
Prognostic significance of PD-L1 expression and CD8+ TILs density for disease-free survival in surgically resected lung squamous cell carcinoma: a retrospective study
Introduction
Lung squamous cell carcinoma (LUSC) is one of the most common pathological types of non-small cell lung cancer (NSCLC) and remains the leading cause of tumor-related deaths globally (1,2). Unlike lung adenocarcinoma (LUAD), which has oncogenic driver changes, the treatment for LUSC made little progress and conventional platinum-based chemotherapy remains the gold standard treatment (3-5). The gold standard for early LUSC treatment is lobectomy with peripheral lymph node dissection (3). However, the recurrence rate within 5 years after standard treatment is still more than 20% (5). Although adjuvant chemotherapy only provided a limited survival benefit (6), complete resection surgery combined with adjuvant chemotherapy was the optimal treatment for prolonging survival. Recently, neoadjuvant and adjuvant using immune checkpoint inhibitors (ICIs) has shown promising results. ICIs targeting programmed cell death protein 1 (PD-1) and its ligand (PD-L1), compared to conventional chemotherapy, have prolonged survival, and the treatment of advanced lung cancer has been brought into a new era (7-10). A preliminary study (11) found that neoadjuvant nivolumab caused 45% of patients to have primary pathological response in early lung cancer.
Tumor mutation burden (TMB) was found having no relationship with pathologic response of neoadjuvant Atezolizumab in the LCM3 study (12), while the response could also be found in patients with negative PD-L1 expression. Multiple retrospective studies (13-15) suggest that the disease-free survival (DFS) may be related to the differentiation and abundance of various immune cells in tumor tissues of LUSC patients. tumor-node-metastasis (TNM) stages (16,17) can predict the prognosis of LUSC, but its accuracy remains unclear. Previous studies (12,18,19) have shown that age, gender, smoking location and so on have certain limitations in predicting DFS of early stage LUSC after surgery. Therefore, it is necessary to comprehensively delineate the genomic landscape and immune pattern of lung cancer patients and elucidate their correlations to illuminate the phenotypes of lung cancer patients that could potentially benefit from immunotherapy, which could facilitate the development of neoadjuvant immunotherapy strategies for NSCLC patients. Several studies have reported data on LUAD, though, situations of LUSC remains largely unknown currently (20,21).
In this study, we reviewed 180 patients underwent completely resection of LUSC without any history of immunotherapy and analyzed the correlations among the TMB, PD-L1 expression, cluster of differentiation 8+ tumor-infiltrating lymphocyte (CD8+ TILs) density, and other clinical parameters with the genomic landscape and survival. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-630/rc).
Methods
Sample collection
Retrospectively, we reviewed the data of 180 patients with histologically confirmed LUSC that had undergone surgical resection of the lung (lobectomy or pulmonectomy), who had not previously received any immunotherapy, at the Shanghai Pulmonary Hospital from 2013 to 2016. Samples with insufficient or poor quality, missing baseline clinicopathological features, mixed histology, or incomplete follow-up data were all excluded. We confirmed the histological type of each case in the medical electronic records. Corresponding formalin-fixed and paraffin-embedded (FFPE) tissues were used for whole-exome sequencing (WES) and immunohistochemistry (IHC) staining. Tissues were used for WES and IHC. Patients’ baseline characteristics were collected, including age, sex, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor maximum diameter, lymph node status and stage, pleural invasion status, vascular invasion status, tumor TNM stage, differentiation degree, chemotherapy regimens and tumor location classification, which were used to test whether the baseline features were biased. We also collected the data of disease free survival (DFS) and overall survival (OS) to analyse the association between biomarkers and prognosis. Survival status and data were followed through telephone and the follow-up time ended on January 31, 2022. Stage TNM was defined with the eighth UICC/AJCC TNM classification (22) and radiographic progression disease (PD) were defined according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (23). DFS refers to the time since initial surgical resection until recurrence. OS is calculated from the date of diagnosis of LUSC to death from any cause or to censoring at the last follow-up visit. This study was conducted in accordance with the provisions of the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. K20-288). Individual consent for this retrospective analysis was waived.
