30–40% of a tumor mass. EGFR mutation status alone is not sufficient to predict outcome. Zhou et al. demonstrated that relatively high EGFR mutation abundance could predict which NSCLC patient subpopulation would have a PFS benefit from EGFR-TKI treatment (30) using direct sequencing and the amplification refractory mutation system (ARMS) assay (with different sensitivity). A qualitative detection method for EGFR mutations is not sufficient to guide precise targeted therapy in clinical practice. Zhao et al. demonstrated that the quantitative abundance of EGFR mutations may be a potential predictor to select patients with acquired resistance to primary EGFR-TKIs that would benefit from EGFR-TKI re-administration (31). Li et al. (32) quantitatively evaluated the abundance of EGFR mutations for patients with advanced NSCLC and showed that the high abundance group had a median PFS benefit, and similar results were also observed in a study by Wang et al. (33). Plasma EGFR mutation abundance was also found to affect the clinical response to first-line EGFR-TKIs in patients with advanced NSCLC (34). Overall survival (OS) is an important index for evaluating antitumor therapy and could evaluate whether the treatment regimens or test indicators can be included in guidelines. However, these previous studies investigated PFS as the clinical outcome, while overall survival (OS) was not explored.
In the current study, we determined the EGFR mutational status of advanced NSCLC patients receiving first-line EGFR-TKI treatment and investigated the correlation between EGFR mutation types, abundance, and the OS and PFS of these patients. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-755/rc).
Methods
Patients
This retrospective cohort study was conducted at the Affiliated Cancer Hospital of Zhengzhou University (Zhengzhou, China), and all the patients were admitted to our hospital between January 2013 and November 2016. Patients were eligible if: (I) they were ≥18 years of age; (II) they had an Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 2 or less; (III) they underwent computed tomography (CT) and had a pathologically confirmed diagnosis of advanced lung adenocarcinoma with EGFR mutation (19DEL or L858R). Patients with severe comorbidities, tumors other than NSCLC, active infection and pulmonary fibrosis, or severe heart disease were excluded. They were followed up every three months until lost or dead.
The study protocol was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2019060) and the study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. Patient consent was not required because of the retrospective nature of the study. Patient data were anonymized.
Patient evaluation
The following clinical parameters were recorded from electronic records by retrospective collection: age, sex, smoking history, ECOG-PS, TNM stage without any treatment, the whole treatment process, EGFR mutation types, and abundance. Tumor stages were determined according to the 7th edition of the TNM classification (35). All patients were evaluated monthly with physical examination including ECOG-PS and routine laboratory investigations. Routine surveillance imaging included chest and abdomen CT and brain magnetic resonance imaging (MRI). Patients underwent bone scans when bone metastasis was suspected. The treatment response assessment was performed 1 month after the initiation of EGFR-TKI treatment and subsequently every 2 months according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) (36) until they developed signs of disease progression or death from any cause. Additional assessment was performed at any time when signs or symptoms suggested disease progression.
EGFR mutation testing
EGFR mutations and abundance in tumor specimens of eligible NSCLC patients without any treatment were analyzed by the ARMS specific mutation quantitation (ARMS+) (32). All samples had sufficient tumor tissues for analysis. If the tumor content was observed to be <50%, the samples were trimmed to satisfy the criteria. Multiple cores per biopsy were analyzed. Microdissection was performed for the formalin-fixed paraffin-embedded (FFPE) blocks to reduce stroma/normal tissue/necrosis contamination. Extracted DNA was from the FFPE block core or slides. If extraction was performed from cores, multiple, spatially diverse cores were taken. If extraction was performed from slides, slides from multiple depths were used. Viable tumor cellularity was checked before performing DNA extraction. Genomic DNA was extracted from 1 FFPE block using a QIAmp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). DNA concentration was adjusted to 20–50 ng/mL. EGFR mutations including exon 19DEL, exon 21 L858R, exon 20 T790M, and exon 18 mutations (G719X) were detected using a Human EGFR Gene Mutations Quantitative Detection Kit (TB002, Beijing ACCB Biotech, Beijing, China). The assay was carried out according to the manufacturer’s protocol with the MX3000P Real-Time PCR system (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA). Mutation abundance was calculated by copies of EGFR mutation divided by copies of EGFR locus and the cut-off values for 19DEL and L858R were 4.9% and 9.5%, respectively (32).
