Resection of a giant bilateral retrovascular intrathoracic goiter causing severe upper airway obstruction, 2 years after subtotal thyroidectomy: a case report and review of the literature
Introduction
Various terms and definitions have been used to describe the presence of thyroid goiter inside the thorax. Terms that are mostly used include: substernal goiter, intrathoracic goiter, retrosternal goiter and mediastinal goiter. In this paper we use the term intrathoracic goiter, which describes not only true retrosternal goiters, but also retrovascular and posterior mediastinal goiters. The most accepted current definition of an intrathoracic or substernal goiter is the presence of 50% or more of the mass of a thyroid goiter inside the thorax (i.e., below the thoracic inlet), as described at least since 1985 by Katlik et al. (1-7).
Katlik et al. in their review (in 1985) reported that intrathoracic goiters were usually adenomatous and benign, having a low incidence of malignancy of 2-3% (1). Mack (8) in his review (in 1995) reported a relatively high incidence of malignancy. The reported incidence of malignancy in retrospective studies varies, reaching 22%. (1,4,5,9-17) (Table 1).
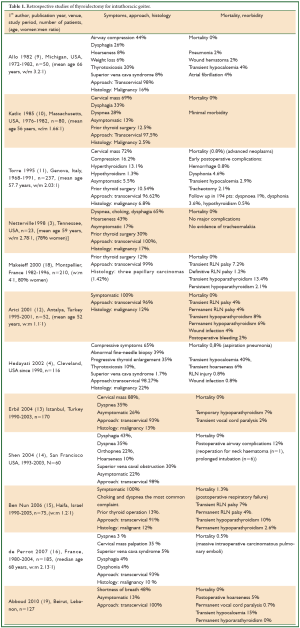
Full table
The systematic review by White et al. concluded that limited (level III/IV) data suggest that the incidence of malignancy in substernal goiters does not exceed the incidence of malignancy in cervical goiters. Family history of thyroid pathology, prior history of cervical radiation, recurrent goiter, and cervical adenopathy are considered risk factors for malignancy in substernal goiters (grade C recommendation) (20).
In retrospective studies, history of previous thyroid surgery has been reported in 10% to 30% of patients operated for intrathoracic goiter (3,10,11,15,18) (Table 1).
The systematic review by White et al. concluded that prospective (level V) data suggest that about 5-10% of operations for intrathoracic goiters are performed in recurrent or persistent disease, but retrospective (level V) data report a higher rate, reaching 37%. The most common initial operations appear to be subtotal or hemithyroidectomy (20).
Benign intrathoracic goiters usually exhibit slow but steady growth, leading to compression of the trachea, the oesophagus, vascular and neural structures, thus producing symptoms, such as dyspnoea, stridor, dysphagia, superior vena cava syndrome, deep vein thrombosis of the subclavian vein, dysphonia, hypophonia, and Horner’s syndrome, while some patients can be asymptomatic (1,7-17,21). A cervical mass can be usually palpated (1,8-16). The severity of symptoms depends on the degree of compression. Intrathoracic goiters may cause more severe compressive symptoms than the cervical goiters, due to the limited space of the thoracic inlet and cage (1,2). Airway obstruction (presenting with stridor) can be life-threatening, particularly if suddenly precipitated by spontaneous or traumatic bleeding into the substernal goiter, or by tracheal infection (1,8). Most patients are euthyroid (1), but intrathoracic goiters can produce symptoms of hyperthyroidism and thyrotoxicosis, and rarely hypothyroidism (4,8,9,11) (Table 1).
Surgery is the indicated treatment, being usually performed through a transcervical approach and resulting in symptomatic relief (1-18,20).
We hereby report a rare case of a recurrent, benign, gigantic, bilateral, retrovascular, intrathoracic goiter, descending into the posterior mediastinum down to the tracheal bifurcation, which caused severe dyspnoea and stridor and required resection through a median sternotomy (Video 1).
