
The overall survival benefit in Chinese ALK+ NSCLC patients received targeted therapies
Introduction
Anaplastic lymphoma kinase (ALK) gene rearrangement is found in approximately 5% of non-small cell lung cancer (NSCLC) patients and has been identified as a driver gene of lung cancer (1,2). Crizotinib, the 1st ALK inhibitor (ALKi), was approved by the Food and Drug Administration for ALK-rearranged metastatic NSCLC in 2011 and became available in 2013 in China. The PROFILE 1029 study indicated that compared to conventional chemotherapy, crizotinib significantly prolonged the progression-free survival (PFS) of east Asian patients (3). The objective response rate (ORR) was up to 87.5%; however, half of the patients developed disease progression after about 11 months (3-5). The final overall survival (OS) analysis from the PROFILE 1014 study suggested that there was an improvement in OS that favored crizotinib rather than chemotherapy, and the longest OS was observed in patients who received subsequent ALKis after crizotinib (6). Second-generation (ceritinib, alectinib, brigatinib, and ensartinib) and 3rd-generation (lorlatinib) ALKis have demonstrated improved efficacy and central nervous system penetration. Recent findings suggest that sequential therapy with ALKis improves the OS of advanced NSCLC patients (6-10). An in vitro study indicated the ability of third-generation inhibitor lorlatinib in preventing emergence of single and subsequently compound ALK mutation (11). The available ALKis have different potencies, differential penetration into the central nervous system, unique safety profiles, and different “spectrums” of activity against particular acquired resistance mutations. Sequential treatment with another ALKi has been proven effective in patients who fail the initial ALKi treatment (12). Sequential use of ALK inhibitors of crizotinib and alectinib was also shown encouraging value in Japanese population (13). Despite the availability of new ALKi therapies, less is known about the outcomes associated with sequencing of applying ALKis, especially among Chinese patients. We conduct the current study to evaluate the outcome of multiple use ALKis in ALK+ NSCLC patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-622/rc).
Methods
Patients
Patients with advanced NSCLC (stage IIIB–IV) harboring the ALK rearrangement who had been diagnosed between February 1, 2012 and November 19, 2019 at Peking University Cancer Hospital, were included in this cohort study. The patients were aged no less than 18 years, with detailed trackable medical and treatment records since the diagnosis with NSCLC. All those who received an ALKi treatment received at least 28 days of the treatment.
Study design
This study collected the data from patient registry of Peking University Cancer Hospital. The clinical characteristics retrospectively obtained or calculated from the database were diagnostic detail (type and stage of the cancer), age (at year of diagnosis), gender, smoking history, histopathological type, brain metastases, and disease progression. The initial anti-tumor therapy and subsequent treatments were collected. The subsequent clinical details such as medical imaging and blood tests would be collected and updated in the routine medical checkup. Data were collected until the death of the patients or the end to follow up for the current study (Jun 30, 2020). The outcomes of the patients, such as OS, PFS will be compared between the groups treated with different strategies, in order to evaluate which one is most benefit to the patients.
Assessments
Tumor stage was reconfirmed according to the American Joint Committee on Cancer 8th Edition of the TNM Classification for Lung Cancer (14). Physical & clinical characters and disease progression was identified by reviewing patients’ charts and radiographic images. ALK rearrangement was determined by ALK Ventana (D5F3) immunohistochemistry (IHC), or fluorescent in-situ hybridization (FISH, Vysis ALK break-apart FISH assay), or next-generation sequencing.
OS was defined as the duration from the 1st ALKi dose to death or final follow-up. For patients who did not receive an ALKi treatment, OS was defined as the duration from the 1st anti-tumor therapy session to death or final follow-up. PFS was defined as the time from starting the treatment of NSCLC by either systemic chemotherapy or ALKis to disease progression or death from any cause. Progression disease (PD) was defined as at least 20% and 5-mm increase in the sum of diameters of target lesions [according to RECIST, version 1.1 (15)]. Oligoprogression was defined as progression in only 1 site; the brain was considered 1 site of oligoprogression regardless of the number of lesions progressing in the brain at the time.
Ethical statement
All techniques were performed in accordance with relevant guidelines and regulations. The study was approved by the Ethics Committee, Peking University Cancer Hospital (No. 2021KT21). All clinical information was provided in a de-identified pattern. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Statistical analysis
The Kaplan-Meier method was used to estimate the median PFS for each treatment group with 95% confidence intervals. The comparison between the treatment groups with respect to survival outcomes was based on a stratified log-rank test at a 5% level of significance (two-sided). A multivariate Cox proportional-hazard analysis was conducted to examine the relationship among the variables with OS. All the statistical tests were two-sided, and a P value <0.05 was considered statistically significant. All the analyses were performed using SPSS software (version 26, IBM Crop., Armonk, NY, USA).