PD-L1 expression
Anti-human PD-L1 antibody (#13684, clone E1L3N, Cell Signaling Technology, Danvers, MA, diluted 1:200) was used to determine PD-L1 expression in accordance with the manufacturer’s recommendations. PD-L1 expression was defined as the percentage of tumor cells showing membranous immunoreactivity (in the central or marginal tumor region). The samples were also retested using another antibody assay (24) (clone SP142, Spring Bioscience, Ventana, Tucson, AZ, diluted 1:100). The cutoff value for dichotomizing PD-L1 positivity or negativity (PD-L1+/−) was 1%. Sections of human placenta tissues and the breast cancer cell line MCF-7 were employed as the positive and negative controls for the PD-L1 IHC staining, respectively.
CD8+ TILs density
The CD8+ TILs density was assessed using a mouse anti-CD8 monoclonal antibody (clone C8144B, DAKO). These lymphocytes contain the CD8 cytoplasmic marker and infiltrate within tumor regions (central or marginal) were defined as CD8+ TILs. Similar to the approach adopted by previous studies (25,26), we identified positive/negative CD8+ TILs density (CD8+ TILs+/−) with a cutoff value of 0%.
Targeted sequencing and TMB calculation
All Genomic deoxyribonucleic acid (DNA) samples included in this study were extracted using the Qiagen DNeasy FFPE DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and a DNA library was constructed. The matched peripheral blood leukocytes were used as the source for germline DNA control. The libraries were prepared using a custom capture panel (Genecast, Beijing, China) that covers the genes associated with cancer diagnosis and prognosis. Afterward, Illumina Novaseq 6000 sequencing systems were used to sequence the captured DNA fragments. The average unique sequence coverage was at least 900× for lymphocytes and 2,000× for tumor tissue samples. Mutect2 was used to detect single nucleotide variants and small indels.
In the mutation calling procedure, each individual’s germline mutations were filtered out during tumor-normal paired sample calling. There was a requirement for ≥20 reads of sequencing depth in each somatic mutation region, and ≥5 reads were required to support the variant call. Conversely, the number of reads supporting the variant in the germline data had to be <5, and the sequencing depth had to be ≥20. The variant data were annotated using ANNOVAR (27).
We then filtered out all common germline mutations with a population frequency of ≥1% using the gnomAD, ExAC, and esp6500 databases. And in order to ensure the data was reliable, common variants and potential background noise from the platform were also excluded. An in-house blacklist from Genecast was used to filter the sequencing-specific errors and background noises. Variants were converted to MAF files using vcf2maf (https://github.com/mskcc/vcf2maf). The clinical evidence levels of mutations were annotated with OncoKB MafAnnotator (https://github.com/oncokb/oncokb-annotator).
The TMB was defined as the number of somatic coding mutations per megabase of genome examined, which included single-base substitution and indels. And we only considered nonsynonymous mutations and introns (synonymous mutations that do not affect amino acid sequences) were excluded. Specifically, mutations belong to the following ENSEMBL’s items: “frame shift del”, “frame shift ins”, “splice site”, “translation start site”, “nonsense mutation”, “nonstop mutation”, “in frame del”, “in frame ins”, and “missense mutation”, which were selected following the instructions of maftools (28).
Statistical analysis
The patient characteristics are presented using descriptive statistics. The continuous variables are presented as the median and interquartile range, and the categorical data are presented as the count and frequency. The comparative analysis of the continuous and categorical variables between the groups was performed with the Wilcoxon rank-sum test for two independent samples and Fisher’s exact test, respectively, using the R package ggpubr. The Kaplan-Meier method was used for the survival analysis, and log-rank test was used to test the significance of differences between survival curves. The univariable and multivariable survival analyses were performed with Cox’s proportional hazards model to generate estimates of the hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) using the R package survminer. The Spearman correlation matrix was calculated and plotted using the R package corrplot. P<0.05 was considered significant.