Case report
A 63 year old man with history of subtotal thyroidectomy 2 years ago, presented with progressively increasing dyspnoea and recent inspiratory stridor. The patient was euthyroid and a cervical mass was barely palpable above the sternum. Posteroanterior chest radiography showed enlargement of the upper and middle mediastinum, mild tracheal deviation to the right, and possible tracheal stenosis, at the level of the aortic arch (Figure 1). Thoracic computed tomography showed a large cervical, and prespinal superior and posterior mediastinal mass. More than 90% of the mass was below the thoracic inlet. Two retrovascular, pre- para- and retro-tracheal lobes were displacing the aortic arch, the anonymous vein, and the trachea, descending to the hila, severely compressing the trachea at the level of the aortic arch (Figure 2). The diagnosis of recurrent intrathoracic goiter was made and operation was promptly scheduled.
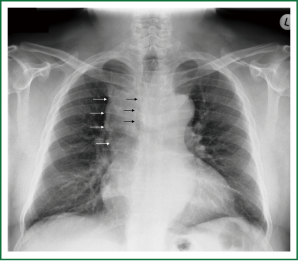 Figure 1. Preoperative chest x-ray. Enlargement of the upper and middle mediastinum (white arrows), mild tracheal deviation to the right, and tracheal stenosis, at the level of the aortic arch (black arrows).
Figure 1. Preoperative chest x-ray. Enlargement of the upper and middle mediastinum (white arrows), mild tracheal deviation to the right, and tracheal stenosis, at the level of the aortic arch (black arrows).
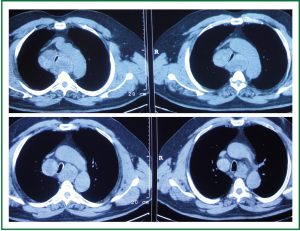 Figure 2. Preoperative thoracic computed tomography. A large prespinal superior and posterior mediastinal mass. Two retrovascular, pre- para- and retro-tracheal lobes are displacing the aortic arch, the anonymous vein, and the trachea, descending to the hila, severely compressing the trachea at the level of the aortic arch.
Figure 2. Preoperative thoracic computed tomography. A large prespinal superior and posterior mediastinal mass. Two retrovascular, pre- para- and retro-tracheal lobes are displacing the aortic arch, the anonymous vein, and the trachea, descending to the hila, severely compressing the trachea at the level of the aortic arch.
Total thyroidectomy was performed through a median sternotomy combined with a cervical collar incision. The lower parathyroid glands and the recurrent laryngeal nerves were visually identified and protected. Intraoperative monitoring of the laryngeal nerves was not used. The 2 lobes were resected within their capsules, completely intact, by extracapsular dissection, gentle traction and digital mobilization. The right lobe was enucleated first, followed by enucleation of the second lobe. The blood supply was from the cervical vessels. The total specimen weight was 290 gr, the maximum length of the right lobe was 12 cm, and the maximum length of the left lobe was 14 cm (Figures 3,4). Histology revealed multinodular goiter without malignancy.
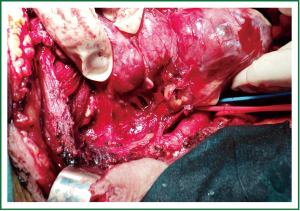 Figure 3. The right lobe has just been delivered from the mediastinum, intact and encapsulated (enucleated).
Figure 3. The right lobe has just been delivered from the mediastinum, intact and encapsulated (enucleated).
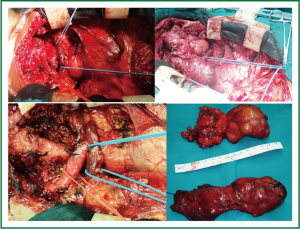 Figure 4. Total thyroidectomy was performed through a median sternotomy combined with a cervical collar incision. The 2 lobes were enucleated and delivered from the mediastinum completely intact. The total specimen weight was 290 gr, the maximum length of the right lobe was 12 cm, and the maximum length of the left lobe was 14 cm.
Figure 4. Total thyroidectomy was performed through a median sternotomy combined with a cervical collar incision. The 2 lobes were enucleated and delivered from the mediastinum completely intact. The total specimen weight was 290 gr, the maximum length of the right lobe was 12 cm, and the maximum length of the left lobe was 14 cm.