Treatments
Patients harboring sensitive EGFR mutations were treated with first-line EGFR-TKIs including icotinib (125 mg 3 times a day), erlotinib (150 mg once a day), and gefitinib (250 mg once a day). EGFR-TKIs were continued until disease progression as defined by RECIST guidelines (36) or death from any cause. Upon progression, patients received second- or further-line platinum-based chemotherapy, single-agent chemotherapy, first-generation EGFR-TKI if EGFR mutation testing performed on the re-biopsied tissue when they developed progression showed sensitive EGFR mutations (either 19DEL or L858R), or best supportive care (BSC).
Study outcomes
The primary outcome of the study was OS, which was calculated from the first day of first-line EGFR-TKI treatment to death from any cause or the last day of follow-up. The secondary outcomes included PFS and ORR. PFS was calculated from the first day of first-line EGFR-TKI therapy to disease progression or death from any cause. ORR was defined as the percentage of patients having either a complete response (CR) or a partial response (PR). DCR was defined as the percentage of patients with stable disease (SD), CR, or PR. The last follow-up was conducted in December 2017.
Statistical analysis
All analyses were performed using the Statistical Package for the Social Sciences 18.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using a χ2 test or Fisher’s exact test. OS and PFS were compared between the 2 groups using the Kaplan-Meier method, and 95% confidence intervals (CI) were presented. Univariate analysis was carried out and variables with P<0.1 (age, performance status, tumor stage, tumor mutation types and abundance) were entered into multivariate Cox regression to evaluate prognostic factors. P<0.05 was considered statistically significant.
Results
Patient demographic and baseline characteristics
The study flow chart is shown in Figure 1. A total of 1,192 NSCLC patients were screened positive for 19DEL or L858R mutations. Among them, 833 patients who did not receive first-line EGFR-TKI therapy were excluded, 20 patients with NSCLC other than adenocarcinoma were not included, and 103 patients with incomplete follow-up data were also excluded. Finally, 236 EGFR-mutated lung adenocarcinoma patients who received first-line EGFR-TKI treatment were included in this retrospective cohort study. The demographic and baseline characteristics of the patients are shown in Table 1. More patients were female (58.1%) and the majority were never smokers (74.6%). Most patients had an ECOG PS score of 0 or 1 (84.7%) and 93.2% were TNM stage IV.
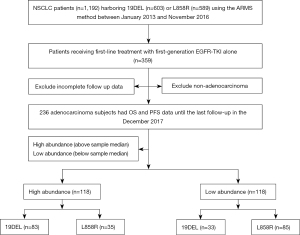
Table 1
| Characteristics | Low abundance, n (%) | High abundance, n (%) | Chi-square (χ2) value | P value |
|---|---|---|---|---|
| Age (years) | 0.623 | 0.430 | ||
| ≤60 | 30 (45.5) | 87 (51.2) | ||
| >60 | 36 (54.5) | 83 (48.8) | ||
| Sex | 0.008 | 0.927 | ||
| Female | 38 (57.6) | 99 (58.2) | ||
| Male | 28 (42.4) | 71 (41.8) | ||
| Smoking history | 0.351 | 0.553 | ||
| Yes | 15 (22.7) | 45 (26.5) | ||
| No | 51 (77.3) | 125 (73.5) | ||
| ECOG-PS | 0.186 | 0.667 | ||
| 0–1 | 57 (86.4) | 143 (84.1) | ||
| 2 | 9 (13.6) | 27 (15.9) | ||
| Mutation types prior to treatment | 31.807 | <0.001 | ||
| 19DEL | 13 (19.7) | 103 (60.6) | ||
| L858R | 53 (80.3) | 67 (39.4) | ||
| TNM stage | 0.774 | 0.379 | ||
| IIB | 6 (9.1) | 10 (5.9) | ||
| IV | 60 (90.9) | 160 (94.1) | ||
| First-line EGFR-TKI | 1.438 | 0.487 | ||
| Erlotinib | 15 (22.7) | 32 (18.8) | ||
| Icotinib | 13 (19.7) | 26 (15.3) | ||
| Gefitinib | 38 (57.6) | 112 (65.9) | ||
| Second- or further-line treatment | 2.448 | 0.485 | ||
| Platinum-based chemotherapy | 25 (37.9) | 80 (47.1) | ||
| Single-agent chemotherapy | 15 (22.7) | 27 (15.9) | ||
| First-generation EGFR-TKI | 21 (31.8) | 48 (28.2) | ||
