
Positron emission tomography/computed tomography and endobronchial ultrasound-guided transbronchial needle aspiration to evaluate the status of N2 in preoperative non-small cell lung cancer: a diagnostic test
Introduction
According to the latest statistics, among all malignant tumors affecting the Chinese population, lung cancer has the highest incidence and mortality, and its incidence is increasing annually (1). Among the various types of lung cancer, non-small cell lung cancer (NSCLC) accounts for about 80% (2). Lymph node metastasis is most common NSCLC metastasis, and tumor cells are usually transferred from the pulmonary lymph nodes to the hilar lymph nodes along the lymphatic drainage route, to the mediastinal lymph nodes, and further to distant metastases. Treatment programs and patient prognosis vary according to the stage of NSCLC of the individual patient. In patients with stage I and II of NSCLC surgical resection is the preferred treatment regimen. For patients with stage IIIA NSCLC, if there is no occurrence of mediastinal lymph node metastasis, surgical treatment is preferred, accompanied by postoperative adjuvant radiotherapy and chemotherapy (3). If mediastinal lymph node metastasis has occurred, preoperative neoadjuvant chemotherapy is necessary and associated with better patient survival than surgery (4). Therefore, for NSCLC patients, to enable accurate staging and formulation of a personalized treatment program and prognosis, precise diagnosis of mediastinal lymph node metastasis is of great significance (5).
For the diagnosis of mediastinal lymph nodes, mediastinoscopy has been considered the gold standard, with the advantage that it can be conducted under direct vision and can obtain pathological specimens. However, patients are required to undergo general anesthesia for mediastinoscopy; it is costly and traumatic for the patient, all of which hinder its wide clinical application. In recent years, positron emission tomography/computed tomography (PET/CT) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) have been used as new metastatic mediastinal lymph node septal tests (6). Both methods are increasingly being used in the diagnosis and treatment of NSCLC, with the advantages of non- or minimal-invasiveness and higher metastatic mediastinal lymph node detection.
According to existing clinical studies, EBUS-TBNA is an effective minimally invasive method for the diagnosis of mediastinal lymph nodes in NSCLC (7). A comparative study of EBUS-TBNA and chest CT and PET scans showed that EBUS-TBNA was more accurate than non-invasive methods such as CT and PET in the chest; PET/CT has more anatomical advantages than PET scanning, is more accurate than PET scanning alone or simple visual-related PET scanning plus CT scans in lung cancer staging (8,9). The PET/CT examination is widely used for lung cancer staging. Fetal CT is still the most popular method of preoperative staging of lung cancer. Both PET/CT and EBUS-TBNA are used as further methods for chest CT diagnosis of mediastinal lymph node-positive NSCLC. The limitation of EBUS-TBNA was that difficulty in locating the lymph nodes might cause to puncture failure and tumor tissue might be not within the puncture sampling (7). The disadvantage of PET/CT was that metastasis risk could only be evaluated indirectly by the metabolic change of lymph nodes and iconography characteristics (8,9). So far, no relevant studies have comparatively evaluated the diagnostic value of PET/CT and EBUS-TBNA for these patients. This study investigated the significance of PET/CT and EBUS-TBNA in patients with NSCLC who underwent preoperative thoracic CT to mediate mediastinal lymph node positivity. The study will help surgeons to have a more accurate preoperative assessment of the status of N2 lymph nodes. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-521/rc).
Methods
Participants
We retrospectively identified 421 consecutive NSCLC patients in our hospital from January 2015 to December 2016. The inclusion criteria were as follows: (I) chest CT showed lung lesions, and the final pathological diagnosis was NSCLC; (II) chest CT prompted ipsilateral mediastinal lymph nodes, and short diameter >1 cm; (III) the findings of all patients who underwent brain magnetic resonance (MR), chest and abdomen CT, and body bone scan, and other tests suggested no distant metastasis; (IV) all patients had no other tumor history, were without fever, cough, and sputum, and other lung infection history, with no recent history of tuberculosis; (V) all patients were without thoracic invasive surgery history and had not undergone chemotherapy and other anti-tumor treatment before the test. Patients were excluded if they had incomplete clinical data. Ultimately, 112 patients who met the inclusion criteria were included in this research. Ethical approval was waived by the ethics committee of The Affiliated Hospital of Qingdao University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was a retrospective study, and the informed consent from the patients and their families was abandoned.