The postoperative course was uneventful, without: bleeding, infection, recurrent laryngeal nerve palsy, cardiorespiratory, or wound complications. The patient was transferred to the Intensive Care Unit and was extubated on the first postoperative day. Airway stenosis was immediately relieved, although a mild degree of tracheal stenosis, attributed to tracheomalacia due to chronic compression, remained. No further intervention was required (Figure 5). Routine short-term treatment with calcium (1 gr daily orally) and 1alpha-hydroxyvitamin D3 (1 μgr daily orally) was administered (as per protocol). The patient was discharged home on the 9th postoperative day on levothyroxin (100 μgr daily orally). Excellent tracheal patency with some distortion of its lumen (increased anteroposterior and decreased transverse diameter at the level of the aortic arch, where severe stenosis was present preoperatively) was noted on computed tomography performed at 1- and 6- month follow-up (Figures 6,7,8). Thirteen months postoperatively the patient remains alive and well, without symptoms or signs of recurrence.
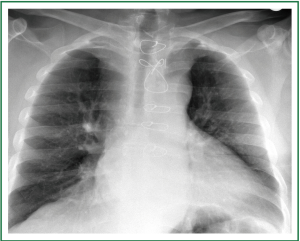 Figure 5. Postoperative chest x-ray, on the 8 postoperative day (the day before discharge). Very satisfactory recovery, without cardiorespiratory complications. Mild residual tracheal stenosis and deviation, attributed to tracheomalakia due to chronic compression, remained. No further intervention was required.
Figure 5. Postoperative chest x-ray, on the 8 postoperative day (the day before discharge). Very satisfactory recovery, without cardiorespiratory complications. Mild residual tracheal stenosis and deviation, attributed to tracheomalakia due to chronic compression, remained. No further intervention was required.
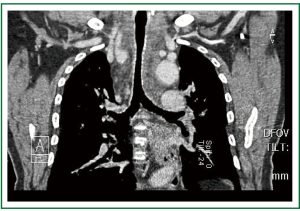 Figure 6. Computed tomography, 1 month postoperatively. Clinically insignificant residual tracheal stenosis, at the level of the aortic arch.
Figure 6. Computed tomography, 1 month postoperatively. Clinically insignificant residual tracheal stenosis, at the level of the aortic arch.
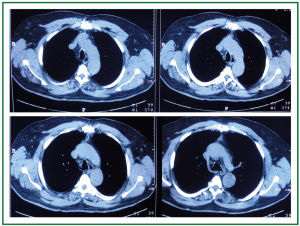 Figure 7. Computed tomography, 6 months postoperatively. Excellent tracheal patency with some distortion of its lumen (increased anteroposterior and decreased transverse diameter at the level of the aortic arch, where severe stenosis was present preoperatively). Complete goiter resection, absence of recurrence.
Figure 7. Computed tomography, 6 months postoperatively. Excellent tracheal patency with some distortion of its lumen (increased anteroposterior and decreased transverse diameter at the level of the aortic arch, where severe stenosis was present preoperatively). Complete goiter resection, absence of recurrence.
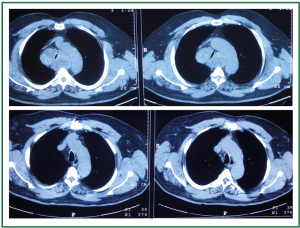 Figure 8. Comparison of preoperative and postoperative thoracic computed tomographies. Notice the degree of tracheal compression and the displacement of the aortic arc preoperatively (up). Anatomy restored postoperatively (down).
Figure 8. Comparison of preoperative and postoperative thoracic computed tomographies. Notice the degree of tracheal compression and the displacement of the aortic arc preoperatively (up). Anatomy restored postoperatively (down).
Discussion
The overall incidence of substernal goiter in countries where routine use of iodized salt is employed is very low. Nevertheless it has been reported that intrathoracic goiters can represent up to 7% of mediastinal tumors, even in regions where they are less common. Large intrathoracic goiters mainly represent neglected and long standing disease (9,11,17,22,23).