| BSC | 5 (7.6) | 15 (8.8) | ||
| Mutation types rebiopsy | 34.732 | <0.001 | ||
| 19DEL | 12 (18.2) | 100 (58.8) | ||
| L858R | 51 (77.3) | 64 (37.6) | ||
| 19DEL/T790M | 1 (1.5) | 1 (0.6) | ||
| L858R/T790M | 1 (1.5) | 3 (1.8) | ||
| T790M | 1 (1.5) | 2 (1.2) |
Yes (smoking history): smokers are defined as current smokers smoking >100 cigarettes/lifetime, or smoking >100 cigarettes/lifetime but abstinence from smoking for less than 1 year on the day before the start of therapy. No (smoking history): no smokers are defined as smoking <100 cigarettes/lifetime. ECOG-PS, Eastern Cooperative Oncology Group performance status; TNM, tumor-node-metastasis; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; BSC, best supportive care; 19DEL/T790M, 19DEL/T790M co-mutations; L858R/T790M, L858R/T790M co-mutations.
EGFR mutation testing revealed 19DEL mutation in 116 (49.2%) patients and L858R mutation in 120 (50.8%) patients. The high and low mutation abundance groups were comparable in terms of demographic and baseline variables. EGFR mutation testing was performed on the re-biopsied tissue when patients developed progression. The results revealed 19DEL mutation in 47.5% (112/236) of patients, L858R mutation in 48.7% (115/236) of patients, 19DEL/T790M co-mutations in 0.8% (2/236) of patients, and L858R/T790M co-mutations in 1.7% (4/236) of patients, while T790M mutation was only present in 1.3% (3/236) of patients (Table 1).
Treatment characteristics
In terms of first-line treatment, 47 patients received erlotinib, 39 received icotinib, and 150 received gefitinib. As the second-line or further-line treatment, 25 (37.9%) and 80 (47.1%) patients received platinum-based combination chemotherapy in the low and the high abundance groups, respectively. Additionally, in the low and high abundance groups, respectively, 15 (22.7%) and 27 (15.9%) patients received single-agent chemotherapy, 21 (31.8%) and 48 (28.2%) patients received first-generation EGFR-TKI treatment, and 5 (7.6%) and 15 (8.8%) received BSC (Table 1).
OS
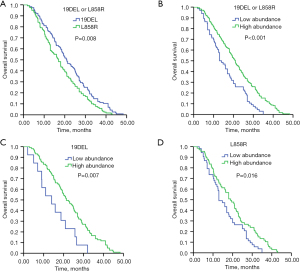
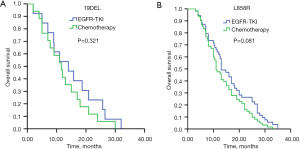
The median follow-up duration was 23.2 months (95% CI: 14.9–26.7 months). Patients carrying 19DEL mutation had markedly longer OS (20.9 months, 95% CI: 17.7–24.1 months) than patients with L858R mutation (17.0 months, 95% CI: 14.4–19.6 months) (P=0.008) (Figure 2A). Patients with high abundance had significantly longer OS (20.9 months, 95% CI: 18.3–23.5 months) than those with low abundance (13.0 months, 95% CI: 10.3–15.7 months) (P<0.001) (Figure 2B). In the subgroup analysis that included 19DEL mutation only, patients with high mutation abundance had significantly longer OS (21.4 months, 95% CI: 17.4–25.3 months) than those with low mutation abundance (14.0 months, 95% CI: 6.1–21.9 months) (P=0.007) (Figure 2C). In the subgroup analysis that included L858R mutation only, patients with high abundance also had significantly longer OS (19.0 months, 95% CI: 14.6–23.4 months) than those with low abundance (13.0 months, 95% CI: 10.0–16.0 months) (P=0.016) (Figure 2D). Further analysis of 19DEL mutation only showed that patients with low mutation abundance who received first-line EGFR-TKI treatment had significantly longer OS (14.0 months, 95% CI: 6.1–21.9 months) than patients who received first-line chemotherapy (11.6 months, 95% CI: 7.7–15.5 months), with no significant difference (P=0.321) (Figure 3A). In the subgroup analysis that included L858R mutation only, patients with low mutation abundance who received first-line EGFR-TKI treatment had significantly longer OS (13.0 months, 95% CI: 10.0–16.0 months) than patients who received first-line chemotherapy (11.0 months, 95% CI: 9.1–12.9 months), with no significant difference (P=0.081) (Figure 3B).