Checking method
PET/CT
Our hospital uses the GE Discovery Elite PET/CT (GE Healthcare, Chicago, IL, USA). Patients were required to fast for a minimum of 6 hours, to achieve controlled fasting blood glucose concentration of under 8 mmol/L. After resting quietly indoors in the supine position for 15 minutes, the parents were injected with 0.12 mci/kg 18F-fluorodeoxyglucose (FDG), after which they continued to rest in the supine position for 1 hour. Patients were asked to void their bladder prior to PET/CT examination. Scanning was conducted with the patient in the supine position, hands over their head, breathing evenly and gently. The scan moved from the top of the head to the upper thigh. The setting for CT scanning was as follows: voltage 120 kV, current 100 mA, pitch 0.813, matrix 512×512, and thickness 2.5 mm. For PET collection, the timing was 1.5 minutes/bed, for a total of 10–11 beds. Data were reconstructed to obtain transverse, coronal, sagittal PET, CT, and fusion images.
EBUS-TBNA
All patients underwent EBUS-TBNA with an Olympus fiber bronchial endoscopy (BF-UC260F-OL8; Olympus, Tokyo, Japan) and their supporting components. Patients received a 2% lidocaine injection for airway surface anesthesia, assumed a supine position on the examination bed, and were administered nasal catheter oxygen. They were monitored with a noninvasive electrocardiogram (ECG) accompanied by blood pressure and finger blood oxygen saturation monitoring. The patient was then checked with the conventional fiber bronchus. The left and right bronchial branches were examined in turn. According to the anatomical position information provided by the PET/CT and the thoracic CT, the directional approach for the biopsy was determined. The biopsy was performed with 2% lidocaine injection through the bronze mirror injection hole in the airway to fortify airway surface anesthesia, in order to facilitate endoscopic ultrasound operation. After the completion of the examination, the patient rested in the supine position for 5 minutes and then underwent ultrasound bronchoscopy examination. The fiber-optic ultrasound bronchoscopy was inserted into the airway through the unilateral nostrils, and the initial location of the first bronchoscopy was measured. The location, size, and relationship with the surrounding blood vessels were measured by ultrasonic probe. After calculating and fixing the needle puncture depth, the target lymph node was punctured under ultrasound guidance. When the target area appeared via the strong echo of the puncture needle, a successful puncture was indicated. The specimens were then collected using the negative pressure suction. Each target lymph node was punctured 1–4 times (average 3 times), and a new needle was used for each location to avoid contamination.
Surgery
For EBUS-TBNA, mediastinal lymph node-negative patients underwent thoracoscopic lobectomy and systemic thoracic lymph node dissection. The 4L and 5–12 groups lymph nodes and surrounding soft tissue were cleaned during the left thoracic surgery. The 2R, 3a, 3p, 4R, and 7–12 lymph nodes and surrounding soft tissues were removed by right thoracic surgery.
Assessment of results
The golden diagnosis of N2 status was postoperative pathological results
If clear malignant tumor cells could be seen, suspicious malignant tumor cells were present, or the type of pathology or differentiation were unable to be identified in the lymph nodes, these lymph nodes were metastasis.
PET/CT image analysis
All lymph nodes were grouped according to the grouping criteria developed by the American Joint Committee for Cancer (AJCC). For each lymph node group, the CT values, short diameter, and the maximum standardized uptake value (SUVmax) of each lymph node were recorded. The PET/CT images were analyzed by double-blind method by two senior physicians of nuclear medicine. According to the size, shape, location, concentration of radionuclides, and the SUVmax, the mediastinal lymph nodes were transferred.
EBUS-TBNA puncture specimens
If the smear revealed a number of lymphocyte clusters, the puncture was deemed a success; if several red blood cells or ciliated columnar cells and less lymphocytes were visible on the smear, the puncture was deemed a failure. If clear malignant tumor cells could be seen in any part of a smear, suspicious malignant tumor cells were present, or the type of pathology or differentiation were unable to be identified, that the results were taken as positive. If the smear of all the puncture sites only revealed rich lymphocytes and no malignant tumor cells, then it was taken as negative. Clinical information and reference standard results were made publicly available.