In Greece iodised salt is routinely used, and management of substernal goiters by cardiothoracic surgeons is extremely rare. In a retrospective study conducted in a tertiary referral centre of Greece, within a series of 591 patients who underwent thyroidectomy there were 37 patients (6.2%) with intrathoracic (descending) goiter. A cervical approach was employed in 97.29% of patients (1 patient required sternotomy, 0.17% of all thyroidectomies); malignancy was revealed in 16.6% (24).
The main indications for surgery in thyroid disease include fear of malignancy, tracheo-esophageal compression, and cosmetic reasons (2). The presence of compressive symptoms is an indication for resection of both cervical and intrathoracic goiters. The severity of dyspnoea is related to the size of the goiter and the degree of compression (but not the deviation) of the trachea, as shown on computed tomography. Compression is more frequently caused by intrathoracic goiters than cervical goiters (1,2,10,22,25).
The presence of a substernal goiter is per se an indication for thyroidectomy, even in the absence of symptoms, because there is no other effective means of preventing the growth of the goiter (to the “bottomless” mediastinal space), as medical therapy is generally unsuccessful (1,2,8,9,17).
Surgical resection is indicated in all patients, with a potential exception of the highest risk patients (1,26). Early and even “aggressive” tracheal decompression has been recommended, particularly in symptomatic patients (8,23). Acute airway distress may require intubation or semi-emergent surgery (22,23).
With surgical resection, the symptomatic patients enjoy relieve of dyspnea and/or dysphagia, while the asymptomatic patients are spared from the small but present risk of life-threatening situations (mainly acute airway compression), and/or the risk of occult thyroid malignancy (1,2,8).
Resection of intrathoracic goiters can generally be accomplished via a transcervical approach (usually through the standard cervical collar incision) (1-4,8-11,17-19,24). Large goiters can be delivered from the mediastinum by (subcapsular) dissection, traction, digital mobilization (by a sweeping motion), and, occasionally, sponge-holding clamps, a spoon, or other instruments, such as the Kocher’s specially made forceps (1,8). Enucleation (26) or extirpation (2) of the gland is preferred, while morcellization or fragmentation is less desirable, to avoid bleeding and dissemination of occult malignancy (1,8).
In fact, the vast majority of intrathoracic goiters are removed through a cervical incision; nevertheless, sternotomy or thoracotomy may be occasionally required (1,2,12,15-17).
The standard cervical approach is considered to be related with less morbidity than the sternotomy or the thoracotomy. A number of surgeons, though, indicated that removal by the cervical approach alone may increase the risk of uncontrollable hemorrhage, recurrent laryngeal nerve injury, and incomplete removal of the goiter (1).
Intrathoracic goiters usually receive blood supply from the cervical thyroid vessels. The “primary intrathoracic goiters” (those with intrathoracic blood supply) may require a sternotomy. Extraction of a posterior mediastinal goiter or a retrovascular goiter may prove difficult, requiring a combined cervical-thoracic or cervical-sternotomy approach (1,26). Apart from primary intrathoracic and posterior mediastinal goiters, malignant goiters and recurrent goiters, often require median sternotomy for safe removal (8).
The systematic review by White et al. concluded that prospective (level V) data suggest that expert surgeons employ an extracervical approach in approximately 2% of cases, to safely remove an intrathoracic goiter. Use of a sternotomy or a thoracotomy appears more likely in the presence of a primary intrathoracic goiter or a goiter larger than the thoracic inlet (20).
Surgical therapy for substernal goiters, performed by experienced surgeons results in full relief of symptoms, with “minimal morbidity and practically zero mortality” (Table 1) (3,5,8-10,12,13,17-19).
Complications of thyroidectomy of intrathoracic (as well as cervical) goiters include transient or permanent recurrent laryngeal nerve palsy or hypothyroidism (1,4,9,12,13,18) (Table 1). Identification of the recurrent laryngeal nerve and the parathyroid glands has been recommended (1,18).