Results
Patient characteristics
A total of 222 patients with the ALK rearrangement were enrolled in this study from February 1, 2012 to November 19, 2019. The median follow-up for OS was 41 months. A total of 20 patients were excluded because of their tumor stage, 19 were lost to follow-up, and 7 were excluded because it was unknown which medication they took. Thus, ultimately, 176 patients were included in the data analysis. Of the 176 patients, 140 received at least one type of ALKi. Notably, 106 patients were treated with crizotinib as the initial ALKi. The characteristics of the patients are shown in Table 1.
Table 1
| Characteristics | Total (n=176) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| Median age | 54 | |
| <45 | 49 | 27.8 |
| 45–65 | 110 | 62.5 |
| >65 | 17 | 9.7 |
| Gender | ||
| Male | 92 | 52.3 |
| Female | 84 | 47.7 |
| Smoking status | ||
| Never-smoker | 107 | 60.8 |
| Current/former-smoker | 56 | 31.8 |
| Missing data | 13 | 7.4 |
| Histology | ||
| Adenocarcinoma | 162 | 92.0 |
| Squamous cell carcinoma | 7 | 4.0 |
| Other | 7 | 4.0 |
| Brain metastasis at diagnosis | ||
| Yes | 38 | 21.6 |
| No | 138 | 78.4 |
| No ALKi | 36 | 20.5 |
| 1 ALKi | 83 | 47.2 |
| 2 ALKis | 41 | 23.3 |
| ≥3 ALKis | 16 | 9.1 |
| Crizotinib as the initial ALKi | 106 | |
| Number of systemic therapies before crizotinib | ||
| 0 | 70 | 66.0 |
| 1 | 28 | 26.4 |
| ≥2 | 8 | 7.5 |
| Next-generation ALKi as the initial ALKi | 34 | |
| Alectinib | 19 | 55.9 |
| Ceritinib | 5 | 14.7 |
| Brigatinib | 8 | 23.5 |
| Ensartinib | 2 | 5.9 |
| Number of systemic therapies before next-generation ALKi | ||
| 0 | 27 | 79.4 |
| 1 | 4 | 11.8 |
| 2 | 3 | 8.8 |
ALK, anaplastic lymphoma kinase; ALKi, ALK inhibitor.
OS in the ALK-rearranged advanced NSCLC patients
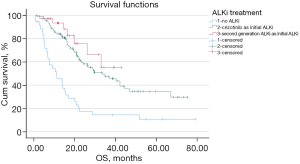
Of the 176 ALK-rearranged NSCLC patients, 106 patients were treated with crizotinib as the initial ALKi, 34 were treated with a 2nd-generation ALKi as the initial ALKi, and 36 were not treated with an ALKi. The median OS (mOS) was 10.3 months [95% confidence interval (CI): 3.3–17.4 months] for patients treated with conventional chemotherapy but no ALKi, 32.9 months (95% CI: 20.1–45.6 months) for patients treated with crizotinib as the initial ALKi, and not reached (NR) for those treated with a next-generation ALKi as the initial ALKi (see Figure 1). A significantly longer OS was observed either in patients treated with crizotinib (P<0.001) or in patients treated with a next-generation ALKi (P<0.001) as the initial ALKi, compared with patients treated with conventional chemotherapy but no ALKi. No significant difference was observed in OS between the patients treated with crizotinib and the patients treated with a next-generation ALKi as the initial ALKi (P=0.287). In the ALK-rearranged patients treated with crizotinib as the initial ALKi, no significant difference was observed in OS in terms of gender, smoking status, and whether the patient was aged above the median or not.
Efficacy of crizotinib
A total of 106 patients were treated with crizotinib as the initial ALKi. Disease progression was observed in 85 patients (80.2%). The ORR was 61.3%, the disease control rate (DCR) was 95.3%, and the median PFS was 10.9 months (95% CI: 9.2–12.5 months). The median duration using crizotinib was 12.6 months (95% CI: 11.0–14.1 months). Of the 106 patients, 33 continued to be treated with crizotinib beyond progressive disease (CBPD). At the time of our analysis, 21 patients were still receiving crizotinib. The mOS was 32.9 months (95% CI: 20.1–45.6 months).
A total of 27 patients were identified as having brain metastases before applying crizotinib, and had a median PFS of 9.2 months (95% CI: 4.4–14.1 months) and a mOS of 28.3 months (95% CI: 13.1–43.5 months). For the 79 patients without brain metastases before initial crizotinib treatment, the median PFS was 11.5 months (95% CI: 9.0–14.0 months), and the mOS was 33.9 months (95% CI: 19.2–48.6 months). There was no significant difference in either PFS or OS between the two groups.