Statistical analysis
All statistical analyses were performed using the software SPSS 21.0 (IBM Corp., Armonk, NY, USA). Pathological results were used as the final diagnosis, and the final pathological results of PET/CT and EBUS-TBNA were compared. We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of PET/CT and EBUS-TBNA assessment of mediastinal lymph nodes. The diagnostic consistency of the two patterns was compared using the McNemar χ2 test. The patients were then divided into two groups according to the pathological type, namely adenocarcinoma group and squamous cell carcinoma group, and the above method was used to calculate and compare. The values of AUC >0.7 suggested that tests have good diagnostic accuracy. All significant comparisons were considered a two-tailed P value <0.05.
Results
A total of 112 eligible patients were retrospectively collected from January 2015 to December 2016, including 71 males and 41 females, aged 40–75 years, with an average age of 58 years. There were 60 cases of adenocarcinoma and 53 cases of squamous cell carcinoma. The lung tumors were located in the left lobe in 46 cases, and in the right lobe in 66 cases. The lymph nodes punctured by EBUS-TBNA were divided into 2 groups, 4 groups, and 7 groups. The general clinical characteristics of the enrolled patients are shown in Table 1.
Table 1
| Variables | Number |
|---|---|
| Patients | 112 |
| Male/female gender | 71/41 |
| Age (mean ± SD), year | 57.93±9.02 |
| Final diagnosis, No. | |
| Adenocarcinoma | 60 |
| Squamous cell carcinoma | 52 |
| Location of target lymph nodes | |
| Right upper paratracheal (#2R) | 9 |
| Right lower paratracheal (#4R) | 35 |
| Left lower paratracheal (#4L) | 12 |
| Subcarinal (#7) | 56 |
| Lymph node short axis by CT (mean ± SD) | 1.46±0.19 |
CT, computed tomography; SD, standard deviation.
Comparison of EBUS-TBNA and surgical results
The EBUS-TBNA examination results yielded diagnosis of mediastinal lymph nodes as positive in 38 cases and negative in 74 cases.
A total of 38 patients whose EBUS-TBNA results were positive received preoperative neoadjuvant chemotherapy and did not undergo surgery directly; 74 patients with negative EBUS-TBNA results underwent thoracoscopic lobectomy and systemic intrathoracic lymph node dissection. The final pathological results confirmed that 4 patients had tumor metastasis in the mediastinal lymph nodes, so those EBUS-TBNA results were false negative. The final pathological results of the remaining 70 patients were negative, which was consistent with the results of the EBUS-TBNA examination, so those EBUS-TBNA results were true negative.
Comparison of EBUS-TBNA with PET/CT results
The PET/CT yielded a diagnosis of mediastinal lymph node positive in 58 cases, and negative in 54 cases. The 30 parents of these 58 positive results were confirmed to be true by EBUS-TBNA and 28 were confirmed to be false positive by surgery. The 8 parents of these 54 negative cases were confirmed by EBUS-TBNA as false negative (6 cases of adenocarcinoma, 2 cases of squamous cell carcinoma), and 4 cases were confirmed to be false (2 cases of adenocarcinoma, 2 cases of squamous cell carcinoma). In general, the sensitivity, specificity, PPV, NPV, and accuracy of NSCLC mediastinal lymph node metastasis diagnosed by PET/CT were 71.4%, 60%, 51.7%, 77.8%, and 64.3%, respectively. The sensitivity, specificity, PPV, NPV, and accuracy of NSCLC mediastinal lymph node metastasis diagnosed by EBUS-TBNA were 90.5%, 100%, 100%, 94.6%, and 96.4%, respectively. The results showed that EBUS-TBNA had a higher diagnostic value for mediastinal lymph nodes than PET/CT in Figure 1A (AUC =0.954 and 0.636, 95% CI =0.912–0.995 and 0.551–0.721, respectively). Comparison of the diagnostic efficacy of the two methods is displayed in Table 2 and Table 3.