Results
Genomic landscape of early stage LUSC
In this study, a total of 180 patients were enrolled, and their baseline clinicopathological characteristics are set out in Table 1. Patients had a median age of 63 years (57–68 years). Of the patients, 170 (94.4%) were male, and 132 (73.3%) had an ECOG PS of 0. Additionally, 135 (75.0%) patients were confirmed to have TNM stage I/II, and most patients (n=125, 69.4%) had a smoking history. Using a cutoff value of 1%, PD-L1+ tumor cells (E1L3N_TC) were observed in 75 (41.7%) patients; a figure higher than that reported for Caucasian LUSC populations (29). In the CHOICE study (which included both Chinese LUSC and LUAD patients), the PD-L1 positive rate was 23.1% using a H-score ≥50, or 63.9% using >1% positivity in tumor cells as a cutoff; figures consistent with those reported in research on Western populations (30,31). As a control, the clone SP142 PD-L1 antibody was used, and the rate of PD-L1+ immune cells (SP142_IC) was 0 [0, 0] in both groups (PD-L1+/–), and the rate of PD-L1+ tumor cells (SP142_TC) was 0 [0, 8] in the PD-L1+ group (E1L3N_TC) and 0 [0, 0] in the PD-L1– group (E1L3N_TC). The median TMB of both the PD-L1+ group (E1L3N_TC) and PD-L1– group (E1L3N_TC) were 9.4 mut/Mb, and the median rate of the CD8+ TILs was 17 [5, 30] in the PD-L1+ group (E1L3N_TC) and 9 [3.5, 21] in the PD-L1– group (E1L3N_TC).
Table 1
| Characteristics | Total | PD-L1+ (E1L3N_TC) | PD-L1– (E1L3N_TC) | P value |
|---|---|---|---|---|
| Age, n (%) | 0.09 | |||
| <65 | 113 (62.8) | 53 (70.7) | 60 (57.1) | |
| ≥65 | 67 (37.2) | 22 (29.3) | 45 (42.9) | |
| Sex, n (%) | 0.53 | |||
| Female | 10 (5.6) | 3 (4.0) | 7 (6.7) | |
| Male | 170 (94.4) | 72 (96.0) | 98 (93.3) | |
| ECOG PS, n (%) | 0.06 | |||
| 0 | 132 (73.3) | 49 (65.3) | 83 (79.0) | |
| 1 | 48 (26.7) | 26 (34.7) | 22 (21.0) | |
| Smoking, n (%) | 1.00 | |||
| Never | 55 (30.6) | 23 (30.7) | 32 (30.5) | |
| Ever | 125 (69.4) | 52 (69.3) | 73 (69.5) | |
| TNM stage, n (%) | 0.86 | |||
| I/II | 135 (75.0) | 57 (76.0) | 78 (74.3) | |
| III | 45 (25.0) | 18 (24.0) | 27 (25.7) | |
| Lymph node invasion, n (%) | 0.14 | |||
| No | 141 (78.3) | 63 (84.0) | 78 (74.3) | |
| Yes | 39 (21.7) | 12 (16.0) | 27 (25.7) | |
| Lymph node stage, n (%) | 0.27 | |||
| 0 | 141 (78.3) | 63 (84.0) | 78 (74.3) | |
| 1 | 25 (13.9) | 7 (9.3) | 18 (17.1) | |
| 2 | 14 (7.8) | 5 (6.7) | 9 (8.6) | |
| Differentiation, n (%) | 0.18 | |||
| Low | 51 (28.3) | 19 (25.3) | 32 (30.5) | |
| Intermediate | 119 (66.1) | 49 (65.3) | 70 (66.7) | |
| High | 10 (5.6) | 7 (9.3) | 3 (2.9) | |
| Maximum diameter | 4 [2.9–5] | 3.5 [2.8–5] | 4 [3–5] | 0.52 |
| Pleural invasion, n (%) | 0.16 | |||
| No | 166 (92.2) | 72 (96.0) | 94 (89.5) | |
| Yes | 14 (7.8) | 3 (4.0) | 11 (10.5) | |
| Venous invasion, n (%) | 0.72 | |||
| 0 | 172 (95.6) | 71 (94.7) | 101 (96.2) | |
| 1 | 8 (4.4) | 4 (5.3) | 4 (3.8) | |
| CD8+ TILs (%) | 11.5 (4, 24) | 17 (5, 30) | 9 (3.5, 21) | 0.002 |
| SP142_IC (%) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.017 |
| SP142_TC (%) | 0 (0–0) | 0 (0–8) | 0 (0–0) | <0.001 |
| TMB | 9.4 (7.5–13.7) | 9.4 (7.6–13.1) | 9.4 (7.5–14.9) | 0.69 |
Continuous variables are shown as median and interquartile range, and categorical variables are shown with number and frequency. ECOG PS, Eastern Cooperative Oncology Group performance status; TNM stage, tumor-node-metastasis stage; TILs, tumor-infiltrating lymphocytes; IC, immune cell; TC, tumor cell; TMB, tumor mutation burden; PD-L1, programmed cell death-ligand 1.