Univariate analysis showed associations between OS and age (≤60) (HR: 0.51, 95% CI: 0.44–0.92, P=0.003), ECOG-PS (0–1) (HR: 0.49, 95% CI: 0.41–0.85, P=0.038), TNM stage (IIIB) (HR: 0.37, 95% CI: 0.21–0.54, P=0.027), 19DEL mutation (HR: 0.42, 95% CI: 0.31–0.69, P=0.009), high abundance (HR: 0.53, 95% CI: 0.41–0.77, P=0.002), DCR (HR: 0.75, 95% CI: 0.65–0.84, P=0.036), and subsequent therapy (chemoradiotherapy or BSC) (HR: 0.60, 95% CI: 0.52–0.76, P=0.025). In the multivariate Cox regression analysis, longer OS was independently associated with age (≤60 years) (HR: 0.64, 95% CI: 0.56–0.75, P=0.032), ECOG-PS (0–1) (HR: 0.50, 95% CI: 0.41–0.73, P=0.041), TNM stage (IIIB) (HR: 0.52, 95% CI: 0.44–0.62, P=0.047), 19DEL mutation (HR: 0.48, 95% CI: 0.39–0.67, P=0.033), high abundance (HR: 0.62, 95% CI: 0.50–0.79, P=0.027), and subsequent therapy (chemoradiotherapy or BSC) (HR: 0.72, 95% CI: 0.58–0.88, P=0.042) (Table 2).
Table 2
| Variables | Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age, years (≤60, >60) | 0.51 (0.44–0.92) | 0.003 | 0.64 (0.56–0.75) | 0.032 | |
| Sex (female, male) | 0.74 (0.53–1.02) | 0.17 | – | – | |
| Smoking history (yes, no) | 0.68 (0.39–0.90) | 0.45 | – | – | |
| ECOG-PS (0–1, 2) | 0.49 (0.41–0.85) | 0.038 | 0.50 (0.41–0.73) | 0.041 | |
| TNM stage (IIIB, IV) | 0.37 (0.21–0.54) | 0.027 | 0.52 (0.44–0.62) | 0.047 | |
| Mutation types (19DEL, L858R) | 0.42 (0.31–0.69) | 0.009 | 0.48 (0.39–0.67) | 0.033 | |
| Abundance (high, low) | 0.53 (0.41–0.77) | 0.002 | 0.62 (0.50–0.79) | 0.027 | |
| EGFR-TKI (erlotinib, icotinib, gefitinib) | 0.67 (0.35–0.82) | 0.059 | – | – | |
| Initial EGFR-TKI response (DCR, PD) | 0.75 (0.65–0.84) | 0.036 | 0.86 (0.68–0.89) | 0.058 | |
| Subsequent therapy (chemoradiotherapy or BSC) | 0.60 (0.52–0.76) | 0.025 | 0.72 (0.58–0.88) | 0.042 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; TNM, tumor-node-metastasis; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; DCR, disease control rate; PD, progressive disease; BSC, best supportive care.