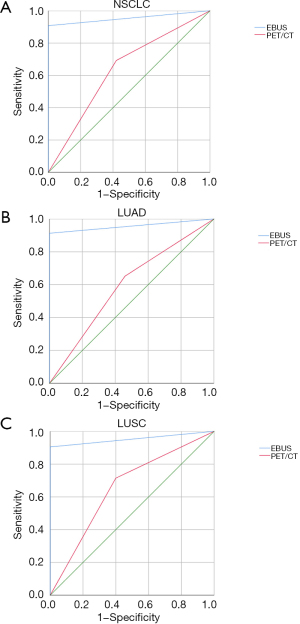
Table 2
| Inspection method | Pathology diagnosis | |
|---|---|---|
| Positive | Negative | |
| PET/CT | ||
| Positive | 30 | 28 |
| Negative | 12 | 42 |
| EBUS-TBNA | ||
| Positive | 38 | 0 |
| Negative | 4 | 70 |
PET/CT, positron emission tomography/computed tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NSCLC, non-small cell lung cancer.
Table 3
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
|---|---|---|---|---|---|
| PET/CT | 71.4% | 60.0% | 51.7% | 77.8% | 64.3% |
| EBUS-TBNA | 90.5% | 100% | 100% | 94.6% | 96.4% |
| P value | 0.013 | <0.001 | <0.001 | <0.001 | <0.001 |
PET/CT, positron emission tomography/computed tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NSCLC, non-small cell lung cancer.
Checking efficacy between PET/CT and EBUS-TBNA according to different histopathological analyses
The diagnosis of mediastinal lymph nodes by PET/CT was prone to false negative results, and the adenocarcinoma was more prone to yield false negative results than other pathological types. In 60 cases of adenocarcinoma cases, PET/CT obtained 8 cases with false negative results, of which 6 cases were confirmed by EBUS-TBNA as positive and 2 cases were confirmed as positive by surgery. In the adenocarcinoma group, the sensitivity, specificity, PPV, NPV, and accuracy of NSCLC mediastinal lymph node metastasis diagnosed by PET/CT were 65.2%, 54.1%, 46.9%, 71.4%, and 58.3%, respectively. The sensitivity, specificity, PPV, NPV, and accuracy of NSCLC mediastinal lymph node metastasis diagnosed by EBUS-TBNA were 91.3%, 100%, 100%, 94.9%, and 96.7%, respectively. The results showed that the sensitivity, specificity, PPV, NPV, and accuracy of EBUS-TBNA were higher than that of PET/CT in Figure 1B (AUC =0.957 and 0.596, 95% CI =0.888–1.000 and 0.448–0.744, respectively). A comparison of the diagnostic efficacy of the two methods is shown in Table 4.
Table 4
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
|---|---|---|---|---|---|
| PET/CT | 65.2% | 54.1% | 46.9% | 71.4% | 58.3% |
| EBUS-TBNA | 91.3% | 100% | 100% | 94.9% | 96.7% |
| P value | 0.041 | <0.001 | <0.001 | <0.001 | <0.001 |
PET/CT, positron emission tomography/computed tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NSCLC, non-small cell lung cancer.
In 52 cases with squamous cell carcinoma, PET/CT obtained 4 false negative results, among which 2 cases were confirmed as positive by EBUS-TBNA, and the other 2 cases were confirmed as positive by surgery. In squamous cell carcinoma, the sensitivity, specificity, PPV, NPV, and accuracy of NSCLC mediastinal lymph node metastasis diagnosed by PET/CT were 78.9%, 66.7%, 57.7%, 84.6%, and 71.2%, respectively. The sensitivity, specificity, PPV, NPV, and accuracy of NSCLC mediastinal lymph node metastasis diagnosed by EBUS-TBNA were 89.5%, 100%, 100%, 94.3%, and 96.2%, respectively. The sensitivity of EBUS-TBNA was greater than that of PET/CT, but the difference was not statistically significant (P=0.48). Both methods had high NPV, but the difference was not statistically significant (P>0.05). The specificity, PPV, and accuracy of EBUS-TBNA were significantly higher than those of PET/CT in Figure 1C (AUC =0.952 and 0.657, 95% CI =0.900–1.000 and 0.553–0.761, respectively). No adverse events were reported from performing PET/CT or EBUS-TBNA.