The rate of recurrent laryngeal nerve palsy after thyroid surgery (applied mainly in cervical goiters) varied greatly; transient palsy has been reported in 0-7% of cases, while permanent palsy has been reported in 0-11%. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid (and parathyroid) surgery has been widely accepted as an adjunct to the gold standard (27) of visual identification (28). Guidelines regarding the use of standard equipment and problem solving algorithms to evaluate signal loss may improve the clinical practice (29).
The incidence of postoperative transient hypoparathyroidism after total thyroidectomy (mainly of cervical goiters) ranged between 3% and 40% (30). Apart from identification, and careful and meticulous dissection for preservation of the parathyroid glands, parathyroid autotransplantation has been suggested to avoid postoperative hypocalcemia and permanent hypothyroidism (30-33). Hedayati et al. performed parathyroid autotransplantation in 37% of patients with intrathoracic goiter (vs. 22% of patients with cervical goiter, P<0.01) and reported transient hypocalcemia in 40% of patients (4).
Reoperative thyroid surgery has been related with higher risk of complications, including temporary or permanent recurrent laryngeal nerve palsy and hypothyroidism (34).
In a recent retrospective review of 200 consecutive thyroidectomies for cervical or intrathoracic goiter, performed in a centre of excellence and including a series of patients with extensive goiter (25), temporary paralysis of the recurrent laryngeal nerve occurred in 1.8% of nerves at risk, being significantly lower with laryngeal nerve monitoring. Permanent hypoparathyroidism occurred in 3% overall. There were no cases of tracheomalacia. Bilateral cervical goiter was identified as a risk factor for difficult intubation, recurrent laryngeal nerve injury and postoperative hypocalcemia (35,36).
The systematic review by White et al. concluded that there may be a higher rate of permanent recurrent laryngeal nerve injury and permanent hypoparathyroidism when total thyroidectomy is performed for treatment of an intrathoracic goiter than for treatment of a cervical goiter alone (recommendation grade C) (20).
Although rare, tracheomalacia secondary to prolonged tracheal compression requires careful postoperative monitoring (17).
The systematic review by White et al. concluded that the presence of an intrathoracic goiter, especially when it is present for more than 5 years and causes significant tracheal compression, is a potential risk factor for tracheomalacia and tracheostomy. Tracheomalacia is an infrequent occurrence, and in many patients can be managed without tracheostomy (20).
Thus, the presence of large, bilateral, recurrent (requiring re-operation), posterior mediastinal intrathoracic goiters, as well as goiters causing significant tracheal compression has been related with postoperative complications and/or need for sternotomy (1,8,18,25,35).
According to the study by Shen et al. [2004] (14) patients with postoperative airway complications had larger goiters (210.7+/-37.0 gr vs. 112.2+/-7.7 gr), and were more likely to exhibit tracheal compression on preoperative imaging (P<0.05).
Abbouad et al. (19) also reported (in 2010) that patients with large thyroid tumors and tracheal compression were more likely to develop postoperative complications.
Shin et al. (25) and Randolph et al. (35) reported (in 2011) a mean specimen size of 10.5±4.8 cm, and a mean weight of 142.9±113.3 grams, characterizing the goiters as extensive, and concluded that surgical complications were associated with large and bilateral goiters, suggesting increased caution in these patients.
In relevant review articles, it has been suggested that a sternotomy may be required for safe removal of posterior mediastinal goiters, recurrent goiters, and goiters larger than the thoracic inlet (1,8,20).