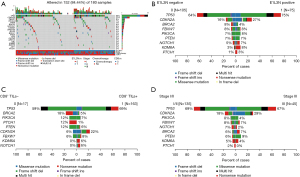
All of the 180 samples were sequenced, and mutations with clinical evidence were identified using the OncoKB database. Altered genes were identified in 152 (84.4%) of the samples (see Figure 1A) and the top 8 altered genes (no less than 5%) were tumor suppressor p53 (TP53), cyclin-dependent kinase inhibitor 2A (CDKN2A), F-box with 7 tandem WD40 (FBXW7), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), phosphatase and tensin homolog (PTEN), BReast-CAncer susceptibility gene 2 (BRCA2), notch homolog protein 1 (NOTCH1), and Lysine demethylase 6A (KDM6A). This was similar to the significantly mutated genes reported in The Cancer Genome Atlas (TCGA) database, which identified 10 genes [i.e., TP53, CDKN2A, PTEN, PIK3CA, Kelch-1ike ECH- associated protein 1 (KEAP1), Mixed Lineage Leukemia 2 (MLL2), Human leukocyte antigen A (HLA-A), Nuclear factor erythroid 2-related factor 2 (NFE2L2), NOTCH1, and Retinoblastomal (RB1)] with a false discovery rate Q value <0.1 (31). Additionally, 14 genes exhibited significant enrichment for the somatic mutation in Caucasians with LUSC (Kadara et al. cohort): TP53, MLL2, PIK3CA, NFE2L2, CDH8, KEAP1, PTEN, ADCY8, PTPRT, CALCR, GRM8, FBXW7, RB1, and CDKN2A (32). In our cohort, the TP53 mutation was most frequently found in 68% of all cases (TCGA: 81%; Kadara et al. cohort: 60%) followed by CDKN2A in 21% of all cases. The mutation rates of TP53 (64% vs. 75%; P=0.145), CDKN2A (16% vs. 27%; P=0.095), BRCA2 (8% vs. 4%; P=0.365), FBXW7 (8% vs. 8%; P=1.000), PIK3CA (7% vs. 8%; P=0.776), KDM6A (3% vs. 8%; P=0.167) were comparable between the PD-L1+ and PD-L1– patients (see Figure 1B). Similarly, gene alterations, including TP53 (59% vs. 69%; P=0.416), BRCA2 (18% vs. 5%; P=0.072), PIK3CA (12% vs. 7%; P=0.353), PTCH1 (12% vs. 1%; P=0.024), PTEN (12% vs. 6%; P=0.316), CDKN2A (6% vs. 22%; P=0.203), FBXW7 (6% vs. 8%; P=1.000), and NOTCH1 (0% vs. 6%; P=1.000), were comparable between the CD8+ TILs+ group and CD8+ TILs− group, and only the gene mutation frequency of PTCH1 was significantly different between the two groups (see Figure 1C), and the contingency coefficient of PTCH1 between CD8+ TILs+ group and CD8+ TILs- group was 0.247, P=0.001. However, the gene variation rate did not differ significantly between the two groups (TNM stages I/II and III), including TP53 (69% vs. 67%; P=0.854), CDKN2A (18% vs. 29%; P=0.136), PIK3CA (8% vs. 4%; P=0.523), FBXW7 (8% vs. 7%; P=1.000), NOTCH1 (7% vs. 2%; P=0.455), BRCA2 (6% vs. 7%; P=1.000), and PTEN (6% vs. 9%; P=0.498; see Figure 1D).
Effects of clinicopathological characteristics on survival
The univariate and multivariate analyses results are set out in Table 2. In the univariate analysis, TNM stage (HR =1.690; P=0.005), pleural invasion (HR =1.838; P=0.037), CD8+ TILs (HR =0.400; P<0.001), and PD-L1 (HR =0.615; P=0.005) were significantly associated with DFS. The multivariate analysis results showed that TNM stage (HR =1.719; P=0.005) was an independent risk factor and both CD8+ TILs (HR =0.433, P=0.002) and PD-L1 (HR =0.700, P=0.046) were independent protective factors for longer DFS.