Of the 106 patients, 36 (34.0%) treated with crizotinib as the initial ALKi had received systemic therapy other than an ALKi before the 1st crizotinib dose, while the rest (66.0%) received crizotinib as the 1st-line therapy. In the patients who received a systemic therapy other than ALKi before the 1st treatment of crizotinib, the ORR was 38.9% (14/36), the DCR was 94.4% (34/36), and the median PFS was 13.2 months (95% CI: 8.8–17.5 months), while for those who received 1st-line crizotinib, the ORR and DCR was 72.9% (51/70) and 95.7% (67/70), and the median PFS was 10.2 months (95% CI: 8.5–11.9 months). The ORR of the patients who received 1st-line crizotinib was significantly higher than that of those who received other systemic therapies before crizotinib (P=0.01). There was no significant difference in PFS between the two groups (P=0.099).
Of the 106 patients, 36 patients received systemic conventional chemotherapy before the 1st treatment of crizotinib. Among those, 28 patients received crizotinib as a 2nd-line therapy, 5 received crizotinib as a 3rd-line therapy, 2 received crizotinib as a 4th-line therapy, and 1 received crizotinib as a 7th-line therapy. Receiving only 1 systemic therapy before the 1st dose of crizotinib did not significantly improve OS compared to receiving 2 or more therapies (P=0.242). A total of 22 patients received no other ALKi than crizotinib and had an mOS of 20.1 months (95% CI: 12.9–27.2 months); the other 14 patients received subsequent ALKis after crizotinib and had an mOS of 28.6 months (95% CI: 14.3–42.9 months); no significant difference was observed between the two groups (P=0.247).
Of the 70 patients who received 1st-line crizotinib, 33 received no other ALKi than crizotinib and had an mOS of 20.3 months (95% CI: 12.3–28.3 months); the other 37 patients received subsequent ALKis after crizotinib, and the mOS was NR, but the mOS in subsequent ALKis group was significantly longer than the crizotinib-only group (P<0.001).
CBPD
Disease progression was observed in 85 patients, who received crizotinib as the initial ALKi (see Table 2). The mOS was 9.1 months (95% CI: 6.9–11.2 months). A total of 33 patients received CBPD, and had a median treatment duration of 7.2 months (95% CI: 0.6–49.6 months). Of the 85 patients with progressive disease (PD), 57 received 1st-line crizotinib (23 of whom received CBPD), 28 received systemic therapies other than ALKi before crizotinib (10 of whom received CBPD), 34 received subsequent therapies other than ALKi (17 of whom received CBPD), and 51 received subsequent ALKis (16 of whom received CBPD) (see Table 3).
Table 2
| Site of progression with crizotinib | Total (n=85) |
|---|---|
| Cerebral | 34 |
| Lung | 36 |
| Pleura | 5 |
| Lymph node | 17 |
| Bone | 6 |
| Liver | 3 |
| Muscle | 1 |
| Subcutaneous | 1 |
| Pericardium | 1 |
| ≥2 organs | 20 |
Table 3
| Characteristics | All patients (N=85) | CBPD (N=33) | No CBPD (N=52) |
|---|---|---|---|
| Cerebral progression | |||
| Yes | 34 | 24 | 10 |
| No | 51 | 9 | 42 |
| Oligoprogression | |||
| Yes | 49 | 25 | 24 |
| No | 36 | 8 | 28 |
| Next-generation ALKis after crizotinib progression | |||
| Yes | 51 | 16 | 35 |
| No | 34 | 17 | 17 |
| Crizotinib mPFS (months) (95% CI) | 9.1 (6.9–11.2) | 9.1 (4.4–13.8) | 8.9 (6.6–11.2) |
| mOS (95% CI) (months) | 29.0 (21.2–36.9) | 32.7 (18.2–47.1) | 29.0 (18.5–39.6) |
CBPD, crizotinib beyond progressive disease; ALKis, anaplastic lymphoma kinase inhibitors; mPFS, median progression-free survival; CI, confidence interval; mOS, median overall survival.
Among all the patients, 34 developed initial cerebral progression in brain. The mOS of patients without cerebral progression was 40.5 months (95% CI: 27.7–53.3 months), and cerebral progression greatly reduced the mOS of patients to 20.1 months (95% CI: 16.3–23.9 months). No statistically significant difference between the two groups was observed (P=0.066). Of the 34 cerebral progression patients, 24 received CBPD, but no significant difference was observed in the mOS between the patients received CBPD or not (mOS: 46.4 vs. 33.1 months; P=0.097).