The posterior mediastinal goiter is rarer than the superior and anterior mediastinal goiters. The term substernal or retrosternal indicates that in most cases the thyroid mass descends behind the sternum, anterior to the great thoracic vessels (prevascular). Hedayati et al. [2002] reported posterior mediastinal goiters in only 6% of their patients with intrathoracic goiter (4). Katlic, Wang and Grillo (1) in their 1985 review reported that posterior mediastinal goiters represent 10-15% of all intrathoracic goiters. Nevertheless, de Perrot et al. [2007] (16) reported prevascular goiters in 38% of patients, retrovascular and paratracheal in 33%, retrotracheal in 27%, and aberrant intrathoracic in 2%. The goiters’ median size was 10 cm (5-23 cm), and only 21% of them were bilateral. De Perrot et al. reported sternotomy in 13 out of 185 patients (7%) mainly due to recurrent goiter, ectopic goiter, or invasive carcinoma (P=0.1, P<0.001, P<0.001, respectively), concluding that sternotomy should be reserved for patients having these risk factors (16).
Thus, it appears that our patient had most of the various described risk factors, including: a very large and bilateral intrathoracic goiter (total specimen weight 290 grams, length 14 cm and 12 cm for the left and right lobe respectively), a recurrent intrathoracic goiter (2 years after subtotal thyroidectomy), a retrovascular, posterior mediastinal goiter, completely encircling the trachea (pre- para- and retro-tracheal), causing severe tracheal compression (dyspnea, stridor, significant stenosis on computed tomography). The goiter was not extremely long standing, being developed within 2 years, while mean durations of 16, 15 and 12.9 years have been reported by Allo et al. [1983] (9), Marruoti et al. [1991] (26), and Torre et al. [1995] (11) respectively. Nevertheless, it can be considered as neglected and underdiagnosed.
Conclusively, we believe that recurrent goiter should be always suspected in patients presenting dyspnoea after thyroidectomy. Absence of a palpable cervical mass and euthyroid state should not be considered as criteria for absence of recurrence. Thoracic imaging should be performed to exclude or confirm the presence of a thoracic goiter and evaluate the degree of compression. Operation should be promptly performed, well before establishment of severe dyspnoea and stridor.
With early intervention, most intrathoracic goiters can be removed through a cervical approach, while tracheomalacia is avoided. Nevertheless, a sternotomy may be preferable in high risk patients, to ensure better visualization, identification of anatomical structures that should be preserved (such as the recurrent laryngeal nerves and the parathyroid glands), better haemostasis, and complete enucleated removal.
Re-operation and resection of a large, bilateral, retrovascular goiter, with more than 90% of its mass below the thoracic inlet, descending to the posterior mediastinum, adjacent to the aortic arch, the descending aorta and the thoracic spine required a median sternotomy that was not associated with thyroidectomy-related or median sternotomy-related morbidity. The patient enjoyed immediate and lasting relief of symptoms, with clinically insignificant residual tracheal stenosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Katlic MR, Wang CA, Grillo HC, Substernal goiter. Ann Thorac Surg. 1985;391-9. [PubMed ]
- Singh B, Lucente FE, Shaha AR, Substernal goiter: a clinical review. Am J Otolaryngol. 1994;409-16. [PubMed ]
- Netterville JL, Coleman SC, Smith JC, Management of substernal goiter. Laryngoscope. 1998;1611-7. [PubMed ]
- Hedayati N, McHenry CR, The clinical presentation and operative management of nodular and diffuse substernal thyroid disease. Am Surg. 2002;245-51. [PubMed ]
- Batori M, Chatelou E, Straniero A, Substernal goiters. Eur Rev Med Pharmacol Sci. 2005;355-9. [PubMed ]