A comparison of the diagnostic efficacy of the two methods is shown in Table 5.
Table 5
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
|---|---|---|---|---|---|
| PET/CT | 78.9% | 66.7% | 57.7% | 84.6% | 71.2% |
| EBUS-TBNA | 89.5% | 100% | 100% | 94.3% | 96.2% |
| P value | 0.48 | <0.003 | <0.001 | 0.058 | <0.001 |
PET/CT, positron emission tomography/computed tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NSCLC, non-small cell lung cancer.
Discussion
In our study, the diagnostic efficacy of EBUS-TBNA was better than that of PET/CT for mediastinal staging of NSCLC patients with mediastinal lymph node >1 cm, and the difference was statistically significant (P<0.01). We found that EBUS-TBNA was more sensitive than PET/CT due to it having higher specificity, PPV, NPV, and accuracy than PET/CT. These results suggest that EBUS-TBNA is an effective minimally invasive method for the diagnosis of mediastinal lymph nodes after PET/CT examination. Our findings are similar to previous study (10), suggesting that EBUS-TBNA has a higher efficacy in the diagnosis of mediastinal lymph nodes in NSCLC than PET/CT.
According to the clinical guidelines for lung cancer diagnosis and treatment (11), pathological results need to be obtained for positive mediastinal lymph nodes detected by PET/CT, because PET/CT may yield false positives. Li et al. (12) reported that the results of EBUS-TBNA diagnosis can be as high as 95.7%, sensitivity is 95.7%, and specificity is 100% when PET/CT has been used to diagnose mediastinal lymph node positive. Our data showed that EBUS-TBNA is a safe and effective method for the diagnosis of mediastinal lymph nodes in both thoracic CT and PET/CT-positive NSCLCs as a method of diagnosis of mediastinal lymphadenopathy. Korevaar et al. has demonstrated that combined use of EBUS and endoscopic ultrasound (EUS) can improve sensitivity in detecting mediastinal metastasis (13).
If there is a possibility of surgery in patients with NSCLC whose PET/CT results show mediastinal lymph node negative, is has remained unclear whether such patients with mediastinal lymph node diagnosis need to undergo invasive re-examination. Although the high NPV of PET/CT examination shows that PET/CT examination of negative cases can avoid mediastinoscopy and other invasive examination, in some clinical studies, the additional invasive investigations are still recommended for further diagnosis (14). Our study also showed that EBUS-TBNA was superior to PET/CT in mediastinal lymph node diagnosis. This suggests that EBUS-TBNA has a higher efficacy in the diagnosis of mediastinal lymph nodes, even if PET/CT-negative cases have a higher diagnostic efficacy. Therefore, we recommend that EBUS-TBNA be performed for patients with mediastinal lymph node-negative findings for PET/CT.
The above results indicate that re-EBUS-TBNA examination of patients with show mediastinal lymph node-negative on PET/CT is meaningful. It remains unclear whether the different pathological types of NSCLC are meaningful in this instance. Therefore, we divided adenocarcinoma and squamous cell carcinoma into two subgroups. It was found that in adenocarcinoma group, EBUS-TBNA detected 6 cases of mediastinal lymph node metastasis in 8 cases which were diagnosed as negative by PET/CT. In the squamous cell carcinoma group, EBUS-TBNA detected 2 cases of mediastinal lymph node metastasis among 4 cases which had been diagnosed as negative by PET/CT. Statistical analysis showed that NPV (94.9%) of EBUS-TBNA was higher than that of PET/CT (71.4%) in the adenocarcinoma group, and the difference was statistically significant. In the squamous cell carcinoma group, NPV (94.3%) of EBUS-TBNA was similar to that of PET/CT (84.6%), and the difference was not statistically significant.