Secondary outcomes
PFS
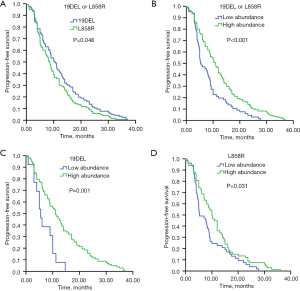
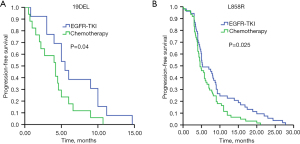
Patients carrying 19DEL mutation had markedly longer PFS (10.2 months, 95% CI: 8.5–11.9 months) than patients with L858R mutation (8.3 months, 95% CI: 7.1–9.5 months) (P=0.046) (Figure 4A). PFS was significantly longer in the high mutation abundance group (11.0 months, 95% CI: 9.7–12.3 months) than the low abundance group (5.3 months, 95% CI: 3.6–7.0 months) (P<0.001) (Figure 4B). In the subgroup analysis that included 19DEL mutation only, patients with high mutation abundance had significantly longer PFS (11.0 months, 95% CI: 8.8–13.1 months) than those with low mutation abundance (5.5 months, 95% CI: 3.7–7.2 months) (P=0.001) (Figure 4C). In the subgroup analysis that included L858R mutation only, patients with high mutation abundance also had significantly longer PFS (10.1 months, 95% CI: 7.8–12.4 months) than those with low mutation abundance (5.3 months, 95% CI: 3.0–7.6 months) (P=0.031) (Figure 4D). Further analysis of 19DEL mutation only showed that patients with low mutation abundance who received first-line EGFR-TKI treatment had significantly longer PFS (5.5 months, 95% CI: 3.7–7.2 months) than patients who received first-line chemotherapy (4.2 months, 95% CI: 2.2–6.1 months) (P=0.04) (Figure 5A). Further analysis of L858R mutation only showed that patients with low mutation abundance who received first-line EGFR-TKI treatment had significantly longer PFS (5.3 months, 95% CI: 3.0–7.6 months) than patients who received first-line chemotherapy (4.6 months, 95% CI: 3.4–5.8 months) (P=0.025) (Figure 5B).
Univariate analysis revealed that 19DEL mutation (HR: 0.52, 95% CI: 0.39–0.89, P=0.019), high mutation abundance (HR: 0.60, 95% CI: 0.51–0.92, P=0.022), and DCR (HR: 0.85, 95% CI: 0.65–0.94, P=0.039) were prognostic determinants of PFS. Multivariate Cox regression analysis showed that 19DEL mutation (HR: 0.56, 95% CI: 0.42–0.60, P=0.041), high mutation abundance (HR: 0.77, 95% CI: 0.66–0.82, P=0.037), and DCR (HR: 0.68, 95% CI: 0.51–0.89, P=0.045) were independent prognostic determinants of PFS (Table 3).
Table 3
| Variables | Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age, years (≤60, >60) | 0.78 (0.64–0.92) | 0.21 | – | – | |
| Sex (female, male) | 0.74 (0.53–0.86) | 0.50 | – | – | |
| Smoking history (yes, no) | 0.69 (0.49–0.81) | 0.36 | – | – | |
| ECOG-PS (0–1, 2) | 0.75 (0.52–0.83) | 0.075 | 0.87 (0.74–0.93) | 0.064 | |
| TNM stage (IIIB, IV) | 0.43 (0.35–0.59) | 0.051 | 0.53 (0.45–0.61) | 0.059 | |
| Mutation types (19DEL, L858R) | 0.52 (0.39–0.89) | 0.019 | 0.56 (0.42–0.60) | 0.041 | |
| Abundance (high, low) | 0.60 (0.51–0.92) | 0.022 | 0.77 (0.66–0.82) | 0.037 | |
| EGFR-TKI (erlotinib, icotinib, gefitinib) | 0.37 (0.28–0.45) | 0.44 | – | – | |
| Initial EGFR-TKI response (DCR, PD) | 0.85 (0.65–0.94) | 0.039 | 0.68 (0.51–0.89) | 0.045 | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; TNM, tumor-node-metastasis; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; DCR, disease control rate; PD, progressive disease.
ORR and DCR
Patients with high mutation abundance had a significantly higher ORR and DCR than those with low mutation abundance in subjects carrying 19DEL or L858R mutations (ORR: 67.10% versus 28.80%, P<0.001; DCR: 94.10% versus 71.20%, P<0.001, respectively). Similar results regarding ORR and DCR were observed in the subgroup analysis of patients carrying 19DEL and L858R mutations (Table 4).