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥65 vs. <65 y) | 1.166 (0.834, 1.632) | 0.369 | |||
| Sex (male vs. female) | 0.891 (0.454, 1.751) | 0.738 | |||
| ECOG PS (1 vs. 0) | 1.103 (0.766, 1.588) | 0.599 | |||
| Smoking (ever vs. never) | 1.036 (0.729, 1.474) | 0.842 | |||
| TNM stage (III vs. I/II) | 1.690 (1.167, 2.446) | 0.005* | 1.719 (1.181, 2.504) | 0.005* | |
| Lymph node invasion (yes vs. no) | 1.385 (0.934, 2.053) | 0.105 | |||
| Lymph node stage (1 vs. 0) | 1.464 (0.909, 2.357) | 0.245 | |||
| Lymph node stage (2 vs. 0) | 1.270 (0.700, 2.306) | 0.245 | |||
| Differentiation (intermediate vs. low) | 1.060 (0.733, 1.532) | 0.817 | |||
| Differentiation (high vs. low) | 1.276 (0.596, 2.732) | 0.817 | |||
| Maximum diameter (≥4 vs. <4) | 1.068 (0.979, 1.164) | 0.140 | |||
| Pleural invasion (yes vs. no) | 1.838 (1.035, 3.261) | 0.037* | 1.751 (0.972, 3.155) | 0.062 | |
| Venous invasion (yes vs. no) | 1.883 (0.877, 4.044) | 0.104 | |||
| CD8+ TILs (>0 vs. 0) | 0.400 (0.238, 0.6724) | <0.001* | 0.433 (0.255, 0.735) | 0.002* | |
| TMB (≥9.4 vs. <9.4) | 1.253 (0.903, 1.738) | 0.177 | |||
| PD-L1 (≥1% vs. <1%) | 0.615 (0.438, 0.864) | 0.005* | 0.700 (0.493, 0.993) | 0.046* | |
Variables with a P value <0.05 in the univariate models were analyzed in the multivariate analysis model. *, P<0.05. DFS, disease-free survival; ECOG PS, Eastern Cooperative Oncology Group performance status; TNM stage, tumor-node-metastasis stage; CD8+, cluster of differentiation 8+; TILs, tumor-infiltrating lymphocytes; TMB, tumor mutation burden; PD-L1, programmed cell death-ligand 1.
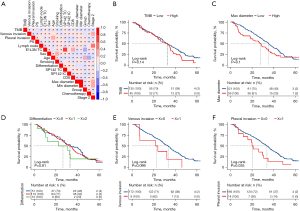
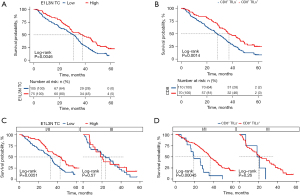
Next, we conducted a survival analysis. The median follow-up time was 105 months, and the median DFS was 33.1 months (95% CI: 28.227, 37.973), while the median OS didn’t reach, which maybe because 75% of the patients were stage I/II. Therefore, only DFS was examined in the survival analysis. As Figure 2A showed, a correlation matrix analysis and cluster analysis of the clinical characteristics were performed. The Kaplan-Meier method was used for the continuous variables that were grouped by the first quartile, median, and third quartile to determine the best cutoff value. The results indicated that patients with different TMB levels (see Figure 2B), maximum diameters (see Figure 2C), differentiation degrees (see Figure 2D), and venous invasion statuses (Figure 2E) had comparable DFS. Notably, pleural invasion (P=0.035) status was significantly correlated with a poorer DFS (see Figure 2F), and high PD-L1 expression on tumor cells tested by the clone E1L3N PD-L1 antibody (E1L3N_TC) (HR =0.615, P=0.0046; see Figure 3A) and CD8+ TILs+ (CD8_IC) (HR =0.400, P=0.0014; see Figure 3B) were significantly associated with longer DFS. A further subgroup analysis revealed that both E1L3N_TC (see Figure 3C) and CD8_IC (see Figure 3D) were significantly correlated with the DFS of stage I/II patients, but not the DFS of stage III patients.