Previous studies have suggested that the different histological types of lung cancer can determine the corresponding mediastinal staging method (15,16). In the negative case of chest CT examination, compared with squamous cell carcinoma, adenocarcinoma has a higher occult mediastinal metastasis rate (17,18). Santos et al. (19) studied 224 patients with early NSCLC, among whom 16 were diagnosed with mediastinal lymph nodes by CT and PET scans. Meanwhile, 16 patients also showed occult mediastinal lymph node metastasis through invasive examination, and the pathological results were adenocarcinoma. Herth et al. (20) showed that mediastinal lymph node metastases were confirmed by EBUS-TBNA in 6 of 100 patients with normal mediastinal lymph nodes examined by chest CT and PET scans. These 6 patients with pathological findings were also adenocarcinoma. In patients with squamous cell carcinoma, mediastinal lymph node-negative patients rarely display occult metastases through PET scan (19,21). This phenomenon may be associated with adenocarcinoma being more prone to mediastinal lymph node metastasis (22), or the FDG uptake rate of adenocarcinoma is lower than that of squamous cell carcinoma (23-25). This suggests that we should conduct careful preoperative staging, even if the PET/CT results are negative. A systemic approach by the sampling of all mediastinal and hilar lymph nodes larger than 5 mm rather than a “targeted approach” whereby only abnormal lymph nodes seen on imaging are sampled, may result in fewer missed lymph nodes and thereby improved sensitivity (7). The use of EBUS-TBNA in the diagnosis of mediastinal lymph node pathology has become increasing prevalent due to its less invasiveness, lower cost, and fewer adverse events compared to surgical lymph node sampling (26). Combined with its high accuracy, it makes for the ideal first step in the diagnosis of pathology in mediastinal lymph nodes. The main aim of our study was to confirm that EBUS-TBNA is a safe and reliable minimally invasive method for patients whose PET/CT results are false positive (27,28).
Based on the results of our research, we offer a suggestion to guide clinicians in choosing the method of diagnosing mediastinal lymph nodes. For patients with abnormal lung shadows on chest CT that are highly suspected of lung cancer, if there are swollen lymph nodes on the chest CT image, PET/CT can be performed first. During the diagnostic bronchoscopy, if the enlarged lymph nodes or the lymph nodes with a positive PET/CT diagnosis are within the range of EBUS-TBNA, that is, in groups 2, 4, and 7 lymph nodes, it is recommended to use EBUS-TBNA to puncture and biopsy the diseased lymph nodes. If the pathological examination results are negative, thoracoscopic surgery can be considered. If the lymph nodes in groups 5, 6, 8, or 9 are enlarged or PET/CT is positive, EUS-fine-needle aspiration (FNA) should be considered.
The available evidence suggests that EBUS-TBNA examination performance is superior to that of PET/CT; however, with the continuous improvement of science and technology, non-invasive examination methods will be perfected. We anticipate that in future, non-invasive methods will be able to achieve diagnostic precision similar to that of the more invasive examination methods, and that they will eventually become an effective diagnostic method of the disease.
Conclusions
The method EBUS-TBNA is currently more accurate than PET/CT for preoperative diagnosis of mediastinal lymph node metastases in NSCLC, and EBUS-TBNA is an effective minimally invasive method after PET/CT scans. The EBUS-TBNA approach is feasible, safe, and effective as a method for the examination of mediastinal lymph nodes when they are diagnosed as positive by PET/CT in clinical stage IIIA patients with NSCLC. Using EBUS-TBNA can help to confirm whether the mediastinal lymph nodes are true negative when patients have been diagnosed mediastinal lymph node-negative by PET/CT, especially if the patient’s pathologic type is adenocarcinoma. For those patients with suspected mediastinal lymph node metastasis, EBUS-TBNA should be preferred method to evaluated the status of mediastinal lymph nodes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-521/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-521/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-521/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was waived by the ethics committee of The Affiliated Hospital of Qingdao University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was a retrospective study, and the informed consent from the patients and their families was abandoned.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Rao G, Pierobon M, Kim IK, et al. Inhibition of AKT1 signaling promotes invasion and metastasis of non-small cell lung cancer cells with K-RAS or EGFR mutations. Sci Rep 2017;7:7066. [Crossref] [PubMed]