Table 4
| Efficacy | 19DEL or L858R (n=236) | 19DEL (n=116) | L858R (n=120) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low abundance | High abundance | χ2 | P | Low abundance | High abundance | χ2 | P | Low abundance | High abundance | χ2 | P | |||
| PR | 19 | 114 | 4 | 72 | 15 | 42 | ||||||||
| SD | 28 | 46 | 6 | 27 | 22 | 19 | ||||||||
| PD | 19 | 10 | 3 | 4 | 16 | 6 | ||||||||
| ORR | 28.80% | 67.10% | 28.311 | <0.001 | 30.80% | 69.90% | 7.825 | 0.01 | 28.30% | 62.70% | 14.030 | <0.001 | ||
| DCR | 71.20% | 94.10% | 23.143 | <0.001 | 76.90% | 96.10% | 7.499 | 0.03 | 69.80% | 91.00% | 8.911 | 0.003 | ||
ORR, overall response rate; DCR, disease control rate; EGFR, epidermal growth factor receptor; PR, partial response; SD, stable disease; PD, progressive disease;
Discussion
Delineation of gene mutations of NSCLC has led to molecularly stratified therapy for NSCLC and has improved lung adenocarcinoma management. The emergence of intratumor heterogeneity, including individual tumor biopsies and spatial separation between biopsies of the same tumor (37), may impact the tumor biopsy strategy, actionable targets, and individualized targeted treatment (37-40). In the present study, multiple cores per biopsy were analyzed. If extracted DNA was from the FFPE block cores, spatially diverse cores were taken. If extraction was from slides, slides from multiple depths were used. Tumor sampling bias may arise due to differences between and within single biopsies or metastatic sites, and between the primary and metastatic sites (37). Heterogeneity is also dynamic and evolves over time (41). To some extent, the repeatability of mutation abundance measurement was not satisfactorily reproducible.
Both the LUX-Lung 3 and LUX-Lung 6 trials demonstrated that first-line afatinib significantly improved the OS of patients with 19DEL mutation, while those with L858R mutation did not show improved OS (42). Several retrospective studies (43-45) and a prospective study (46) also showed that 19DEL mutation was associated with better efficacy than L858R mutation in patients receiving first-line EGFR-TKIs. A previous study also showed that osimertinib was equally effective for NSCLC patients with various abundance of T790M mutation (47). The current study indicated that patients carrying 19DEL mutation had better OS and PFS than those with L858R mutation in response to first-line EGFR-TKI therapy. However, this was not observed in a retrospective cohort study which showed that the median OS of patients treated with a first-line EGFR-TKI with 19DEL and L858R mutation was 32.7 and 24.7 months, respectively (95% CI: 16.8–48.6 and 20.8–28.5 months, respectively, P=0.174) (48).
The status of EGFR mutation types is not well studied, quantification of EGFR mutations for molecular stratification of NSCLC patients has only recently drawn the attention of clinical investigators. In the present study, we carried out quantitative analysis of 19DEL and L858R mutations in advanced lung adenocarcinoma patients on EGFR-TKI therapy. Our study demonstrated that high EGFR mutation abundance correlated with the clinical outcomes of NSCLC patients receiving EGFR-TKI therapy. In addition, our study showed that 88.8% (103/116) of patients with 19DEL mutation exhibited high mutation abundance, while 55.8% (67/120) of patients with L858R mutation exhibited high mutation abundance. The OS benefit of patients with 19DEL mutation may be due to the higher proportion of high mutation abundance in patients with L858R mutation.
Patients with high EGFR mutation abundance had significantly longer OS and PFS versus those with low EGFR mutation abundance of 19DEL or L858R, and similar results were obtained from our subgroup analysis in patients with high mutation abundance carrying 19DEL only or L858R only mutations. These findings indicate that EGFR mutation abundance could be used to help stratify NSCLC patients for EGFR-TKI therapy. We further observed that EGFR mutation abundance was significantly associated with response to EGFR-TKIs. ORR and DCR were significantly higher in patients with high mutation abundance than those with low mutation abundance, as well as with 19DEL or L858R mutation. Our observations were similar to a previous study (32). Our study also showed that patients with low mutation abundance who received first-line EGFR-TKIs had significantly longer PFS than patients who received first-line standard chemotherapy. These results were similar to those of the blind independent central review (BICR) (49), but there was no significant difference in OS, as confounding factors such as subsequent treatment were present.