Discussion
Lung cancer is one of the most pathogenic and fatal cancer diseases in the world (33), and LUSC is a major pathological subtype of lung cancer. Surgery is the standard treatment for early stage LUSC; however, its recurrence rate remains high after surgery. In the last decade, immunotherapy, especially ICIs that target the PD-1/PD-L1 axis, has brought the treatment strategies of advanced lung cancer into a new era. Exploring the relationship between immune patterns and prognosis is of great significance for neoadjuvant or adjuvant immunotherapy in patients with early LUSC.
We examined 180 samples using targeted sequencing, and the important somatic mutations we reported are consistent with those reported in previous studies (31,32,34,35). Understanding the interaction between the genetic landscape, TME patterns, and clinicopathological parameters may help promote the development of treatment for early stage or advanced non-small cell lung cancer (NSCLC). PTCH1 gene suppressed the Hedgehog signaling pathway, which plays an important role in cell embryonic development, by inhibiting a key signal transducer Smoothened receptor (SMO) (36,37). Previous studies have shown that PTCH1 inhibits the cell cycle and plays a critical role in cancer progression and metastasis (38). Wan et al. found that the low expression of PTCH1 may promote the progression of NSCLC (39). Our results of the sequencing and IHC staining analysis revealed that only PTCH1 gene mutation frequency was significantly lower in patients with CD8+ TILs+. And the results of the Kaplan-Meier survival analysis confirmed that higher CD8 infiltration were significantly correlated with longer DFS. These results suggest that lower PTCH1 gene mutation and CD8+TILs+ may be beneficial background for LUSC patients to have better prognosis.
Higher PD-L1 expression was also significantly with longer DFS and the DFS data was only comparable between high and low TMB groups. Similar to previous reports (40,41), intense lymphocytic infiltration and lower TMB were significantly associated with better DFS. Jiang et al. (42) reported that TMB combined with CD8+ TILs and/or PD-L1 could successfully stratify the prognosis of surgically resected LUSC patients and found a significant association between CD8+ TILs density and PD-L1 expression. In our study, the multivariate analysis suggested that stage was an independent risk factor for DFS. Thus, we conducted a subgroup analysis to examine the effects of CD8+ TILs and PD-L1 expression on DFS based on different TNM stages, and found that the two factors were significantly correlated with the DFS of stage I/II patients but not the DFS of stage III patients. Given these results, a comprehensive analysis of these biomarkers in predicting the prognosis of early LUSC patients is warranted.
In summary, this study explored the gene molecular characteristics of patients with surgically resected LUSC who had not undergone immunotherapy previously and its association with clinicopathological parameters. The results suggested that only PTCH1 gene mutation frequency was correlated with CD8+ TILs density. Intense CD8+ TILs density and high PD-L1 expression were associated with a longer DFS. Our findings provide insights into the precision treatment of early LUSC. These preliminary results need to be further validated, and the mechanisms need to be further explored with an enlarged sample size.
Acknowledgments
Funding: This work was supported by the Shanghai Key Specialty of Respiratory Disease and Shanghai Major Disease Multidisciplinary Diagnosis and Treatment Capacity Building Project.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-630/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-630/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-630/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the provisions of the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. K20-288). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Gandara DR, Hammerman PS, Sos ML, et al. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res 2015;21:2236-43. [Crossref] [PubMed]
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. [Crossref] [PubMed]
- Langer CJ, Obasaju C, Bunn P, et al. Incremental Innovation and Progress in Advanced Squamous Cell Lung Cancer: Current Status and Future Impact of Treatment. J Thorac Oncol 2016;11:2066-81. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol 2010;28:35-42. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Han H, Silverman JF, Santucci TS, et al. Vascular endothelial growth factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann Surg Oncol 2001;8:72-9. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Pilotto S, Sperduti I, Leuzzi G, et al. Prognostic Model for Resected Squamous Cell Lung Cancer: External Multicenter Validation and Propensity Score Analysis exploring the Impact of Adjuvant and Neoadjuvant Treatment. J Thorac Oncol 2018;13:568-75. [Crossref] [PubMed]
- Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [Crossref] [PubMed]
- Mlecnik B, Bindea G, Pagès F, et al. Tumor immunosurveillance in human cancers. Cancer Metastasis Rev 2011;30:5-12. [Crossref] [PubMed]
- Zeng Z, Yang F, Wang Y, et al. Significantly different immunoscores in lung adenocarcinoma and squamous cell carcinoma and a proposal for a new immune staging system. Oncoimmunology 2020;9:1828538. [Crossref] [PubMed]
- Stiles BM, Rahouma M, Hussein MK, et al. Never smokers with resected lung cancer: different demographics, similar survival. Eur J Cardiothorac Surg 2018;53:842-8. [Crossref] [PubMed]
- Imai H, Kaira K, Minato K. Clinical significance of post-progression survival in lung cancer. Thorac Cancer 2017;8:379-86. [Crossref] [PubMed]
- Ock CY, Keam B, Kim S, et al. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin Cancer Res 2016;22:2261-70. [Crossref] [PubMed]
- Chalela R, Curull V, Enríquez C, et al. Lung adenocarcinoma: from molecular basis to genome-guided therapy and immunotherapy. J Thorac Dis 2017;9:2142-58. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Armato SG 3rd, Nowak AK. Revised Modified Response Evaluation Criteria in Solid Tumors for Assessment of Response in Malignant Pleural Mesothelioma (Version 1.1). J Thorac Oncol 2018;13:1012-21. [Crossref] [PubMed]
- Takada K, Okamoto T, Toyokawa G, et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer 2017;104:7-15. [Crossref] [PubMed]
- Yang H, Shi J, Lin D, et al. Prognostic value of PD-L1 expression in combination with CD8+ TILs density in patients with surgically resected non-small cell lung cancer. Cancer Med 2018;7:32-45. [Crossref] [PubMed]
- Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer 2016;55:7-14. [Crossref] [PubMed]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010;38:e164. [Crossref] [PubMed]
- Mayakonda A, Lin DC, Assenov Y, et al. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res 2018;28:1747-56. [Crossref] [PubMed]
- Yu H, Chen Z, Ballman KV, et al. Correlation of PD-L1 Expression with Tumor Mutation Burden and Gene Signatures for Prognosis in Early-Stage Squamous Cell Lung Carcinoma. J Thorac Oncol 2019;14:25-36. [Crossref] [PubMed]
- Zhang XC, Wang J, Shao GG, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun 2019;10:1772. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Kadara H, Choi M, Zhang J, et al. Whole-exome sequencing and immune profiling of early-stage lung adenocarcinoma with fully annotated clinical follow-up. Ann Oncol 2017;28:75-82. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Choi M, Kadara H, Zhang J, et al. Mutation profiles in early-stage lung squamous cell carcinoma with clinical follow-up and correlation with markers of immune function. Ann Oncol 2017;28:83-9. [Crossref] [PubMed]
- Okamoto T, Takada K, Sato S, et al. Clinical and Genetic Implications of Mutation Burden in Squamous Cell Carcinoma of the Lung. Ann Surg Oncol 2018;25:1564-71. [Crossref] [PubMed]
- Sriperumbudur A, Breitzig M, Lockey R, et al. Hedgehog: the key to maintaining adult lung repair and regeneration. J Cell Commun Signal 2017;11:95-6. [Crossref] [PubMed]
- Hasanovic A, Mus-Veteau I. Targeting the Multidrug Transporter Ptch1 Potentiates Chemotherapy Efficiency. Cells 2018;7:107. [Crossref] [PubMed]
- Li Y, Zhang D, Chen C, et al. MicroRNA-212 displays tumor-promoting properties in non-small cell lung cancer cells and targets the hedgehog pathway receptor PTCH1. Mol Biol Cell 2012;23:1423-34. [Crossref] [PubMed]
- Wan X, Kong Z, Chu K, et al. Co-expression analysis revealed PTCH1-3’UTR promoted cell migration and invasion by activating miR-101-3p/SLC39A6 axis in non-small cell lung cancer: implicating the novel function of PTCH1. Oncotarget 2018;9:4798-813. [Crossref] [PubMed]
- Owada-Ozaki Y, Muto S, Takagi H, et al. Prognostic Impact of Tumor Mutation Burden in Patients With Completely Resected Non-Small Cell Lung Cancer: Brief Report. J Thorac Oncol 2018;13:1217-21. [Crossref] [PubMed]
- Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:1223-30. [Crossref] [PubMed]
- Jiang T, Shi J, Dong Z, et al. Genomic landscape and its correlations with tumor mutational burden, PD-L1 expression, and immune cells infiltration in Chinese lung squamous cell carcinoma. J Hematol Oncol 2019;12:75. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