A total of 49 patients who received crizotinib as the initial ALKi developed oligoprogression, and 36 developed systematic progression. Prolonged OS was associated with oligoprogression (oligoprogression: n=49, mOS 41.2 months, 95% CI: 19.6–62.7 months; systemic progression: n=36, mOS 18.2 months, 95% CI: 12.8–23.6 months; P=0.006). Of the patients with oligoprogression, 25 received CBPD (mOS 28.3 months, 95% CI: 9.4–47.2 months), while the other 24 did not (mOS 41.1 months, 95% CI: 34.1–48.1 months); thus, there were no meaningful advantage in using CBPD (P=0.948). A total of 8 patients with systemic progression received CBPD (mOS 39.2 months, 95% CI: 19.5–77.6 months) while the other 28 did not (mOS 18.2 months, 95% CI: 13.6–22.9 months), the application of CBPD showed no significant effect either (P=0.360).
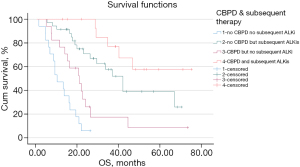
A total of 33 disease progressive patients received CBPD, of whom 17 received no ALKi other than crizotinib, and 16 received CBPD and subsequent next-generation ALKis. A total of 35 patients did not receive CBPD but did receive subsequent next-generation ALKis, while the other 17 patients received neither CBPD nor a subsequent next-generation ALKi. The analysis showed that patients who received neither CBPD nor a subsequent next-generation ALKi had shorter OS than those who received CBPD but no subsequent next-generation ALKi (mOS: 9.7 vs. 20.3 months; P=0.015), no CBPD but subsequent next-generation ALKis (mOS: 9.7 vs. 41.1 months; P<0.001), and CBPD with subsequent next-generation ALKis (mOS: 9.7 months vs. NR; P<0.001). The OS of the patients who received no CBPD but who received subsequent next-generation ALKis was significantly longer than that of those who received CBPD but who did not receive subsequent next-generation ALKis (mOS: 41.1 vs. 20.3 months; P=0.006) (see Figure 2). In patients who received subsequent next-generation ALKis, the use of CBPD appeared to have no effect on OS (mOS: NR vs. 41.1 months; P=0.093).
He multivariable Cox regression revealed that systemic therapy before crizotinib was significantly associated with an increased risk of death [hazard ratio (HR) 1.89, 95% CI: 1.06–3.39; P=0.032]. Furthermore, longer PFS of crizotinib (the PFS of crizotinib longer than the median), the sequential therapy of ALKis and CBPD were found to be significantly associated with a decreased risk of death. Other variables, including sex, age, smoking status, and oligoprogression of crizotinib, were not significant (see Table 4).
Table 4
| Variables | Tested | Reference | Multivariable analysis | |
|---|---|---|---|---|
| HR (95% CI) | P value | |||
| Age | > Median | ≤ Median | – | NS |
| Gender | Male | Female | – | NS |
| Smoking status | Never | Former/current | – | NS |
| Systemic therapy prior to crizotinib | Yes | No | 1.89 (1.06–3.39) | 0.032 |
| PFS of crizotinib | > Median | ≤ Median | 0.26 (0.13–0.50) | 0.000 |
| Sequential therapy of ALK inhibitors | Yes | No | 0.17 (0.09–0.34) | 0.000 |
| CBPD | Yes | No | 0.46 (0.25–0.87) | 0.017 |
| Oligoprogression of crizotinib | Yes | No | – | NS |
OS, overall survival; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival; ALK, anaplastic lymphoma kinase; CBPD, crizotinib beyond progressive disease; NS, not significant.
Sequential therapy of ALKis
A total of 57 patients received sequential therapy using ALKis, of whom 51 received a crizotinib-led sequential therapy, and the other 6 received a 2nd-generation ALKi-led sequential therapy. In the 51 patients treated with crizotinib-led sequential therapy, 37 received 1st-line crizotinib, and the other 14 received non-ALKi systemic therapies before crizotinib (mOS: NR vs. 28.6 months; P=0.042).
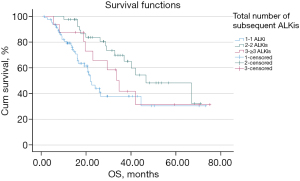
The mOS of the patients who received at least 1 ALKi (n=140) was 33.9 months (95% CI: 24.9–42.9 months). More specifically, the mOS of patients who received 1 ALKi was 21.3 months (n=83, 95% CI: 17.9–24.7 months), the mOS of patients who received 2 ALKis was 45.8 months (n=41, 95% CI: 25.6–66.1 months), and the mOS of patients who received ≥3 ALKis was 32.9 months (n=16, 95% CI: 24.4–41.4 months) (see Figure 3). Compared to patients who received 1 ALKi, a survival benefit was observed in patients who received 2 ALKis (P=0.003), but not in patients who received ≥3 ALKis (P=0.366).