- Canneto B, Ferraroli G, Falezza G, et al. Ideal conditions to perform EBUS-TBNA. J Thorac Dis 2017;9:S414-7. [Crossref] [PubMed]
- Watanabe SI, Nakagawa K, Suzuki K, et al. Neoadjuvant and adjuvant therapy for Stage III non-small cell lung cancer. Jpn J Clin Oncol 2017;47:1112-8. [Crossref] [PubMed]
- Yoon TH, Lee CH, Park KS, et al. Preoperative Risk Factors for Pathologic N2 Metastasis in Positron Emission Tomography-Computed Tomography-Diagnosed N0-1 Non-Small Cell Lung Cancer. Korean J Thorac Cardiovasc Surg 2019;52:221-6. [Crossref] [PubMed]
- Guarize J, Casiraghi M, Donghi S, et al. Endobronchial Ultrasound Transbronchial Needle Aspiration in Thoracic Diseases: Much More than Mediastinal Staging. Can Respir J 2018;2018:4269798. [Crossref] [PubMed]
- Leong TL, Loveland PM, Gorelik A, et al. Preoperative Staging by EBUS in cN0/N1 Lung Cancer: Systematic Review and Meta-Analysis. J Bronchology Interv Pulmonol 2019;26:155-65. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Akhurst T. Staging of Non-Small-Cell Lung Cancer. PET Clin 2018;13:1-10. [Crossref] [PubMed]
- Hwangbo B, Kim SK, Lee HS, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009;135:1280-7. [Crossref] [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S.
- Li SB, He JX, Li SY, et al. Value of endobronchial ultrasound-transbronchial needle aspiration biopsy for diagnosis of PET-CT positive mediastinal lymph nodes. Zhonghua Zhong Liu Za Zhi 2012;34:613-5. [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Melek H, Gunluoglu MZ, Demir A, et al. Role of positron emission tomography in mediastinal lymphatic staging of non-small cell lung cancer. Eur J Cardiothorac Surg 2008;33:294-9. [Crossref] [PubMed]
- Slavova-Azmanova NS, Lizama C, Johnson CE, et al. Impact of the introduction of EBUS on time to management decision, complications, and invasive modalities used to diagnose and stage lung cancer: a pragmatic pre-post study. BMC Cancer 2016;16:44. [Crossref] [PubMed]
- Guo D, Ni Y, Lv X, et al. Distribution and prognosis of mediastinal lymph node metastases of nonsmall cell lung cancer. J Cancer Res Ther 2016;12:120-5. [Crossref] [PubMed]
- Deng HY, Zeng M, Li G, et al. Lung Adenocarcinoma has a Higher Risk of Lymph Node Metastasis than Squamous Cell Carcinoma: A Propensity Score-Matched Analysis. World J Surg 2019;43:955-62. [Crossref] [PubMed]
- Diebels I, Hendriks JMH, Van Meerbeeck JP, et al. Evaluation of mediastinoscopy in mediastinal lymph node staging for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2021;32:270-5. [Crossref] [PubMed]
- Santos FS, Verma N, Marchiori E, et al. MRI-based differentiation between lymphoma and sarcoidosis in mediastinal lymph nodes. J Bras Pneumol 2021;47:e20200055. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [Crossref] [PubMed]
- Motoyama S, Sato Y, Wakita A, et al. Approaches to resection of recurrent solitary mediastinal lymph nodes after esophagectomy. Esophagus 2021;18:700-3. [Crossref] [PubMed]
- Fibla JJ, Molins L, Simon C, et al. The yield of mediastinoscopy with respect to lymph node size, cell type, and the location of the primary tumor. J Thorac Oncol 2006;1:430-3. [Crossref] [PubMed]
- Park SG, Lee JH, Lee WA, et al. Biologic correlation between glucose transporters, hexokinase-II, Ki-67 and FDG uptake in malignant melanoma. Nucl Med Biol 2012;39:1167-72. [Crossref] [PubMed]
- Vesselle H, Salskov A, Turcotte E, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol 2008;3:971-8. [Crossref] [PubMed]
- Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 2004;22:3255-60. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Dietrich CF, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part I: endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis 2015;7:E311-25. [PubMed]
- Jenssen C, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis 2015;7:E439-58. [PubMed]
(English Language Editor: J. Jones)