Hence, both EGFR mutation types and mutation abundance are associated with outcome of EGFR-TKI treatment for advanced lung adenocarcinoma. These observations warrant confirmation in a large prospective study in the future. Exploration of the biological mechanisms of the differences between high and low mutation abundance should be undertaken. With respect to spatial diversity in genomic instability processes, evidence suggests that opportunities to exploit such mechanisms therapeutically may be limited for patients with NSCLC (50).
Our study has several limitations. First, our study is a single center study, with the majority of patients being Chinese. This limits the generalizability of our findings. Second, our study was a retrospective cohort study and could not establish a causal relationship. The mutation abundance described in the present study was based on ARMS plus technology. Droplet digital PCR (ddPCR) has become a well-developed method for rapidly and quantitatively assessing EGFR mutations with higher specificity and sensitivity (51-53).
In conclusion, both EGFR mutation types and mutation abundance may be prognostic determinants of lung adenocarcinoma patients receiving first-line EGFR-TKIs, and 19DEL and high EGFR mutation abundance may predict significantly longer OS and PFS of stage IIIB/IV lung adenocarcinoma patients receiving first-line EGFR-TKI therapy.
Acknowledgments
Funding: The study was supported by Henan Province Health and Youth Subject Leader Training Project (No. [2020]60); Leading Talent Cultivation Project of Henan Health Science and Technology Innovation Talents (No. YXKC2020009); ZHONGYUAN QIANREN JIHUA (No. ZYQR201912118); Henan International Joint Laboratory of drug resistance and reversal of targeted therapy for lung cancer (No. [2021]10); Henan Medical Key Laboratory of Refractory lung cancer (No. 2020]27); Henan Refractory Lung Cancer Drug Treatment Engineering Technology Research Center (No. [2020]4); National Natural Science Foundation of China (NSFC) (No. 81872204); Huilan Charity Fund (No. HL-HS2020-129); Key Science and Technology Research Project of Henan Provincial Department of Science and Technology (No. 212102310117); Project Jointly Built of Provincial Department by Henan Provincial Health Commission (No. SB201901106); Project Jointly Built by Henan Provincial Health Commission (No. LHGJ20200158).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-755/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-755/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-755/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2019060) and the study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. Patient consent was not required because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boch C, Kollmeier J, Roth A, et al. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open 2013;3:e002560. [Crossref] [PubMed]
- Yu Q, Huang F, Zhang M, et al. Multiplex picoliter-droplet digital PCR for quantitative assessment of EGFR mutations in circulating cell-free DNA derived from advanced non-small cell lung cancer patients. Mol Med Rep 2017;16:1157-66. [Crossref] [PubMed]
- Peng M, Weng YM, Liu HL, et al. Clinical Characteristics and Survival Outcomes for Non-Small-Cell Lung Cancer Patients with Epidermal Growth Factor Receptor Double Mutations. Biomed Res Int 2018;2018:7181368. [Crossref] [PubMed]
- Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol 2015;16:e447-59. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Roskoski R Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res 2019;139:395-411. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [Crossref] [PubMed]
- Cascone T, Martinelli E, Morelli MP, et al. Epidermal growth factor receptor inhibitors in non-small-cell lung cancer. Expert Opin Drug Discov 2007;2:335-48. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354-6. [Crossref] [PubMed]
- Chiu CH, Ho HL, Chiang CL, et al. Clinical characteristics and treatment outcomes of lung adenocarcinomas with discrepant EGFR mutation testing results derived from PCR-direct sequencing and real-time PCR-based assays. J Thorac Oncol 2014;9:91-6. [Crossref] [PubMed]
- Azzoli CG, Baker S Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 2005;353:207-8. [Crossref] [PubMed]