Discussion
ALK rearrangement is a driver gene in NSCLC (16). Without ALKis, patients harboring ALK rearrangement suffer shorter OS, even compared to EGFR-wild type (WT)/ALK-WT patients (17). In this study, we described the treatment patterns and outcomes of patients treated with ALKis from February 1, 2012 to November 19, 2019.
In this cohort, 36 eligible patients did not receive ALKis. Of these patients, 7 had been diagnosed before January 22, 2013, at which time the ALKi became officially available in the Chinese market; the others did not receive ALKis due to economic difficulties, but it was highly recommended by their physicians. Crizotinib has been included in the National Medical Insurance System of China since October 10, 2018. This reduces the price of crizotinib by 70%. In addition to the insurance deductible, patients paid no more than 10% of original price before 2018. From 2019, all eligible patients were able to receive affordable ALKi treatment.
At the time of the analysis, 31 of the 36 patients who did not receive ALKi treatment died, and had a mOS of 10.3 months. Conversely, the mOS of patients who received at least 1 type of ALKi was 32.9 months. The OS of the ALK-rearranged patients was prolonged by ALKi treatment, no matter which ALKi. A total of 5 (13.9%) ALK-rearranged patients who did not receive any ALKi treatment were still alive at the time of this analysis, of whom 3 had received palliative surgery (2 received palliative lobectomy and were followed up for 78.9 and 63.7 months each; 1 received palliative lobectomy and hepatectomy and was followed up for 62.0 months). This suggests that the palliative debulking operation may improve patient OS. A favorable 5-year OS of 31–42.7% after debulking surgery (consisting of primary tumor resection with or without resection of the disseminated nodules as much as possible) followed by systemic therapy or observation in NSCLC patients has been reported (18-20). Another study revealed that debulking surgery is a more beneficial treatment than thoracotomy for stage I and IV NSCLC patients (21). Unfortunately, the current study could not examine whether the combination of ALKi and debulking surgery resulted in better survival due to data limitations. This may become a major subject of our future analyses.
Of the patients, 60.2% (106/176) were treated with crizotinib as the initial ALKi, as it has been the only ALKi available for a long time. We found that patients using crizotinib as the initial ALKi had an ORR of 61.3% and a DCR of 95.3%, and these figures were similar to those reported in a phase-I clinical trial (PROFILE 1001) (22). A total of 70 patients received 1st-line crizotinib, and had a similar ORR to that reported in the PROFILE1014 study (6). The other 36 patients received a systemic therapy other than ALKi before treatment with crizotinib, and their ORR was slightly lower than that reported in the PROFILE1007 study (23). We presume that this difference is due to a statistical bias (i.e., fewer subjects). The statistics revealed that the use of 1st-line crizotinib was associated with a significantly longer ORR, but a similar DCR.
Several clinical trials and meta-analyses have found an increase in PFS among patients treated with crizotinib compared to those treated with conventional chemotherapy (3,4,24), but no significant increase in OS has been found (6,23,25). The lack of benefit in terms of OS is probably due to the cross-over between the control and experimental arms, and the subsequent therapies. As the sequential therapy of ALKis has been widely accepted, it is difficult to evaluate whether patients could benefit from crizotinib in terms of OS. Some of the patients in our study who received crizotinib did not receive other ALKi therapy, which enabled us to evaluate the effect of crizotinib on OS. Patients who took crizotinib as the only ALKi had prolonged OS compared to those who only received conventional chemotherapy. Further, without subsequent ALKi therapy, the use or lack of use of systemic therapy before crizotinib did not affect PFS or OS.
CBPD and subsequent next-generation ALKi therapy are 2 possible and important strategies of crizotinib-based treatment. There has been debate as to which is more favorable in terms of prolonging OS. Several studies have demonstrated that CBPD may provide a survival benefit to patients with advanced ALK-rearranged NSCLC (26,27). A single-center, retrospective study reported that 74.6% of 201 ALK-rearranged NSCLC patients acquired resistance to crizotinib, and 58 (38.7%) of these continued CBPD. The median treatment duration was 20.7 months. The mOS in the CBPD group was 61.0 months as compared to 41.4 months in the non-CBPD group, but the difference was not statistically significant (28). In another study of 194 ALK-rearranged NSCLC RECIST-defined PD patients, 120 (62%) continued CBPD, and the CBPD patients demonstrated significantly longer OS from the time of initial PD (median: 16.4 vs. 3.9 months; P<0.0001) and from the time of initial crizotinib treatment (median: 29.6 vs. 10.8 months; P<0.0001). The multiple-covariate Cox regression analysis revealed that CBPD continued to be significantly associated with improved OS after adjusting for relevant factors (29).