- Batori M, Chatelou E, Straniero A., Surgical treatment of retrosternal goiter. Eur Rev Med Pharmacol Sci. 2007;265-8. [PubMed ]
- Pace-Asciak P, Higgins K., Management of intrathoracic goitre. Can J Surg. 2008;E111-2. [PubMed ]
- Mack E., Management of patients with substernal goiters. Surg Clin North Am. 1995;377-94. [PubMed ]
- Allo MD, Thompson NW, Rationale for the operative management of substernal goiters. Surgery. 1983;969-77. [PubMed ]
- Katlic MR, Grillo HC, Wang CA, Substernal goiter. Analysis of 80 patients from Massachusetts General Hospital. Am J Surg. 1985;283-7. [PubMed ]
- Torre G, Borgonovo G, Amato A, Surgical management of substernal goiter: analysis of 237 patients. Am Surg. 1995;826-31. [PubMed ]
- Arici C, Dertsiz L, Altunbas H, Operative management of substernal goiter: analysis of 52 patients. Int Surg. 2001;220-4. [PubMed ]
- Erbil Y, Bozbora A, Barbaros U, Surgical management of substernal goiters: clinical experience of 170 cases. Surg Today. 2004;732-6. [PubMed ]
- Shen WT, Kebebew E, Duh QY, Predictors of airway complications after thyroidectomy for substernal goiter. Arch Surg. 2004;656-9. [PubMed ]
- Ben Nun A, Soudack M, Best LA, Retrosternal thyroid goiter: 15 years experience. Isr Med Assoc J. 2006;106-9. [PubMed ]
- de Perrot M, Fadel E, Mercier O, Surgical management of mediastinal goiters: when is a sternotomy required?. Thorac Cardiovasc Surg. 2007;39-43. [PubMed ]
- Newman E, Shaha AR, Substernal goiter. J Surg Oncol. 1995;207-12. [PubMed ]
- Makeieff M, Marlier F, Khudjadze M, Substernal goiter. Report of 212 cases. Ann Chir. 2000;18-25. [PubMed ]
- Abboud B, Sleilaty G, Mallak N, Morbidity and mortality of thyroidectomy for substernal goiter. Head Neck. 2010;744-9. [PubMed ]
- White ML, Doherty GM, Gauger PG, Evidence-based surgical management of substernal goiter. World J Surg. 2008;1285-300. [PubMed ]
- Leuchter I, Becker M, Mickel R, Horner’s syndrome and thyroid neoplasms. ORL J Otorhinolaryngol Relat Spec. 2002;49-52. [PubMed ]
- Shaha AR, Burnett C, Alfonso A, Goiters and airway problems. Am J Surg. 1989;378-80 ;:-; . [PubMed ]
- Shaha AR, Surgery for benign thyroid disease causing tracheoesophageal compression. Otolaryngol Clin North Am. 1990;391-401. [PubMed ]
- Bizakis J, Karatzanis A, Hajiioannou J, Diagnosis and management of substernal goiter at the University of Crete. Surg Today. 2008;99-103. [PubMed ]
- Shin JJ, Grillo HC, Mathisen D, The surgical management of goiter: Part I. Preoperative evaluation. Laryngoscope. 2011;60-7. [PubMed ]
- Maruotti RA, Zannini P, Viani MP, Surgical treatment of substernal goiters. Int Surg. 1991;12-7. [PubMed ]
- Dralle H, Sekulla C, Lorenz K, Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg. 2008;1358-66. [PubMed ]
- Higgins CC, Surgical anatomy of the recurrent laryngeal nerve with especial reference to thyroid surgery. Ann Surg. 1927;827-38. [PubMed ]
- Randolph GW, Dralle H, Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;S1-16. [PubMed ]
- Lang BH, Yih PC, Ng KK, A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg. 2012;1300-6. [PubMed ]
- Lo CY, Lam KY, Postoperative hypocalcemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: a comparative study. Surgery. 1998;1081-6. [PubMed ]
- Lo CY, Lam KY, Routine parathyroid autotransplantation during thyroidectomy. Surgery. 2001;318-23. [PubMed ]
- D’Avanzo A, Parangi S, Morita E, Hyperparathyroidism after thyroid surgery and autotransplantation of histologically normal parathyroid glands. J Am Coll Surg. 2000;546-52. [PubMed ]
- Calò PG, Pisano G, Medas F, Risk factors in reoperative thyroid surgery for recurrent goitre. Our experience. G Chir. 2012;335-8. [PubMed ]
- Randolph GW, Shin JJ, Grillo HC, The surgical management of goiter: Part II. Surgical treatment and results. Laryngoscope. 2011;68-76. [PubMed ]
- Touzopoulos P, Karanikas M, Zarogoulidis P, Current surgical status of thyroid diseases. J Multidiscip Healthc. 2011;441-9. [PubMed ]