- Chen Z, Feng J, Saldivar JS, et al. EGFR somatic doublets in lung cancer are frequent and generally arise from a pair of driver mutations uncommonly seen as singlet mutations: one-third of doublets occur at five pairs of amino acids. Oncogene 2008;27:4336-43. [Crossref] [PubMed]
- Ulivi P, Chiadini E, Dazzi C, et al. Nonsquamous, Non-Small-Cell Lung Cancer Patients Who Carry a Double Mutation of EGFR, EML4-ALK or KRAS: Frequency, Clinical-Pathological Characteristics, and Response to Therapy. Clin Lung Cancer 2016;17:384-90. [Crossref] [PubMed]
- Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014;346:256-9. [Crossref] [PubMed]
- de Bruin EC, McGranahan N, Mitter R, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 2014;346:251-6. [Crossref] [PubMed]
- Zhou Q, Zhang XC, Chen ZH, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:3316-21. [Crossref] [PubMed]
- Zhao ZR, Wang JF, Lin YB, et al. Mutation abundance affects the efficacy of EGFR tyrosine kinase inhibitor readministration in non-small-cell lung cancer with acquired resistance. Med Oncol 2014;31:810. [Crossref] [PubMed]
- Li X, Cai W, Yang G, et al. Comprehensive Analysis of EGFR-Mutant Abundance and Its Effect on Efficacy of EGFR TKIs in Advanced NSCLC with EGFR Mutations. J Thorac Oncol 2017;12:1388-97. [Crossref] [PubMed]
- Wang H, Zhang M, Tang W, et al. Mutation abundance affects the therapeutic efficacy of EGFR-TKI in patients with advanced lung adenocarcinoma: A retrospective analysis. Cancer Biol Ther 2018;19:687-94. [Crossref] [PubMed]
- Wang X, Liu Y, Meng Z, et al. Plasma EGFR mutation abundance affects clinical response to first-line EGFR-TKIs in patients with advanced non-small cell lung cancer. Ann Transl Med 2021;9:635. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 2010;195:W221-8. [Crossref] [PubMed]
- Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res 2012;72:4875-82. [Crossref] [PubMed]
- Yap TA, Gerlinger M, Futreal PA, et al. Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med 2012;4:127ps10. [Crossref] [PubMed]
- Swanton C, Larkin JM, Gerlinger M, et al. Predictive biomarker discovery through the parallel integration of clinical trial and functional genomics datasets. Genome Med 2010;2:53. [Crossref] [PubMed]
- Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer 2013;108:479-85. [Crossref] [PubMed]
- Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012;120:1067-76. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [Crossref] [PubMed]
- Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:3908-14. [Crossref] [PubMed]
- Wang H, Huang J, Yu X, et al. Different efficacy of EGFR tyrosine kinase inhibitors and prognosis in patients with subtypes of EGFR-mutated advanced non-small cell lung cancer: a meta-analysis. J Cancer Res Clin Oncol 2014;140:1901-9. [Crossref] [PubMed]
- Kim DW, Lee SH, Lee JS, et al. A multicenter phase II study to evaluate the efficacy and safety of gefitinib as first-line treatment for Korean patients with advanced pulmonary adenocarcinoma harboring EGFR mutations. Lung Cancer 2011;71:65-9. [Crossref] [PubMed]
- Pan G, Chen K, Yu X, et al. The correlation between the abundance of EGFR T790M mutation and osimertinib response in advanced non-small cell lung cancer. Transl Cancer Res 2021;10:2895-905. [Crossref] [PubMed]
- Yao ZH, Liao WY, Ho CC, et al. Real-World Data on Prognostic Factors for Overall Survival in EGFR Mutation-Positive Advanced Non-Small Cell Lung Cancer Patients Treated with First-Line Gefitinib. Oncologist 2017;22:1075-83. [Crossref] [PubMed]
- Wu YL, Saijo N, Thongprasert S, et al. Efficacy according to blind independent central review: Post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EGFR mutation-positive advanced NSCLC. Lung Cancer 2017;104:119-25. [Crossref] [PubMed]
- Burrell RA, McGranahan N, Bartek J, et al. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013;501:338-45. [Crossref] [PubMed]
- Zhu G, Ye X, Dong Z, et al. Highly Sensitive Droplet Digital PCR Method for Detection of EGFR-Activating Mutations in Plasma Cell-Free DNA from Patients with Advanced Non-Small Cell Lung Cancer. J Mol Diagn 2015;17:265-72. [Crossref] [PubMed]
- Feng WN, Gu WQ, Zhao N, et al. Comparison of the SuperARMS and Droplet Digital PCR for Detecting EGFR Mutation in ctDNA From NSCLC Patients. Transl Oncol 2018;11:542-5. [Crossref] [PubMed]
- Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014;20:1698-705. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