As the efficacy of next-generation ALKis has been shown in the treatment of post-crizotinib tumors (30-34), crizotinib-led sequential therapy with next-generation ALKis has been widely accepted. A retrospective study demonstrated that patients receiving crizotinib followed by a next-generation ALKis after progression had an mOS of 86 months, while patients with progression on crizotinib who did not receive next-generation ALKis had an mOS of 52 months (9). In the randomized phase-III PROFILE 1014 trial, patients who received 1st-line crizotinib achieved a 4-year OS of 56.6%, and the longest OS was observed in patients who received subsequent ALKis at progression, with a 4-year OS of approximately 80%, compared to an OS of approximately 25% among those who did not receive subsequent ALKi treatment at progression (6). In the French retrospective CLINALK study, subsequent next-generation ALKi was correlated with better survival outcomes in the multivariate analysis. Patients who received crizotinib-led sequential ALKi therapy had a median post-PD survival time of 25.0 months and an mOS time from metastatic disease diagnosis of 89.6 months (8). Xu et al. studied 138 patients with advanced ALK-rearranged NSCLC resistant to crizotinib, and found a significant difference in OS among subsequent therapies in the non-liver progression patients (mOS: 27.6 months with next-generation ALKis, 13.3 months with crizotinib continuation, and 10.8 months with chemotherapy, respectively, P=0.019). CBPD together with local therapy might be a feasible strategy for patients with progression in the brain beyond crizotinib resistance, and next-generation ALKis (mOS: 28.9 months with CBPD vs. 32.8 months with next-generation ALKis, P=0.204). Next-generation ALKis tend to provide a survival benefit to patients with non-liver progression (35).
It is not yet clear whether patients who receive both CBPD and subsequent next-generation ALKis would obtain a greater benefit in terms of OS. We observed disease progression in 85 patients who received crizotinib as the initial ALKi. Treatment strategies with progressive disease include: CBPD but no subsequent next-generation ALKis (n=17), CBPD and subsequent next-generation ALKis (n=16), no CBPD but subsequent next-generation ALKis (n=35), neither CBPD nor subsequent next-generation ALKis (n=17). Our analysis showed that patients who received neither CBPD nor subsequent next-generation ALKis had shorter OS than those who received CBPD but no subsequent next-generation ALKis (mOS: 9.7 vs. 20.3 months; P=0.015), no CBPD but subsequent next-generation ALKis (mOS: 9.7 vs. 41.1 months; P<0.001), and CBPD with subsequent next-generation ALKis (mOS: 9.7 months vs. NR; P<0.001). Thus, both CBPD and subsequent next-generation ALKis could have a survival benefit. Patients who received only subsequent next-generation ALKis had a prolonged OS compared to those who received only CBPD (mOS: 41.1 vs. 20.3 months; P=0.006), which suggests that the subsequent next-generation ALKis could have a greater survival benefit than CBPD. In patients who received subsequent next-generation ALKis, CBPD did not have an obvious positive effect on OS (mOS: NR vs. 41.1 months; P=0.093). Further, as the mOS of the patients who received both CBPD and subsequent next-generation ALKis was NR, we intend to continue tracking their disease progress.
In our multivariable Cox regression analysis, systemic chemotherapy before crizotinib was associated with an increased risk of death. Similar results have also been found in other studies (10,36). Thus, the early use of crizotinib (ahead of systemic chemotherapy) is important in crizotinib-led sequential ALKi therapy. However, for some unexpected reasons, such as drug unreachable, a failure to conduct genetic tests of the ALK fusion gene, or early implication of anti-tumor therapy on patients’ request while waiting for the genetic test results, patients will receive systemic chemotherapy before crizotinib. Given the negative effect of systemic therapy before sequential ALKi therapy on outcomes, the immediate testing for biomarkers after a diagnosis of advanced NSCLC and the early commencement of crizotinib is critical in crizotinib-led sequential ALKi therapy. We found that 3 variables (i.e., a PFS of crizotinib longer than the median, the sequential therapy of ALKis, and CBPD) were associated with a decreased risk of death. Further, CBPD and subsequent next-generation ALKi therapy could improve the outcomes of post-crizotinib patients. A PFS of crizotinib longer than the median may be a predictive index of a good prognosis. Of course, administering ALKi as soon as the genetic testing results of ALK-rearrangement are received, avoiding conventional systemic chemotherapy, and proper implication of CBPD and other ALKi as sequential therapy may lead to the most prolonged OS.
Most patients (51/57) who received a sequential therapy of ALKis received crizotinib as the initial ALKi, and only 6 were initially treated with a 2nd-generation ALKi. There was no significant difference in the OS between the two groups. Among the patients who received the crizotinib-led sequential therapy, the analysis demonstrated prolonged OS in patients who received 1st-line crizotinib and subsequent ALKis compared to those who received systemic therapy other than ALKis before crizotinib (mOS: NR vs. 28.6 months, P=0.042). Thus, the early use of crizotinib was positively associated with a benefit in terms of OS. Additionally, there was no significant difference in OS between patients who received crizotinib-led sequential therapy and those who received 2nd-generation ALKi-led sequential therapy, regardless of whether they received systemic treatment before crizotinib or not. Thus, clinical professionals should pay more attention to the early application of ALKis to eligible patients than to the type of ALKi. Notably, only 6 patients received 2nd-generation ALKi-led sequential therapy, accounting for 22.2% of the patients who received a 2nd-generation ALKi as the initial ALKi. The median PFS of the initial ALKi in the 6 patients treated with 2nd-generation ALKi-led sequential therapy was 13.3 months (95% CI: 9.5–17.1 months), which was much shorter than that in other clinical trials (34.8 months with alectinib in the ALEX study, 25.8 months with ensartinib in an eXalt3 study, and 24 months with brigatinib in the ALTA-1L study) (37-39). Given that the median follow-up period was 14.1 months, which did not reach the median PFS of most 2nd-generation ALKis, it is still too early to draw a conclusion about the best strategy for sequential ALKi therapy. Follow-up research needs to be conducted to gather more data.
As more and more 2nd- and 3rd-generation ALKis have been approved, the utilization patterns of the ALKi-related therapies have become an urgent problem. In this study, most patients were treated with crizotinib-led sequential therapy. This was mainly because crizotinib was the 1st ALKi approved by the National Medical Products Administration (NMPA) in 2013 and was the only ALKi available until 2018. Longer OS was observed in patients receiving sequential therapy of 2 types of ALKi but not in patients receiving 3 or more types. It appears that the OS of the ALK+ patients was mainly related to the first 2 types of ALKis. It is thought to be associated with the development mechanism of ALKi resistance. Many types of single mutations that occur in the ALK kinase domain can lead to ALKi resistance, and each ALKi appears to be associated with a specific acquired ALK mutation. The ALK G1202R mutation can develop resistance to most of the 1st- and 2nd-generation ALKis. Fortunately, G1202R/del is uncommon, and is only found in <2% of crizotinib-treated patients (34,40). Conversely, for the patients who previously received 1 or more 2nd-generation tyrosine kinases inhibitors, G1202R/del was the predominant ALK mutation, and was detected in 53% and 55% of circulating-free deoxyribonucleic acid and tumor tissue samples, respectively (34). According to the published data, tumor cells harboring the ALK G1202R mutation were insensitive to most of the 1st- and 2nd-generation ALKis, except lorlatinib.
Under our current strategy, we recommend shifting to another genomic-blinded sequential ALKi treatment if 2 or more resistance mutations are encountered. This may reduce the effectiveness of sequential therapy and could affect OS. Further, the small number of the enrolled subjects may have also led to a statistical bias. As few patients received sequential therapy of 3 or more ALKis, further research needs to be conducted to gather more data. Treatment guidelines recommend the use of multiple ALKis rather than chemotherapy. Currently, alectinib, brigatinib, or lorlatinib have been recommended for 1st-line therapy as the “preferred drugs” (41). It is likely that more and more patients will receive next-generation ALKi-led sequential therapy. Follow-up studies need to be conducted to examine the following issues: the selection of the initial ALKi; the effect of the initial duration of treatment on OS; and the strategy for sequential ALKi therapy. It may be difficult to draw a conclusion, as targeted therapy is highly individualized and long-term follow-up data are required.
Conclusions
Crizotinib and next-generation ALKis improve the OS of advanced NSCLC patients with the ALK rearrangement. The sequential therapy of ALKis may maximize the benefit of OS. The results of this study support the use of sequential ALKi therapy. However, further research needs to be conducted to determine whether there is a positive correlation between the number of sequential ALKis and prolonged survival. Receiving chemotherapy before sequential ALKi therapy produced no survival benefit. Thus, the 1st-line use of ALKi and sequential ALKi therapy may be the most efficient strategy for improving the outcomes of patients with the ALK rearrangement. A PFS of crizotinib longer than the median, the sequential therapy of ALKis, and CBPD may improve OS. Further studies will gradually reveal the effect of ALKi treatment strategies on the outcomes of advanced NSCLC patients with the ALK rearrangement.
Acknowledgments
Funding: The study was funded by Wu JiePing Medical Foundation (Award Number: 320.6750.2020-19-34 to Guangming Tian).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-622/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-622/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-622/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. Erratum in: N Engl J Med 2011 Feb 10;364(6):588. [Crossref] [PubMed]
- Griesinger F, Eberhardt W, Nusch A, et al. Biomarker testing in non-small cell lung cancer in routine care: Analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021;152:174-84. [Crossref] [PubMed]
- Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1539-48. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Ito K, Hataji O, Kobayashi H, et al. Sequential Therapy with Crizotinib and Alectinib in ALK-Rearranged Non-Small Cell Lung Cancer-A Multicenter Retrospective Study. J Thorac Oncol 2017;12:390-6. [Crossref] [PubMed]
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [Crossref] [PubMed]
- Pacheco JM, Gao D, Smith D, et al. Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:691-700. [Crossref] [PubMed]
- Waterhouse DM, Espirito JL, Chioda MD, et al. Retrospective Observational Study of ALK-Inhibitor Therapy Sequencing and Outcomes in Patients with ALK-Positive Non-small Cell Lung Cancer. Drugs Real World Outcomes 2020;7:261-9. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Chiari R, Metro G, Iacono D, et al. Clinical impact of sequential treatment with ALK-TKIs in patients with advanced ALK-positive non-small cell lung cancer: Results of a multicenter analysis. Lung Cancer 2015;90:255-60. [Crossref] [PubMed]
- Asao T, Fujiwara Y, Itahashi K, et al. Sequential Use of Anaplastic Lymphoma Kinase Inhibitors in Japanese Patients With ALK-Rearranged Non-Small-Cell Lung Cancer: A Retrospective Analysis. Clin Lung Cancer 2017;18:e251-8. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Lee JK, Park HS, Kim DW, et al. Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancer. Cancer 2012;118:3579-86. [Crossref] [PubMed]
- Li C, Kuo SW, Hsu HH, et al. Lung adenocarcinoma with intraoperatively diagnosed pleural seeding: Is main tumor resection beneficial for prognosis? J Thorac Cardiovasc Surg 2018;155:1238-1249.e1. [Crossref] [PubMed]
- Iida T, Shiba M, Yoshino I, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2015;10:1076-82. [Crossref] [PubMed]
- Yun JK, Kim MA, Choi CM, et al. Surgical Outcomes after Pulmonary Resection for Non-Small Cell Lung Cancer with Localized Pleural Seeding First Detected during Surgery. Thorac Cardiovasc Surg 2018;66:142-9. [Crossref] [PubMed]
- Wu Y, Huang Z, Rong T. Debulking operation for non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 1997;19:442-4. [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Nishio M, Kim DW, Wu YL, et al. Crizotinib versus Chemotherapy in Asian Patients with ALK-Positive Advanced Non-small Cell Lung Cancer. Cancer Res Treat 2018;50:691-700. [Crossref] [PubMed]
- Cruz BD, Barbosa MM, Torres LL, et al. Crizotinib Versus Conventional Chemotherapy in First-Line Treatment for ALK-Positive Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncol Ther 2021;9:505-24. [Crossref] [PubMed]
- Hong X, Chen Q, Ding L, et al. Clinical benefit of continuing crizotinib therapy after initial disease progression in Chinese patients with advanced ALK-rearranged non-small-cell lung cancer. Oncotarget 2017;8:41631-40. [Crossref] [PubMed]
- Liu C, Yu H, Long Q, et al. Real World Experience of Crizotinib in 104 Patients With ALK Rearrangement Non-small-cell Lung Cancer in a Single Chinese Cancer Center. Front Oncol 2019;9:1116. [Crossref] [PubMed]
- Yang G, Ma D, Xu H, et al. Treatment duration as a surrogate endpoint to evaluate the efficacy of crizotinib in sequential therapy for patients with advanced ALK-positive non-small cell lung cancer: A retrospective, real-world study. Cancer Med 2019;8:5823-30. [Crossref] [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Xu H, Ma D, Yang G, et al. Sequential therapy according to distinct disease progression patterns in advanced ALK-positive non-small-cell lung cancer after crizotinib treatment. Chin J Cancer Res 2019;31:349-56. [Crossref] [PubMed]
- Morcos PN, Nueesch E, Jaminion F, et al. Exposure-response analysis of alectinib in crizotinib-resistant ALK-positive non-small cell lung cancer. Cancer Chemother Pharmacol 2018;82:129-38. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J Clin Oncol 2020;38:3592-603. [Crossref] [PubMed]
- Tan DSW, Geater S, Yu CJ, et al. Ceritinib Efficacy and Safety in Treatment-Naive Asian Patients With Advanced ALK-Rearranged NSCLC: An ASCEND-4 Subgroup Analysis. JTO Clin Res Rep 2021;2:100131. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer. Version 1. 2022. December 7, 2021.


